Abstract
The aim of the paper was to determine potential of using grape pulp, marc and must in the beer production process. Samples were fermented using non-Saccharomyces yeasts (Dekkera bruxellensis 3429, Metschnikowia pulcherrima MG970690) and Saccharomyces cerevisiae Safale US-05 was used as a control. Grape marc was obtained by pressing grape must with a press. Subsequently, the grape marc, must and pulp were pasteurized and, together with wort, volumetrically introduced into fermentation flasks for fermentation. Mass changes taking place during the process were analyzed. Real extract, alcohol, free amino nitrogen (FAN) content, pH, color, sugars and organic acid profile were determined in obtained beers. The research has shown that the yeasts Dekkera bruxellensis 3429 fermented similarly to Saccharomyces cerevisiae Safale US-05. The yeast D. bruxellensis 3429 produced more alcohol in the finished beers in most cases and assimilated more FAN than M. pulcherrima MG970690. The D. bruxellensis 3429 strain most effectively used L-malic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the modern beer market is vast and constantly offering new types of beers, starting with low-alcohol beers, through flavored beers and ending with low-calorie beers, searching for new beer brewing techniques and methods has become very popular [1]. The innovations have increasingly involved changes in yeasts used to ferment wort. For many years, the species Saccharomyces cerevisiae/Saccharomyces pastorianus have been used in brewing, however, they are starting to be replaced by unconventional strains, which aims at meeting new consumer requirements. Non-Saccharomyces yeasts feature low fermentation capacity and higher ethanol stress sensitivity. They can also cause beer cloudiness and problems with its filtering and viscosity. On the other hand, the compounds that these microorganisms produce are capable of giving beer original flavor and aroma. Using such yeasts is a solution that can be considered bioflavoring, that is, using biological methods to synthesize flavors [2].
Modern consumer market offers a wide variety of beer styles: ale, lager, porter, stout, lambic, waisse, etc. The brewing industry features not only big beer producers, but also smaller, local breweries. Increasing competition and development of the industry force brewers to seek new production methods and paths. Innovations involving the use of sequencing technology, genomics and transcriptomics to create yeasts with desired phenotype features are one of them. Four streams have been determined within the innovations: synthetic hybridization of the strains Saccharomyces cerevisiae and non-cerevisiae Saccharomyces; the use of engineering techniques to improve wort fermentation efficiency; seeking new Saccharomyces cerevisiae strains in nontraditional sources (for instance in fermented food); the use of yeasts other than Saccharomyces cerevisiae to improve beer aroma [3]. An example of strains used in wort inoculation together with Saccharomyces cerevisiae can be Saccharomyces eubayanus. Its potential was noticed by the Heineken brewery which created a product exclusively using this species of yeast. The limited product edition the brewery created was called “Wild Lager” [4]. Another factor that leads to the evolution of the brewing industry are changes in consumer needs. An example of that can be an increased demand for light (up to 3.5% vol.) beers which has resulted in perfecting light beers. However, this phenomenon is characteristic of North America and Australia, it has not been so popular in Europe. The reason for such a popularity of light beer is its low bitterness compared to traditional beer. The low bitterness level stems from the lack or low concentration of certain compounds among which there are glycerol, oligosaccharides, polyphenols, iso-α-acids, fusel alcohols or trihydroxy fatty acids. Apart from this, light beer is produced using a variety of methods. One method is adding glucoamylase to wort just before or during its fermentation. The aim of such an addition is to decompose residual carbohydrates and dextrins to fermentable sugars, which makes beer less caloric. Some research on the use of genetically modified yeasts has also been conducted to improve yeast fermentation efficiency. The genetic modifications involve the introduction of amylolytic genes, which improve fermentation efficiency, into the yeast genome [5]. Current innovations are also largely focused on improving productivity, saving energy and creating novel products with the use of, for instance, unconventional yeasts and immobilized cells or with the use of novel brewing techniques such as high pressure fermentation [6].
Dekkera bruxellensis yeast contributes to the production of the unique organoleptic profile of the Belgian Lambic and Gueuze beers. Additionally, they have aroma enhancing properties of some red wines, and are also involved in the production of kombucha, cider and many other products [7]. D. bruxellensis are capable of fermenting a wide variety of sugars, both monosaccharides and complex carbohydrates. This is because this species has good ß-d-glucosidase activity. This enzyme is responsible for the hydrolysis of dextrins, which are residual sugars in beer, and also found in fruit, hops, wood, barrels used for the maturation of beers or wines [8]. D. bruxellensis are capable of producing high concentrations of volatile ethyl esters such as ethyl acetate, ethyl lactate and ethyl caprate. This contributes to the floral or fruity character of the beers [9, 10]. This species also produces flavoring substances such as acetic acid, isovaleric acid and phenolic compounds including 4-ethylphenol, 4-ethylguaiacol. The obtained metabolites contribute to the formation of the organoleptic profiles of Belgian Lambic beers and wines, for example the French Château de Braucastel. They give them a characteristic sour aftertaste [7, 11]. The above-mentioned advantages may contribute to an increase in the share of this yeast, especially in the production of special beers.
Metschnikowia pulcherrima is a common yeast found on the surface of fruit, flowers and insects, often present during spontaneous fermentation [12, 13]. M. pulcherrima exhibit good activity of hydrolytic, proteolytic and ß-glucosidase enzymes [13, 14]. The ability to create extracellular lipases and proteases contributes to better adaptation of cells to environmental conditions. The above-mentioned enzymes contribute to the production of various aromatic compounds [15]. The advantage of high proteolytic activity is the reduced turbidity in beer or wine. The secretion of pulcherriminic acid and 2-phenylethanol by M. pulcherrima has an antimicrobial effect and contributes to the inhibition of the growth of undesirable yeasts in the fermentation medium, such as Pichia, Candida, Torulaspora, Kluyveromyces or Hanseniaspora [16]. They are present as a permanent part of the microbiota of musts and wines and are involved in the initial stages of spontaneous fermentation. Their content is up to 18% of all yeasts involved in this process [17]. M. pulcherrima may in the future be the basis for the production of low-alcohol beverages with a rich aromatic profile, thanks to the ability of these yeasts to produce low concentrations of ethanol during sugar fermentation, as well as good enzymatic activity.
The constantly developing brewing industry is persistently seeking innovations and an original trend of combining beer and grapes can also be an example of such. In the production of such beer, a variety of methods can be applied. Grapes can be added in different beer brewing stages, for example in mashing or after wort has already been prepared. The fruit is usually used in the form of must [18]. Grape marc obtained after pressing grape must with a press is used to a much lesser extent. Its impact on taste profile of beer is much more subtle. Yet grape skins contain a lot of taste and aroma due to the tannins present in them. Introducing grape marc into beer can give it a winey character and original tartness. Information on fermenting beer with an addition of grape pulp is also scarce in literature. The same as with grape marc, grape must is more frequently used in such production. However, the use of grape pulp as an addition can be equally innovative, especially when using unconventional yeasts that produce compounds which have a profound impact on aroma-taste profile of fermented beverages. As grape marc is commonly considered to be waste, its use along with grape must as whole grape pulp can be seen as an innovation in the brewing industry.
The aim of the paper was to determine potential of using grape pulp, marc and must in the beer production process. Samples were fermented using non-Saccharomyces yeasts (Dekkera bruxellensis 3429, Metschnikowia pulcherrima MG970690) and Saccharomyces cerevisiae Safale US-05 was used as a control. Grape marc was obtained by pressing grape must with a press. Subsequently, the grape marc, must and pulp were pasteurized and, together with wort, volumetrically introduced into fermentation flasks for fermentation. Mass changes taking place during the process were analyzed. Real extract, alcohol, free amino nitrogen (FAN) content, pH, color, sugars and organic acid profile were determined in obtained beers.
Materials and methods
Materials
The yeast strains Saccharomyces cerevisiae Safale US-05, Dekkera bruxellensis 3429 and Metschnikowia pulcherrima MG970690 from the own collection of the Department of Fermentation Technology and Microbiology of the University of Agriculture in Kraków were used in the study. To make the Summer Ale style wort the malts Viking Pale Ale Malt (Viking Malt), Viking Pilsner Malt (Viking Malt), Viking Wheat Malt (Viking Malt) as well as the hop pellets Iunga PL 2019 (10% aa) and Crystal US 2017 (3% aa) were used. The white vine variety Solaris from the Srebrna Góra vineyard in Kraków was used for the study.
Preparation of wort, grape marc, must and pulp
Wort was prepared by heating 12.8 L of water to the temperature of 67 °C and subsequently adding 3 kg of the Viking Pale Ale Malt (Viking Malt), 1 kg of the Viking Pilsner Malt (Viking Malt) and 0.25 kg of the Viking Wheat Malt (Viking Malt). Mash was kept at the temperature of 64 °C for 60 min. The temperature was then raised to 77 °C and the mash continued to be kept at such temperature for 1 min. An iodine test was performed to determine if all starch had been saccharified. The mash was then transferred to a filter tank and left to develop a layer of spent grain. Subsequently the mash was filtered with liquor of 72 °C, yielding 27.5 L of wort. Then the wort was boiled for an hour. 25 g of the Iunga PL 2019 (10%) hop pellets and 25 g of the Crystal US 2017 (3%) hop pellets were added in the beginning of boiling and 10 min prior to the end of it, respectively, to obtain an appropriate degree of hopping (approximately 18 IBU). After boiling, the wort was left to cool down (extract 12.1°P). Grape marc was obtained by pressing grape must and it was subsequently pasteurized (100 °C, 15 min) together with must and pulp.
Inoculation and fermentation
Pure yeast cultures were passaged in triplicate. In the first stage, the strains were multiplied on the Sabouraud agar (Biocorp, Poland) slants for 24 h. Then the strains were transferred to 10 mL of Sabouraud Broth (Biocorp, Poland). After another 24 h dynamic propagation of the strains was conducted in 200 mL Sabouraud Broth (Biocorp, Poland) on a water bath shaker (120 rpm, 20 °C) for 48 h. After the multiplication process, the dry yeast mass was determined on a moisture analyzer and an appropriate amount of yeast slurry was centrifuged (10 min, 4989×g/min). Sediment obtained from centrifugation of the yeast slurry was washed with sterile water, centrifuged again under the same conditions and introduced to wort and wort with an addition of grape marc, must and pulp.
The basic raw material for fermentation was wort (extract 12.1°P, 18 IBU) and wort with the addition of grape marc, must and pulp. The samples of 0.3 L were fermented in 0.5 L glass flasks. The wort and appropriate volumes of grape marc, must and pulp were introduced into them (according to the variants below). The multiplied yeast slurry was introduced in an amount of 0.5 g d.w./L. The S. cerevisiae Safale US-05 yeast strain was used as a control. After carefully closing the flasks and attaching fermentation tubes filled with glycerin, the system was additionally sealed with parafilm. The fermentation process was conducted for 14 days at the temperature of 20 °C.
The fermentation was conducted using the yeast strains Saccharomyces cerevisiae Safale US-05, Dekkera bruxellensis 3429 and Metschnikowia pulcherrima MG970690 in the following variants (each sample in triplicate): wort; wort + 20% addition of grape marc/must/pulp; wort + 40% addition of grape marc/must/pulp.
Methods
Determination of fermentation dynamics
The fermentation rate was determined based on a mass loss of samples weighted every 24 h with 0.01 g accuracy. Results from three independent repetitions were presented as a percentage loss of the fermentation media mass.
Determination of real extract and alcohol content
Alcohol concentration in final beer was determined using the pycnometric method. For this purpose, the sample after fermentation was distilled. The obtained distillate was filled up to 100 g with distilled water, its density was determined and the concentration of ethanol was read from the adequate tables (Analytica EBC Methods 9.2.1, Analytica EBC Methods 9.4), (Analytica EBC, European Brewery Convention, 1998).
Determination of FAN content
Free amino nitrogen (FAN) was measured using ninhydrin-based methods with the use of the absorbance measurement at 570 nm (Beckman DU-650 UV – Vis) according to the method: 8.10 Free Amino Nitrogen in wort by Spectrophotometry (IM) (Analytica EBC, European Brewery Convention, 1998).
Determination of color
The color of the filtered samples was determined spectrophotometrically (Beckman DU-650 UV–Vis) at a wavelength of 430 nm (according to Analytica EBC Methods 8.5 and Analytica EBC Methods 9.6).
Determination of organic acids
Organic acids analysis was carried out on a Perkin-Elmer (USA) FLEXAR chromatograph equipped with a pump system, and a UV/Vis (monitored at 210 nm). Malic, tartaric, succinic, lactic, citric and acetic acids (Sigma-Aldrich) were determined using Rezex ROA-Organic Acid Aminex HPX-87H (300 mm, 18 cm × 7.8 mm). Samples were eluted isocratically at 40 °C with a mobile phase (0.005 mol/L H2SO4) at a flow rate of 0.4 mL/min.
Determination of sugars
Analyses were performed using a Shimadzu (Japan) NEXERA XR with an RF-20A refractometric detector. The separation was performed on a Asahipak NH2P-50 250 × 4.6 mm Shodex column (Showa Denko Europe, Germany), thermostated at 30 °C. The mobile phase was an aqueous solution of acetonitrile (70% v/v), while the isocratic elution profile (0.8 mL/min) lasted for 20 min. For quantitative determination, standard curves were prepared for the respective standards: fructose, glucose, sucrose, and maltose.
Statistical analysis
The results have been presented as the arithmetic mean of three repetitions, standard deviation included. Moreover, a repeated measures ANOVA and a Tukey's (HSD) multiple range test at the significance level of α = 0.05 have been performed.
Results and discussion
Characteristics of wort and wort with the addition of grape marc, must and pulp
Table 1 shows the results of analyses concerning pH, free amino nitrogen (FAN) content and color of wort and wort with the addition of grape marc, must and pulp. The lowest FAN content was determined in the variant with the addition of grape marc (97.3 mg/L; 42.1 mg/L) and the highest one was determined in the variant with the addition of grape must (124 mg/L; 136 mg/L). It has been assumed that the optimal FAN content in wort equals to approximately 130 mg/L and in grape must to approximately 190 mg/L [19]. Low FAN content in the variants before fermentation can be a sign of weak parameters of the used malt. It has also been observed that the addition of grape must increase FAN content in the samples (Tab. 1). The wort without any addition featured the darkest color (13.8 EBC). Wort color depends mainly on the malt used for its preparation. The addition of grape marc, must and pulp made the wort color lighter.
Fermentation dynamics
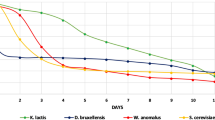
Figure 1 shows the fermentation process of the wort without any addition inoculated with the yeasts D. bruxellensis 3429, M. pulcherrima MG970690 and S. cerevisiae Safale US-05. It has been observed that the strains D. bruxellensis 3429 adapted to the environment the fastest. On the other hand, beers fermented with S. cerevisiae Safale US-05 showed the highest degree of attenuation. The species M. pulcherrima MG970690 featured the weakest fermentation properties (Fig. 1).
Figure 2 shows the fermentation process of the wort with 20% and 40% grape pulp addition. The species S. cerevisiae Safale US-05 and D. bruxellensis 3429 adapted to the environment the fastest and the strains M. pulcherrima MG970690 the slowest. There was a significant mass loss in the samples fermented with S. cerevisiae Safale US-05 and D. bruxellensis 3429 for the first four days of fermentation. The lowest mass loss throughout the whole fermentation process was observed in the wort with 20% grape pulp addition inoculated with D. bruxellensis 3429, whereas the highest one in the wort with 40% grape pulp addition inoculated with this species. What is interesting, mass loss in the 40% addition samples inoculated with D. bruxellensis 3429 was more rapid than for 20% addition ones (Fig. 2). It can be assumed that the increased pulp amount had a crucial impact on the faster and more intensive initiation of the fermentation process for the species D. bruxellensis 3429. The yeast D. bruxellensis 3429 was shown to ferment comparable to Saccharomyces cerevisiae Safale US-05 in the wort with the addition of grape pulp. The slowest adaptation to the environment and the lowest fermentation efficiency was observed in the species M. pulcherrima MG970690 (Fig. 2). It is possible that it was due to slow nitrogen absorption by this species [17].
Figure 3 shows the fermentation process of the wort with the addition of grape must. It has been observed that the higher the content of grape must in the variant was, the higher sample mass loss was. The fermentation process of the wort inoculated with D. bruxellensis 3429 was turbulent in the first days and the sample mass loss was significant. The mass loss stabilized and the fermentation process slowed down on average in the fifth day, which probably means that most sugars had been fermented (Fig. 3). A similar phenomenon was observed in the control samples (Fig. 1). The species M. pulcherrima MG970690 (Fig. 3) featured less intensive fermentation process compared to the remaining strains, which might be a sign of longer adaptation and slower sugars fermentation process by the species. In the species the fermentation process was not as turbulent as in the others and the mass decreased gradually and by small amount (Fig. 3).
Figure 4 shows the fermentation process of the wort with the addition of grape marc. In the wort fermented with D. bruxellensis 3429 and Saccharomyces cerevisiae Safale US-05 the sample mass loss was observed already on the first day. The fermentation process of the wort inoculated with these species was similar to the one of the wort with the addition of grape must (Fig. 3). The slowest adaptation to the environment and the lowest fermentation efficiency was observed in the species M. pulcherrima MG970690 (Fig. 4). Nardi et al. [20] proved that Saccharomyces ferment faster than Dekkera, which has not been shown by the conducted studies (Figs. 1, 2, 3 and 4). The higher the amount of grape must/pulp/marc was in the samples, the higher the number of carbon sources, the most key factor in the yeast metabolism, was. The rate of carbohydrate conversion increased, which directly influenced the speed of the entire process and its course.
Alcohol and real extract content
Real extract content determines the total amount of dissolved substances, i.e., unfermented carbohydrates, proteins, glycerol, beta-glucans, organic acids, amino acids, polyphenols and inorganic substances [21]. These compounds provide beer with full taste and subtle sweetness. Table 2 shows the real extract content in the tested samples. The least significant real extract content differences were observed in beers attenuated with S. cerevisiae Safale US-05 and D. bruxellensis 3429 (Tab. 1). On the other hand, the greatest differences were observed in M. pulcherrima MG970690 (35.1–61.8 g/L). Compared to beers fermented with M. pulcherrima MG970690, lower real extract content occurred in the samples fermented with D. bruxellensis 3429, which could be related to the β-glucosidase activity present in this species of yeast. The activity allows the assimilation of complex sugars. Typically, beers feature a residual extract content of less than 1% (highly attenuated beers, e.g., lambic) to about 10%. A high amount of residual sugars provides beer with a full and sweet taste, while a low amount allows for a lighter mouthfeel [22, 23].
The main compound obtained in the fermentation process is ethanol. Brewing yeasts can produce from 3 to 6% volume of this compound and its content depends on the species used and the conditions of its growth. Typically, the average beer alcohol content is from 4.8 to 5.2% volume [22]. Table 2 shows different alcohol content in the tested samples. The higher the amount of grape must/pulp/marc was in the samples, the higher the percentage alcohol content in beer was. This was due to the greater number of carbon sources that the microorganisms metabolized into ethanol. In the beers without any additions the lowest ethanol content was observed in the beers fermented with D. bruxellensis 3429 (3.32% (v/v). On the other hand, M. pulcherrima MG970690 and S. cerevisiae US-05 produced 3.87% (v/v) and 3.73% (v/v), respectively. Blomqvist et al. [24] proved that the ethanol production efficiency of D. bruxellensis 3429 is similar to that of S. cerevisiae Safale US-05, and often even higher. This is confirmed by the results presented in Table 2 for beers with additions. The beers with 20% addition of grape pulp, must and marc contained from 4.53% to 4.91% (v/v) of alcohol. A higher addition of grape pulp, must and marc (40%) lead to a higher ethanol production (from 5.53% to 5.86% (v/v)). The species M. pulcherrima MG970690 decomposed available sugars to a similar extent as D. bruxellensis 3429. The only exception was the beer with 40% addition of grape must in which the amount of alcohol produced by M. pulcherrima MG970690 was significantly lower (Table 2). It is believed that many strains of this species are able to produce up to 4.4% (v/v) of ethanol, sometimes reaching values up to 6–7% (v/v) [17]. According to Michel et al. [25], non-Saccharomyces yeasts can be used for the production of beers with low alcohol content (0.5–1.2% v/v) and non-alcoholic beers (< 0.5% v/v), which the data shown in Table 2 do not confirm.
pH
The decrease of the pH value is caused by the production of metabolites by yeast that contribute to the increase in the acidity of beverages. The reactions that lead to this include, among others, deamination reactions in which acids are formed, as well as the consumption of phosphates by yeast and the assimilation of ammonium and hydrogen ions. The acidity is, however, mainly affected by the formation of organic and inorganic acids. The former ones are divided into volatile and non-volatile ones. Acetic, propionic, isobutyric, butyric, valeric and caprylic acids are volatile acids that, in high concentrations, affect the sour and salty taste of beer. On the other hand, oxalic, citric, malic, lactic, fumaric, pyruvic and succinic acids are the main non-volatile acids [25]. The yeasts D. bruxellensis 3429 produce a lot of acetic acid and caprylic and capric fatty acids, thus they can be used to produce sour beers [2, 7]. The highest pH values were noted in beers without the addition of grapes. In the variants with the addition of grape marc, pulp and must the value of this parameter decreased (Table 3). The obtained results are similar to the values obtained for fruit beers analyzed by Nardini and Garaguso [26] in which pH was within the range of 3.56–4.87. The obtained results may be influenced by different yeast abilities to utilize nitrogen compounds or to produce metabolites, contributing to an increase in the acidity of beverages. The changes that lead to this include, among others deamination reactions leading to the formation of acids, consumption of phosphates dissolved in the wort and the assimilation of ammonium and hydrogen ions, followed by their release into beer [27, 28].
Free amino nitrogen (FAN)
Free amino nitrogen (FAN) is defined as the sum of amino acids, ammonium ions and low molecular weight peptides in wort. They are produced in the process of protein proteolysis during the malting and mashing processes [29]. The presence of free amino acids in wort is necessary for the proper structure of yeast which at the same time influences the proper conduct of fermentation by these microorganisms. During the fermentation process, brewing yeast strains can utilize up to 50% of the amino nitrogen present in wort. It is believed that bottom fermentation yeasts assimilate less of it than top fermentation yeasts [30]. Therefore, the content of free amino nitrogen in the finished beer is expected to be low, which proves that the fermentation has been conducted well and the finished drink has good organoleptic properties. Table 3 shows the content of free amino nitrogen in the samples. The assimilation of nitrogen compounds in the beers without any additions by the species D. bruxellensis 3429 and M. pulcherrima MG970690 was comparable to the one by S. cerevisiae Safale US-05 (59.5 mg/L and 66.9 mg/L, respectively) (Table 3). Comparing the beers with additions, the yeasts D. bruxellensis 3429 assimilated free amino nitrogen significantly better than M. pulcherrima MG970690. According to Tiukova [31], D. bruxellensis is capable of assimilating nitrogen from alternative sources. It is capable of using nitrates when other resources of this element are exhausted. Grape must features a high content of nitrogen compounds, the amount of which depends on the variety of grapes and the time of their harvest. Most of them, as much as 40%, are ammonium ions easily absorbed by yeast [32]. M. pulcherrima MG970690 assimilated ions in the beers with the addition of grape pulp and marc better than in the beers with the addition of grape must. This may be due to the extended fermentation initiation time, failure to assimilate possible nitrogen compounds, or the process being terminated too quickly [19].
Color
Beer color is mainly influenced by the raw materials used for its brewing (especially malt), as well as by the oxidation of polyphenols derived from malt and hops. The color is specified in EBC or SRM units [33]. It can be completely different from one beer style to another: starting from light yellow lager, for which the EBC value is 2–4, through golden (6–18 EBC), amber (20–30 EBC), to dark stout (70–140 EBC) [34]. Table 3 shows different colors of the tested samples. The highest value of EBC was found in the beers without additions (8.51–10.5 EBC) and with grape must (8.13–10.13 EBC). The samples attenuated with the addition of grape pulp and marc featured lower values (Table 3). The darkening of alcoholic beverages may be the result of, among others, Maillard reactions during which not only flavor compounds but also dyes are produced. Caramelization or oxidation reactions can also cause beer browning [35]. Moreover, it could be related to a higher FAN content in the beers tested. FAN has been proven to affect beer color. Excessive amount of free amino nitrogen can darken it. Another factor that affects the drink color is the time of boiling wort. The longer wort boiling is, the darker beer color is. The fermentation time also has an influence (the longer the process, the brighter the drink).
Organic acids
Organic acids in wort, grape pulp, marc and must
Table 4 shows the organic acid profile in grape must, pulp and marc and in wort and wort with additions. A high content of tartaric and malic acids was found in grape must and marc (Table 4). They are the main organic acids found in grapes. Tartaric acid is usually present in grapes in concentrations of 5–10 g /L, while the content of L-malic acid in ripe fruit usually ranges between 2 and 6.5 g /L. Its particularly large content (25 g /L) can occur in grapes harvested in cold climate [36]. The lowest malic acid content among the tested samples was found in wort (Table 4). The greater the addition of grape must, pulp and marc in the wort was, the higher the content of malic and tartaric acids in the samples was (Table 4). The highest amount of citric acid was observed in grape marc, while the lowest in grape must (Table 4). This proves that grape peel is the most abundant in it. A small amount of acetic acid was found in the analyzed samples (Table 4). The only exception was grape marc. The grape marc introduced into the wort was fresh, therefore it was not possible that it had been acescent.
Organic acids in beers
Organic acids in beer come from wort, but many of them are also produced by yeast metabolism. The formation and excretion of organic acids contribute to lowering the pH of the fermenting wort. They give beer sour or salty taste. The main organic acids found in beer are citric, acetic, lactic, pyruvic, malic and succinic acids. Organic acids are largely derived from the incomplete TCA cycle that occurs during anaerobic repressed growth of yeast. Some of them are derived from amino acid catabolism. Lactate comes from the reduction of pyruvate. The concentration of some organic acids can change during the fermentation process. It has been proven that pyruvate is removed into wort during early fermentation. Later in the process, this acid is reabsorbed by the yeasts and acetic acid is removed [37, 38].
Table 5 shows the profile of organic acids in the tested beers. The lowest content of organic acids was found in the beers without any additions, while the highest in the samples with grape marc (40%). Large amounts of tartaric acid was also noted in beers with 40% addition of grape pulp (D. bruxellensis 3429 and S. cerevisiae Safale US-05). Tartaric acid is resistant to degradation by microorganisms during the fermentation process, while malic and citric acids can be partially metabolized by yeast and bacteria, which reduces the acidity of wine [39, 40]. Our studies also showed no considerable differences in the tartaric acid content in the non-fermented samples and beers (Tables 4, 5). The tested yeasts decomposed malic acid to a considerable extent (Table 5). Saccharomyces yeasts exhibit different degradation abilities of L-malic acid during alcoholic fermentation (up to 3 g/L). The ability depends, among others, on the growth temperature of these microorganisms [41]. A high content of acetic acid was found in the beers with 20% addition of grape marc (Table 5). Yeast can produce small amounts of the acid, which may lead to an increase in the acidity of wine/beer compared to its level in must/wort. Significant amount of lactic and succinic acids was also found in the tested beers (Table 5). It is believed that the average content of these acids in beers is 50–300 mg/L and 50–150 mg/L, respectively [37], however, in the tested beers the amount of the acids found was higher (Table 5).
Sugars
Sugars in wort, grape pulp, marc and must
In the examined grape must, grape pulp and marc, a comparable content of glucose and fructose was found (Table 6). The actual concentration of glucose and fructose in grape must is from 80 to 130 g/L for each of the sugars separately. In addition, the grapes also contain traces of sucrose (2–10 g/L), rhamnose (up to 0.4 g/L) and arabinose (up to 1.5 g/L) [42]. The fructose content in the wort is in the range of 1.0–1.5 g/L [43]. In the analyzed wort, this sugar was detected at the level of 2.60 g/L (Table 6). In turn, glucose was present in the amount of 9.61 g/L (Table 6). Data reported in the literature show a range of this parameter between 8 and 10 g/L [43]. With the introduction of grape must, marc and grape pulp into the wort, the amount of glucose and fructose increased in the tested variants (Table 6). Among the sugars in a typical wort, maltose is present in the highest amount [43, 44]. Maltose in the tested wort exceeded the level reported in the literature (33–54 g/L) [43]. Most likely this was due to the type of malt used.
Sugars in beers
No glucose content was found in the tested beers or its value was at a very low level (Table 7), which proves that non-Saccharomyces yeast showed a similar glucose fermentation efficiency compared to S. cerevisiae Safale US-05. The obtained results are within the range given by the literature data for glucose (0–8 g/L) in beers after fermentation [45]. The highest content of maltose was found in beers with a 20% addition of marc (Table 7). In beers with grape must inoculated with the yeast D. bruxellensis 3429 and M. pulcherrima MG970690, a lower maltose content was found compared to beers fermented with yeast S. cerevisiae Safale US-05 (Table 7). Both S. cerevisiae and D. bruxellensis yeasts have variable maltose fermentation abilities [46]. The yeast D. bruxellensis 3429 showed the weakest fructose fermentation capacity. Its high content was found in beers with the addition of grape must and marc (Table 7). Typical content of glycerol in beers is in the range of 1–3 g/L [47, 48]. Glycerol is a by-product of yeast metabolism, therefore it was not found in the wort (Table 6). Its content in individual beers was significantly diversified (Table 7). Literature data indicate a higher content of glycerol in beers fermented with non-Saccharomyces yeast, compared to traditional brewer’s yeast [49]. This statement is in line with the results obtained. The exception was the beer inoculated with S. cerevisiae Safale US-05 yeast, without the addition of grapes, which showed a higher glycerol content in relation to the non-Saccharomyces strains (Table 7). On the other hand, the highest glycerol content was found in beers with the addition of grape marc (40%), inoculated with D. bruxellensis 3429 (Table 7).
Comparing the course of the fermentation dynamics with the use of sugars by the tested yeasts, it was found that despite the different adaptation times of the strains to the environment, all the tested yeasts carried out the fermentation satisfactorily. The non-Saccharomyces yeast showed a similar ability to attenuate and utilize the ingredients contained in the wort as S. cerevisiae Safale US-05. Despite the longer fermentation time of M. pulcherrima MG970690 strain and lower weight loss compared to the other trials, these yeasts used most of the available sugars in the fermenting worts (Figs. 1, 2, 3 and 4, Table 7).
Conclusions
The results have shown that the yeasts Dekkera bruxellensis 3429 fermented similarly to Saccharomyces cerevisiae Safale US-05. The greater addition of grape must, pulp and marc in wort resulted in the higher values of real extract, alcohol and free amino nitrogen. The highest content of organic acids was observed in the beers with the addition of grape marc and pulp, which related to a high content of such acids (tartaric and citric acid) in grape skins. It has been determined that the tested non-Saccharomyces strains are capable of producing beers with the required physicochemical parameters.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Sannino C, Mezzasoma A, Buzzini P, Turchetti B (2019) Non-conventional yeasts for producing alternative beers. In: Non-conventional yeasts: from basic research to application. Springer, Cham, pp 361–388
Capece A, Romaniello R, Siesto G, Romano P (2018) Conventional and non-conventional yeasts in beer production. Fermentation 4:38. https://doi.org/10.3390/fermentation4020038
Iattici F, Catallo M, Solieri L (2020) Designing new yeasts for craft brewing: when natural biodiversity meets biotechnology. Beverages 6:3. https://doi.org/10.3390/beverages6010003
Gibson B, Geertman J, Hittinger CT et al (2017) New yeasts—new brews: modern approaches to brewing yeast design and development. FEMS Yeast Res 17:1–13. https://doi.org/10.1093/femsyr/fox038
Blanco CA, Caballero I, Barrios R, Rojas A (2014) Innovations in the brewing industry: light beer. Int J Food Sci Nutr 65:655–660. https://doi.org/10.3109/09637486.2014.893285
Kellershohn J, Russell I (2015) Innovations in alcoholic beverage production. In: Advances in bioprocess technology. Springer, Cham, pp 423–433
Schifferdecker AJ, Dashko S, Ishchuk OP, Piškur J (2014) The wine and beer yeast Dekkera bruxellensis. Yeast 31:323–332. https://doi.org/10.1002/yea.3023
Steensels J, Daenen L, Malcorps P et al (2015) Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int J Food Microbiol 206:24–38. https://doi.org/10.1016/j.ijfoodmicro.2015.04.005
Piškur J, Ling Z, Marcet-Houben M et al (2012) The genome of wine yeast Dekkera bruxellensis provides a tool to explore its food-related properties. Int J Food Microbiol 157:202–209. https://doi.org/10.1016/j.ijfoodmicro.2012.05.008
de Barros PW, Teles GH, Peña-Moreno IC et al (2019) The biotechnological potential of the yeast Dekkera bruxellensis. World J Microbiol Biotechnol 35:1–9. https://doi.org/10.1007/s11274-019-2678-x
Basso RF, Alcarde AR, Portugal CB (2016) Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res Int 86:112–120. https://doi.org/10.1016/j.foodres.2016.06.002
Lachance M (2016) Metschnikowia: half tetrads, a regicide and the fountain of youth. Yeast 33:563–574. https://doi.org/10.1002/yea.3208
Pawlikowska E, Gregiel D (2017) Niekonwencjonalne drożdże Metschnikowia pulcherrima i ich zastosowanie w biotechnologii. Postępy Mikrobiol 56:405–415
Vicente J, Ruiz J, Belda I et al (2020) The genus Metschnikowia in enology. Microorganisms 8:1038. https://doi.org/10.3390/microorganisms8071038
Padilla B, Gil JV, Manzanares P (2016) Past and future of non-Saccharomyces yeasts: from spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front Microbiol 7:411. https://doi.org/10.3389/fmicb.2016.00411
Muccilli S, Restuccia C (2015) Bioprotective role of yeasts. Microorganisms 3:588–611. https://doi.org/10.3390/microorganisms3040588
Morata A, Loira I, Escott C et al (2019) Applications of Metschnikowia pulcherrima in wine biotechnology. Fermentation 5:63. https://doi.org/10.3390/fermentation5030063
Walklate J (2011) Uncorking the past: the quest for wine, beer, and other alcoholic beverages. Time Mind 4:111–114
Hill AE, Stewart GG (2019) Free amino nitrogen in brewing. Fermentation 5:22. https://doi.org/10.3390/fermentation5010022
Nardi T, Remize F, Alexandre H (2010) Adaptation of yeasts Saccharomyces cerevisiae and Brettanomyces bruxellensis to winemaking conditions: a comparative study of stress genes expression. Appl Microbiol Biotechnol 88:925–937. https://doi.org/10.1007/s00253-010-2786-x
Olšovská J, Šterba K, Vrzal T (2019) Nutritional composition and energy value of different types of beer and cider. Kvas Prum 65:32–37. https://doi.org/10.18832/kp2019.65.32
Garrett O (2012) The Oxford Companion to Beer. Oxford University Press, New York
Moktaduzzaman M, Galafassi S, Capusoni C et al (2015) Galactose utilization sheds new light on sugar metabolism in the sequenced strain Dekkera bruxellensis CBS 2499. FEMS Yeast Res 15:1–9. https://doi.org/10.1093/femsyr/fou009
Blomqvist J, Eberhard T, Schnürer J, Passoth V (2010) Fermentation characteristics of Dekkera bruxellensis strains. Appl Microbiol Biotechnol 87:1487–1497. https://doi.org/10.1007/s00253-010-2619-y
Michel M, Kopecká J, Meier-Dörnberg T et al (2016) Screening for new brewing yeasts in the non-Saccharomyces sector with Torulaspora delbrueckii as model. Yeast 33:129–144. https://doi.org/10.1002/yea.3146
Nardini M, Garaguso I (2020) Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem 305:125437. https://doi.org/10.1016/j.foodchem.2019.125437
Mochaba FM, Torline PA, Vundla W et al (1999) Slurry pH as an indicator of yeast autolysis. Proc Convers Inst Brew Afr Sect 7:205–207
Kunze W (1999) Technologia piwa i słodu. Piwo-chmiel. VLB, Berlin
Lekkas C, Stewart GG, Hill AE et al (2007) Elucidation of the role of nitrogenous wort components in yeast fermentation. J Inst Brew 113:3–8. https://doi.org/10.1002/j.2050-0416.2007.tb00249.x
Ferreira IM, Guido LF (2018) Impact of wort amino acids on beer flavour: a review. Fermentation 4:23. https://doi.org/10.3390/fermentation4020023
Tiukova I (2014) Dekkera bruxellensis, a non-conventional ethanol production yeast. Doctoral Thesis, Uppsala
Beltran G, Novo M, Rozes N et al (2004) Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentations. FEMS Yeast Res Res 4:625–632. https://doi.org/10.1016/j.femsyr.2003.12.004
Koren D, Vecseri BH, Kun-Farkas G et al (2020) How to objectively determine the color of beer? J Food Sci Technol 57:1183–1189. https://doi.org/10.1007/s13197-020-04237-4
Shellhammer TH (2009) Beer color. In: Beer: a quality perspective. Elsevier Inc., Amsterdam, pp 213–227
Daniels R (1998) Designing great beers: The ultimate guide to brewing classic beer styles. Brewers Publications
Volschenk H, Van Vuuren HJJ, Viljoen-Bloom M (2006) Malic acid in wine: origin, function and metabolism during vinification. S Afr J Enol Viticult 27:123–136. https://doi.org/10.21548/27-2-1613
Briggs DE, Brookes PA, Stevens R, Boulton CA (2004) Brewing: science and practice. Woodhead Publishing Limited, Cambridge
Santalad A, Teerapornchaisit P, Burakham R, Srijaranai S (2007) Capillary zone electrophoresis of organic acids in beverages. LWT-Food Sci Technol 40:1741–1746. https://doi.org/10.1016/j.lwt.2007.01.007
Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius IS (2005) Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res 11:139–173. https://doi.org/10.1111/j.1755-0238.2005.tb00285.x
Moreno-Arribas MV, Polo MC (2009) Wine chemistry and biochemistry, 1st edn. Springer, New York
Vilela-Moura A, Schuller D, Mendes-Faia A, Côrte-Real M (2008) Reduction of volatile acidity of wines by selected yeast strains. Appl Microbiol Biotechnol 80:881. https://doi.org/10.1007/s00253-008-1616-x
Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A (2006) Handbook of enology, volume 1: The microbiology of wine and vinifications. Wiley, New York
Hough JS, Hough JS (1991) The biotechnology of malting and brewing. Cambridge University Press
Serrano R (1977) Energy requirements for maltose transport in yeast. Eur J Biochem 80:97–102. https://doi.org/10.1111/j.1432-1033.1977.tb11861.x
Bamforth CW (2005) Beer, carbohydrates and diet. J Inst Brew 111:259–264. https://doi.org/10.1002/j.2050-0416.2005.tb00681.x
Blomqvist J, Passoth V (2015) Dekkera bruxellensis—spoilage yeast with biotechnological potential, and a model for yeast evolution, physiology and competitiveness. FEMS Yeast Res 15:fov021. https://doi.org/10.1093/femsyr/fov021
Esslinger HM (2009) Handbook of brewing: processes, technology, markets. Wiley
Zhao X, Procopio S, Becker T (2015) Flavor impacts of glycerol in the processing of yeast fermented beverages: a review. J Food Sci Technol 52:7588–7598. https://doi.org/10.1007/s13197-015-1977-y
Contreras A, Hidalgo C, Henschke PA et al (2014) Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl Environ Microbiol 80:1670–1678. https://doi.org/10.1128/AEM.03780-13
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MC-S: conceptualization, methodology, validation; SS: software; MC-S, ZT, KK: formal analysis and investigation; MC-S: original draft preparation; MC-S, SS and PS: review and editing; MC-S, SS: visualization. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Compliance with ethics requirements
All authors were compliant andfollowed the ethical guidelines, according to the requirements of European Food Research and Technology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cioch-Skoneczny, M., Królak, K., Tworzydło, Z. et al. Characteristics of beer brewed with unconventional yeasts and addition of grape must, pulp and marc. Eur Food Res Technol 249, 699–711 (2023). https://doi.org/10.1007/s00217-022-04166-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04166-w