Abstract
Dry fractionated legume protein ingredients are gaining attention as alternatives to conventional solvent extracted legume proteins, being more resource efficient and often exhibiting novel functional properties. However, lack of knowledge about the relationship between composition and functionality limit a more wide-spread use of dry-fractionated legume protein in applications. In this study, lentil fractions of different degrees of refinement were prepared using air classification having protein and starch contents of 16–59% and 4–64%, respectively. The dry fractionated lentil fractions could emulsify and stabilize 10 wt% oil-in-water emulsions, while a conventional lentil protein isolate used for comparison was not able to form stable emulsions. The latter had significantly larger mean droplet diameters (around 20 µm) due to droplet flocculation than emulsions made with the different lentil fractions ranging between 0.3 and 5.5 µm. Similar surface charges (between −22 and −31 mV) indicated that the discrepancy could be ascribed to differences in steric repulsion and mechanical strength of the interfacial layers between conventionally and dry fractionated lentil. Storage stability tests of emulsions stabilized with dry fractionated samples resulted in separation into a low and higher density phase with the individual droplets being stable against coalescence in both phases. The phase separation was attributed to gravimetrical sedimentation of larger insoluble components accumulating in the denser phase, which was impacted by the degree of refinement by air classification. The results highlight the potential of dry fractionation for the production of sustainable ingredients with unique composition and functionality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing world-wide demand for protein is one of the major challenges for the global food system [1]. It is generally accepted that this demand can only be satisfied with a high proportion of plant-based proteins. Accordingly, as more and more consumers request plant-based alternatives, the food industry is developing a growing product range of plant protein ingredients. The application of plant proteins (e.g., legume or cereal) in modern food products usually requires previous refinement of the raw material to obtain a protein concentrate or isolate. To date, the refinement is mostly accomplished with wet fractionation techniques, which traditionally involve alkaline extraction, followed by isoelectric precipitation and subsequent drying [2]. However, in the last years, dry fractionation has been increasingly investigated as a class of alternative protein refinement techniques. They generally do not require any solvents, and include air classification [3], electrostatic separation [4], or shear induced separation [5]. We here focus on air classification due to its prevalent use in ingredient manufacture and its good scalability. The working principle of air classification is based on a separation of particles by size and density [3]. Starch-rich legumes such as pea or lentil are well suited for protein enrichment by air classification due to the size differences between starch granules and protein bodies. Initially, these structures are liberated by a milling process yielding a finely milled flour that can then be separated in a protein-rich and a starch-rich fraction by air classification. Variation of the air classification settings allows for the production of ingredients that vary in their protein content and thus in their degree of refinement.
Dry fractionation was shown to be more sustainable and economic, because no water or solvents are required, which later necessitates an energy consuming drying step [3], and can have higher protein recovery than wet processes, since not all proteins are dissolved and precipitated in these processes and thus cannot be fully recovered. In addition. there are differences in functionality compared to wet fractionated protein ingredients. Wet fractionation yields protein fractions that have characteristic solubility curves with low solubilities around the isoelectric point of the extracted proteins. This is because the extraction often uses precipitation at the isoelectric point to form protein sediments that is then separated by filtration. Furthermore, wet fractionated protein ingredients may contain high amounts of insoluble protein aggregates due to dehydration processes like spray or drum drying. Moreover, during drying, proteins may be exposed to high product temperatures that can cause changes in the state of proteins, i.e., a loss of nativity, which in turn affects their functional properties [6, 7].
In contrast, dry fractionation constitutes a milder extraction technique that subjects proteins to less severe stresses. However, the purity of dry fractionated ingredients is lower compared to wet fractionated protein isolates, and ingredients are comprised of a multitude of other potentially functional components, such as polysaccharides and/or polyphenols. Interestingly, complex compositions may also provide for unique properties, that make them better suited for certain applications than ingredients that are purer. For example, such ingredients have proven to be quite suitable in forming fibers in shear cells [8, 9] to be used in the manufacture of meat analogues. In emulsions, the protein could form an interfacial layer and act as emulsifier, while the residual carbohydrates could simultaneously serve as stabilizing thickening agents. Using this in food products will require a better understanding of the functional properties in relation to their composition. To that purpose, we used air classification to prepare lentil protein ingredients containing different amounts of protein and analyzed their characteristics and composition. This was followed by examination of the emulsifying properties and emulsion stability of the dry fractionated samples, as well as emulsions made with wet extracted lentil protein isolate for comparison. We hypothesized that dry fractionated protein concentrates differ in their emulsifying properties compared to conventionally wet fractionated protein isolate due to their more complex composition. Furthermore, we expected that the degree of refinement by air classification affects its functionality. The aim of the study was to broaden the knowledge on how the degree of refinement by dry fractionation impacts their functional properties as an emulsifier for oil-in-water emulsions. Results may facilitate an increased use of the resource-efficient dry fractionation process in the food industry.
Materials and methods
Materials
Small brown lentils (Lens culinaris Medikus) of the regional variety “Alb Leisa – Die Kleine” were purchased from Lauteracher Alb-Feld-Früchte (Lauterach, Germany). Lentil protein isolate (LPI) of the red lentil variety „Itaca “ was kindly provided by Fraunhofer IVV (Freising, Germany) containing 82.1% protein of total weight. Miglyol 812 N, a medium chain triacylglyceride mixture was obtained from Cremer Oleo GmbH & Co. KG (Hamburg, Germany). Sodium phosphate monobasic monohydrate (purity ≥ 99%) and sodium phosphate dibasic anhydrous (purity ≥ 99%) were purchased from Merck (Darmstadt, Germany), respectively, from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). Sodium hydroxide (purity ≥ 99%), ortho-phosphoric acid (purity ≥ 85%), and sodium dodecyl sulphate (SDS) (purity ≥ 99%) were obtained from Carl Roth GmbH & Co. KG (Karlsruhe, Germany).
Air classification and characterization of protein fractions
Fractionation
The hulls of the seeds were removed using a horizontal stone mill (360 W Queen 1, Hawos Kornmühlen GmbH, Bad Homburg, Germany). The hulled lentils were finely milled at a rotational speed of 12,000 rpm with an ultra-centrifugal mill (ZM 200, Retsch GmbH, Haan, Germany) equipped with a ring sieve of 0.25 mm perforation. The received lentil flour was air classified (50 ATP, Hosokawa-Alpine, Augsburg, Germany) at an air flow of 65 m3/h with a feed rate between 0.75 and 1 kg/h in batches of about 500 g. The classifier wheel speed was decreased in the steps 11,000, 9000, 7000, 3000 rpm, whereby the respective remaining (coarse) fraction was air classified again with the lower classifier wheel speed. Consequently, four fine fractions 11000F, 9000F, 7000F, 3000F and a coarse fraction 3000C were obtained. In the following we subsumed these fractions as lentil flour fractions (LFF). The yield was defined as the ratio of the respective fraction to the mass of all obtained fractions. Thus, losses during air classification (about 11%) were not considered.
Compositional analysis
Protein content was determined with the Dumas combustion method (Dumatherm N Pro, C. Gerhardt GmbH & Co. KG, Königswinter, Germany). A nitrogen conversion factor of 6.25 was used to calculate the protein content. Starch content analysis by polarimetry was performed according to VO (EG) Nr. 152/2009 III L. The amino acid profile was determined according to VO (EG) Nr. 152/2009 III F & G. Values were normalized to the total percentage of amino acids. Dry matter content was analyzed by drying 2 g of coarsely milled lentils overnight at 105 °C in an oven.
Bulk density
Bulk density was determined following DIN EN ISO 60:2000–01 2000 using a bulk density measuring device (SMG 697, Powtec Maschinen und Engineering GmbH, Remscheid, Germany).
Particle size
Particle size analysis of the lentil flour and air classified fractions was done using a laser diffraction instrument (Mastersizer 2000, Malvern Instruments, Worcestershire, UK). The instrument measures the intensity of scattered light as a function of the scattering angle. The Mie theory is then used to estimate the particle size distributions from the scattering patterns.
Emulsion preparation and analysis
Homogenization
10 wt% oil-in-water emulsions with 1, 2, and 3 wt% of the fractions 7000F, 9000F, and 11000F as well as with 1 and 2 wt% LPI were prepared. The samples were dispersed in 0.01 M sodium phosphate buffer at pH 7 and stirred overnight at room temperature. The next day, the pH was readjusted with sodium hydroxide and ortho-phosphoric acid and oil was added. Coarse emulsions were first produced using a high shear blender (Silent Crusher, Heidolph Instruments GmbH and Co. KG, Schwabach, Germany) for 2 min at 15,000 rpm, followed by a subsequent three pass homogenization with a microfluidizer (LM10, Microfluidics Corporation, Westwood, MA, USA) at 1000 bar. The samples were analyzed shortly after homogenization.
Storage stability
Aliquots of freshly prepared emulsions were stored for 2 days at 4 °C and analyzed thereafter. Samples were differentiated in an upper phase, a middle phase (serum) and a lower phase (Fig. 1). The upper and lower phases were carefully separated using a pipette and then further analyzed. The middle phase was comprised predominately of water and was not further analyzed.
Physical appearance and optical microscopy
Pictures of all emulsion samples were taken using a digital camera. Microscope images of the samples were taken at 100-fold magnification with an optical light microscope (AXIO Scope.A1 Light Microscope, Carl Zeiss MicroImaging GmbH, Jena, Germany) equipped with a camera (Canon Powershot G10 Digital camera, Canon, Tokyo, Japan).
Size distributions
Particle size distributions of the emulsions were measured with a static light scattering device (Horiba LA-950, Retsch Technology GmbH, Haan, Germany). Emulsions were diluted to about 0.005 wt% with 0.01 M sodium phosphate buffer (pH 7) to avoid multiple scattering effects. Refractive indices of 1.52 and 1.33 were used for the oil droplets and the dispersion medium, respectively.
ζ-potential
The ζ-potential of the emulsions was determined using a particle electrophoresis instrument (Nano-ZS, Malvern Instruments, Malvern, U.K.). There, the emulsions were diluted to an oil concentration of 0.1 wt% with 0.01 M sodium phosphate buffer (pH 7). The Smoluchowski equation (Eq. 1) was used to calculate the ζ-potential [10]:
where ɛ is the relative permittivity, UE is the particle mobility, and η is the dynamic viscosity.
Statistics
All experiments were performed at least in duplicate. Measurements were repeated at least in triplicates and means and standard deviations were calculated from these (Microsoft Excel, Microsoft Corporation, Redmond, Washington State, USA). The measurements were statistically analyzed with a one-way analysis of variance (ANOVA) combined with a Tukey-post-hoc test using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA). Statistically significantly different values (α = 0.05) were denoted with different letters.
Results and discussion
Air classification and composition of lentil flour fractions
Milled lentil flour was air classified into five different fractions by varying the classifier wheel speed. The bulk density and particle size of the obtained lentil flour fractions (LFF) decreased with increasing classifier wheel speed (Table 1). Protein bodies (1–3 µm) are much smaller than starch granules (± 20 µm) [11], thus the LFF contents of protein increased, and that of starch decreased with decreasing particle size. However, this correlation did not hold true for fraction 3000C, since insufficiently milled lentil particles, i.e., not properly detached starch granules and protein bodies, accumulated in this fraction. Consequently, there is potential for increasing the yield of the air classification process by adapting the milling process. However, too fine milling can lead to particle agglomeration due to Van der Waals forces, and can crush the starch granules to the size of protein bodies, leading to a decreased separation efficiency [12], and poorer ingredient quality.
The protein content ranged from 16.2 to 59.1% and the starch content from 63.7 to 4.4% (Table 1). The dry matter content of the lentils was determined as 89.74 ± 0.28%, and thus the calculated protein content in the dry matter was about 65.8% in fraction 11000F, and 55.3% in fraction 9000F. In previous studies, the protein content in the dry matter of air classified lentil flour was 57.9%, 64.6%, 49.3%, and 58.5%, respectively [11, 13,14,15]. Our values were slightly higher, probably because of the higher initial protein content. The wet fractionated lentil protein isolate (LPI) that we use for comparison purposes had a protein content of 82.1% and was devoid of starch (Table 1). LPI and LFF were prepared from two different lentil varieties, namely, “Itaca” and “Alb Leisa”, respectively. Since it is known that the amino acid composition can potentially influence the functionality [16] we compared the amino acid compositions of LPI and LFF. Results thereof are shown in Table 2. The composition was similar and thus in this case the functionality should not be affected by the variety. The normalized amino acid composition is comparable with values from literature [17, 18], consisting mainly of glutamic acid, aspartic acid, arginine, leucine, and lysine.
Properties of oil-in-water emulsions manufactured with LPI and LFF
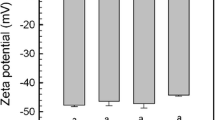
We prepared 10 wt% oil-in-water emulsions using 1, 2 wt% LPI and 1, 2, 3 wt% of the LFF 7000F, 9000F, and 11000F. Accordingly, the emulsions had different contents of protein and starch as shown in Table 3. The emulsions prepared with LPI immediately phase separated and flocculated after homogenization, while samples prepared with LFF showed no phase separation shortly after homogenization regardless of the protein content (Fig. 2). The droplet diameter d32 of the emulsions prepared with LPI (about 20 µm) was significantly (p < 0.05) larger than with LFF (between 0.3 and 5.5 µm) (Table 3). Indeed, microscopy revealed large agglomerates in the LPI-stabilized emulsions, which could be broken up by the addition of SDS (0.5 wt%) to the emulsions reducing the droplet diameter d32 to about 0.2 µm (Fig. 3). The values of LFF are in line with the results of a previous study using also dry fractionated lentil fractions [19]. For lentil protein isolate similar droplet diameters were reported in the literature when SDS or Tween 20 was added to disrupt droplet flocs [20, 21] and likewise larger droplet diameters were measured when no SDS was added [22]. The surface charge of the emulsions was comparable, i.e., the ζ-potential of emulsions made with LPI ranged between −22 and −31 mV, while it was around −30 mV for emulsions made with LFF (Table 3). Earlier studies reported emulsions having ζ-potential values at pH 7 of around −40 mV with lentil protein isolate [20, 23] and around −20 mV with dry fractionated lentil fractions [19]. Our results indicate that the surface charge of the oil droplets was not the cause for the diverging emulsifying properties of LPI and LFF, suggesting that differences in steric repulsion and mechanical strength of the interfacial layer may play a role. The differences could be caused by the isoelectric precipitation and heat exposure used during downstream processing of the isolate. Such treatments are known to alter the legumin/vicilin ratio, respectively, the globulin/albumin ratio [3, 24] and lead to structural alterations, such as the loss of nativity [25]. Nevertheless, the mentioned differences in emulsifying properties between wet and dry fractionation might be at least partly related to the presence of other components, such as starch, fibers, and polyphenols in LFF.
Optical microscopy images (100-fold magnification) of 10 wt% oil-in-water emulsion stabilized by 2 wt% LPI without (A) and with (B) addition of SDS shortly after homogenization. The addition of SDS led to the separation of agglomerates and reduced the droplet diameter d32 from 17.9 ± 4.1 to 0.18 ± 0.01 µm. Scale bar: 50 µm
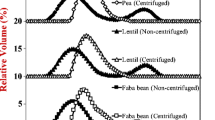
Although all emulsions prepared with LFF were stable shortly after homogenization, they yielded different average droplet diameters d32. The droplet diameter correlated negatively with the protein content, indicating that in some cases the interface was insufficiently covered (Fig. 5) [26]. In all emulsions prepared with LFF, impurities were visible under a light microscope (Fig. 4). The multimodal particle size distributions (Fig. 5) indicated a heterogeneous composition of oil droplets and larger particles, such as starch granules and protein aggregates [21]. The amount of these impurities was larger in the fractions 9000F and 7000F. Since some of these impurities may impact the stability of the emulsions [27], we, therefore, investigated the behavior of the emulsions during storage.
Storage stability
The emulsions were assessed after storage of 48 h at 4 °C. Visual examination of the emulsions revealed phase separation in all samples, which is slightly visible in the pictures but more evident when using backlighting in combination with proper positioning of test tubes (Fig. 2). The emulsions separated into an upper phase, a serum, and a lower phase (Fig. 1). Upper and lower phases were carefully separated and further analyzed. The emulsions made with LPI did not develop a lower phase and remained phase separated in an upper phase and a serum. Microscopy showed that the upper phases of all emulsions were free of impurities, unlike the freshly prepared emulsions (Fig. 4). Static light scattering confirmed the absence of particles larger than 10 µm in the upper phases (Fig. 5). Larger particles had, therefore, sedimented into the lower phases, in which the median particle diameter d0.5 was larger than 10 µm and no particles smaller than 1 µm were present (Fig. 5). Microscopy confirmed the inhomogeneity of the mixture, with large aggregated or flocculated structures in the lower phases (Fig. 4). The protein content of the sample containing 3 wt% 11000F was slightly higher in the lower phase (1.86 ± 0.01%) than in the upper phase (1.67 ± 0.01%). The differences in protein content suggested that undissolved protein sedimented and accumulated in the lower phase.
Being aware of the complex composition of LFF we further looked for the presence of additional components, such as cell wall material and undissolved carbohydrates. Indeed, the lower phase of emulsions prepared with 7000F contained many more starch granules than the low-starch fractions 9000F or 11000F (Fig. 4). In addition, the level of the lower phase was much higher when using 7000F, which we attributed to an overall higher content of non-protein components. The observed phase separation was not related to classical emulsion destabilization mechanisms. The average droplet diameter d32 of the upper phases did not significantly change due to storage aside from a small decrease, and in essence, the two phases each were stable emulsions albeit with a different composition. This was likely related to the inclusion of aggregates and insoluble particles in or around some emulsion droplets causing density to increase and leading to sedimentation. Variation of the protein content had no impact on the stability of the oil droplet size in the upper phase (Fig. 5), suggesting that classical destabilization mechanisms, such as coalescence, Ostwald ripening or flocculation did not play a significant role [26]. Creaming might have played a role at low protein content and larger oil droplet sizes, as the formation of a serum phase in emulsions with 7000F indicated (Fig. 2). Nevertheless, we assumed that the observed phase separation in LFF-stabilized emulsions was mainly driven by the gravimetric sedimentation of large insoluble components according to Stokes Law. A similar behavior was observed in a previous study with suspensions of air classified pea fractions [28]. The authors observed several solid and gel-like layers of sedimented insoluble components, such as starch, fiber, and protein.
Air classification thus leads to the production of a range of fractions that are capable of emulsion stabilization, but ratios of protein to carbohydrates in these ingredients play a major role in the subsequent storage behavior. Elimination of larger non-protein materials leads to more stable emulsions, but as of yet, a phase separation cannot be avoided entirely. LFF possess a complex composition of proteins, carbohydrates, and other components, which add and change functionality. As our results show, the use of LFF in concentrated emulsions such as plant-based mayonnaise alternatives, salad dressings, and spreads may give excellent stability. Due to their complex composition LFF potentially could fulfill multiple functions in such products, acting not only as emulsifier, but also as thickening agent and maybe even as colorant. However, the application in dilute emulsions will require further refinement to avoid phase separation. The removal of the insoluble components by centrifugation was already successfully applied for dry fractionated legume [19], but this process necessitates the wetting of the material and requires a subsequent drying process eliminating key benefits that dry fractionation offers. An alternative could be a high-pressure homogenization of suspended LFF to yield a more homogeneous suspension that could be used for emulsion preparation, i.e., using a two stage production process. A previous study found that such treatments can increase the emulsifying properties of protein isolates [29]. However, preliminary studies on this showed, that a preceding homogenization of LFF (1000 bar, three cycles) in fact increased the average diameter d32 of the emulsions containing 3 wt% 11000F from 0.24 ± 0.02 µm to 1.57 ± 0.34 µm. The contradictory behavior of the wet fractionated LPI and the dry fractionated LFF should be elucidated in further studies.
Conclusions
Air classification was used to produce lentil flour fractions with different composition. Their application in the manufacture of a 10 wt% oil-in-water emulsion showed different emulsifying properties compared to wet separated lentil protein isolate. The droplet sizes of LFF-stabilized emulsions were much lower than of LPI-stabilized emulsions, because LPI-stabilized emulsions were prone to droplet flocculation. LFF was initially able to stabilize emulsions, while LPI was not, but LFF-stabilized emulsions phase separated after storage, which could not be ascribed to classical destabilization mechanisms. Instead, we attributed the destabilization to gravimetric sedimentation of larger particles and aggregates, dragging some the associated oil droplets with it. These insoluble components are typically removed during a wet fractionation process but remained to a certain degree in the dry fractionated fractions. A variation of process parameters, such as classifier wheel speed can be used to alter the degree of refinement and thus the degree of gravimetric separation in dilute emulsions. Overall, our results show the potential of air classified lentil flour fractions to serve as sustainable ingredients with unique functional properties. However, LFF are possibly more suited for use in concentrated emulsions, in which higher viscosity and hydrodynamic interactions between particles limit the effect of gravimetric sedimentation. Further studies are suggested to investigate functional properties in such systems to better elucidate the relationship between composition and functionality of LFF.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, Jochen Weiss, upon reasonable request.
Code availability
Not applicable.
References
Aiking H, de Boer J (2018) The next protein transition. Trends Food Sci Technol 105:515–522. https://doi.org/10.1016/j.tifs.2018.07.008
Boye J, Zare F, Pletch A (2010) Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res Int 43(2):414–431. https://doi.org/10.1016/j.foodres.2009.09.003
Schutyser MAI, van der Goot AJ (2011) The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci Technol 22(4):154–164. https://doi.org/10.1016/j.tifs.2010.11.006
Xing Q, de Wit M, Kyriakopoulou K, Boom RM, Schutyser MAI (2018) Protein enrichment of defatted soybean flour by fine milling and electrostatic separation. Innov Food Sci Emerg Technol 50:42–49. https://doi.org/10.1016/j.ifset.2018.08.014
Peighambardoust SH, Hamer RJ, Boom RM, van der Goot AJ (2008) Migration of gluten under shear flow as a novel mechanism for separating wheat flour into gluten and starch. J Cereal Sci 48(2):327–338. https://doi.org/10.1016/j.jcs.2007.10.005
Peng W, Kong X, Chen Y, Zhang C, Yang Y, Hua Y (2016) Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocoll 52:301–310. https://doi.org/10.1016/j.foodhyd.2015.06.025
Sharif HR, Williams PA, Sharif MK, Abbas S, Majeed H, Masamba KG, Safdar W, Zhong F (2018) Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants – a review. Food Hydrocoll 76:2–16. https://doi.org/10.1016/j.foodhyd.2017.01.002
Dekkers BL, Nikiforidis CV, van der Goot AJ (2016) Shear-induced fibrous structure formation from a pectin/SPI blend. Innov Food Sci Emerg Technol 36:193–200. https://doi.org/10.1016/j.ifset.2016.07.003
Geerts MEJ, Dekkers BL, van der Padt A, van der Goot AJ (2018) Aqueous fractionation processes of soy protein for fibrous structure formation. Innov Food Sci Emerg Technol 45:313–319. https://doi.org/10.1016/j.ifset.2017.12.002
Hunter RJ (1986) Foundations of colloid science. Oxford University Press, Oxford
Tyler RT, Youngs CG, Sosulski FW (1981) Air classification of legumes. I. Separation efficiency, yield, and composition of the starch and protein fractions. Cereal Chem 58(2):144–148
Pelgrom PJM, Vissers AM, Boom RM, Schutyser MAI (2013) Dry fractionation for production of functional pea protein concentrates. Food Res Int 53(1):232–239. https://doi.org/10.1016/j.foodres.2013.05.004
Elkowicz K, Sosulski FW (1982) Antinutritive factors in eleven legumes and their air-classified protein and starch fractions. J Food Sci 47(4):1301–1304. https://doi.org/10.1111/j.1365-2621.1982.tb07673.x
Pelgrom PJM, Boom RM, Schutyser MAI (2015) Method development to increase protein enrichment during dry fractionation of starch-rich legumes. Food Bioprocess Technol 8(7):1495–1502. https://doi.org/10.1007/s11947-015-1513-0
Sosulski F, Youngs CG (1979) Yield and functional properties of air-classified protein and starch fractions from eight legume flours. J Am Oil Chem Soc 56(3):292–295. https://doi.org/10.1007/bf02671477
Evans M, Ratcliffe I, Williams PA (2013) Emulsion stabilisation using polysaccharide–protein complexes. Curr Opin Colloid Interface Sci 18(4):272–282. https://doi.org/10.1016/j.cocis.2013.04.004
Bhatty RS, Slinkard AE, Sosulski FW (1976) Chemical composition and protein characteristics of lentils. Can J Plant Sci 56(4):787–794. https://doi.org/10.4141/cjps76-128
Nosworthy MG, Medina G, Franczyk AJ, Neufeld J, Appah P, Utioh A, Frohlich P, House JD (2018) Effect of processing on the in vitro and in vivo protein quality of red and green lentils (Lens culinaris). Food Chem 240:588–593. https://doi.org/10.1016/j.foodchem.2017.07.129
Gumus CE, Decker EA, McClements DJ (2017) Formation and stability of ω-3 oil emulsion-based delivery systems using plant proteins as emulsifiers: lentil, pea, and faba bean proteins. Food Biophys 12(2):186–197. https://doi.org/10.1007/s11483-017-9475-6
Joshi M, Adhikari B, Aldred P, Panozzo JF, Kasapis S, Barrow CJ (2012) Interfacial and emulsifying properties of lentil protein isolate. Food Chem 134(3):1343–1353. https://doi.org/10.1016/j.foodchem.2012.03.029
Primozic M, Duchek A, Nickerson M, Ghosh S (2017) Effect of lentil proteins isolate concentration on the formation, stability and rheological behavior of oil-in-water nanoemulsions. Food Chem 237:65–74. https://doi.org/10.1016/j.foodchem.2017.05.079
Ladjal-Ettoumi Y, Boudries H, Chibane M, Romero A (2015) Pea, chickpea and lentil protein isolates: physicochemical characterization and emulsifying properties. Food Biophys 11(1):43–51. https://doi.org/10.1007/s11483-015-9411-6
Wang Y, Pillai PKS, Nickerson MT (2019) Effect of molecular mass and degree of substitution of carboxymethyl cellulose on the formation electrostatic complexes with lentil protein isolate. Food Res Int 126:108652. https://doi.org/10.1016/j.foodres.2019.108652
Can Karaca A, Low N, Nickerson M (2011) Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int 44(9):2742–2750. https://doi.org/10.1016/j.foodres.2011.06.012
Geerts MEJ, Nikiforidis CV, van der Goot AJ, van der Padt A (2017) Protein nativity explains emulsifying properties of aqueous extracted protein components from yellow pea. Food Struct 14:104–111. https://doi.org/10.1016/j.foostr.2017.09.001
McClements DJ (2016) Food emulsions: principles, practices, and techniques, 3rd edn. CRC Press, Taylor & Francis Group, Boca Raton
Vogelsang-O’Dwyer M, Zannini E, Arendt EK (2021) Production of pulse protein ingredients and their application in plant-based milk alternatives. Trends Food Sci Technol 110:364–374. https://doi.org/10.1016/j.tifs.2021.01.090
Pelgrom PJM, Boom RM, Schutyser MAI (2015) Functional analysis of mildly refined fractions from yellow pea. Food Hydrocoll 44:12–22. https://doi.org/10.1016/j.foodhyd.2014.09.001
Primozic M, Duchek A, Nickerson M, Ghosh S (2018) Formation, stability and in vitro digestibility of nanoemulsions stabilized by high-pressure homogenized lentil proteins isolate. Food Hydrocoll 77:126–141. https://doi.org/10.1016/j.foodhyd.2017.09.028
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
MF: writing—original draft, conceptualization, investigation, data curation, visualization, formal analysis. ML: conceptualization, writing—review & editing. CW: investigation, conceptualization. MK: investigation. RB: writing—review & editing, resources. JW: supervision, conceptualization, writing—review & editing, resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Funke, M., Loeffler, M., Winkelmeyer, C. et al. Emulsifying properties of lentil protein preparations obtained by dry fractionation. Eur Food Res Technol 248, 381–391 (2022). https://doi.org/10.1007/s00217-021-03883-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03883-y