Abstract
Mechanical properties of sugar beet are important during harvesting and processing. To potentially correlate mechanical properties with structural features of cell wall polymers, four different Beta varieties (Beta vulgaris L.) were characterized for their mechanical properties and cell wall composition. In addition, the influence of nitrogen fertilization was analyzed. Additional nitrogen fertilization only slightly influenced mechanical properties and cell wall composition. Hardly any structural differences of cell wall polysaccharides were observed for all Beta varieties. Slight differences in alcohol insoluble residue and protein contents, in amino acid profiles of proteins, and in esterification degree of pectins were found. The Beta varieties differed in their contents of cell wall bound phenolic components, and particularly in their dry matter contents. Analyses of mechanical properties of the Beta varieties demonstrated differences in tissue firmness and compressive strength. However, no evidence was found that the observed differences of cell wall composition are responsible for the divergent mechanical properties, with the exception of water contents that were higher in samples with less compressive strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugar beet roots are exposed to various mechanical strains during harvest, cleaning and transport. Breakage of whole beet parts and root tip breakage do not only result in yield losses, but injuries also offer entry points for pathogens, too [1, 2]. Thus, damaged beets are more intensely infested with storage pathogens resulting in higher sugar losses during storage [3]. Therefore, the susceptibility to injuries appears to be a major determinant for sugar losses during storage.
It can be assumed that the genotypic differences that have been detected for these traits [3, 4] are associated with differences in the strength of the storage root tissue. Mechanical properties of sugar beet have recently been studied by Nedomova et al. [5], but only as related to changes during storage. It is not clear yet whether sugar beet genotypic differences in mechanical properties can be identified with a texture analyzer, which is commonly used in the analysis of fruits, vegetables and processed foods [5, 6].
Differences in the mechanical properties of beet root tissues may potentially be related to varying tissue/cell wall composition, as already observed for e.g. asparagus. Herppich et al. [7] analyzed time and temperature dependent effects on mechanical and chemical properties (pectic fractions and lignin) during storage of white asparagus. They showed that decreasing tissue stiffness correlates with an increase of water insoluble pectic substances, but they did not observe a relationship between mechanical properties and lignin content. In addition, Rodriguez et al. [8] analyzed the texture of asparagus during storage as maximum shear force that was associated with changes of polysaccharide composition and lignin content. However, an obvious impact of a single structural feature on the mechanical properties was not determined.
The objective of the study was to identify genotypic differences in the mechanical properties of beet root tissues and to relate those to compositional changes in the cell wall. To get a broad range of beet material, four Beta varieties grown with different nitrogen fertilizer applications were included in this study. Nitrogen application usually results in a decline of dry matter and potentially changes beet compositional characteristics.
Materials and methods
Plant material
In 2017 a field trial with four replicates was carried out in a completely randomized block design in Sieboldshausen (Niedersachsen, Germany). Factors varied were Beta variety and nitrogen application. The Beta varieties were sugar beet (Beta vulgaris subsp. Vulgaris var. altissima) with a sugar content type (high-sugar, Finola) and a yield type (high-yield, Daphna), fodder beet (Beta vulgaris subsp. Vulgaris var. crassa) (Ribambelle) and beetroot (Beta vulgaris subsp. Vulgaris var. vulgaris) (Alto). Two nitrogen treatments were applied with no (N0) or 300 kg nitrogen ha−1 (N300) applied as calcium ammonium nitrate (CAN) directly after sowing in April. The plots were kept free of weeds, pests, and diseases according to regional recommendations.
Plots were 21.6 m2 (6 rows a 8 m). In October, three rows in the center of the plot (row no. 2, 3, 4) were harvested (10.8 m2) providing about 90 roots per plot. Roots were washed, and subsequently root yield was determined (fresh matter). From every plot, homogenous beet brei was prepared by sawing. The brei was shock-frozen at − 70 °C and stored at − 20 °C for later analysis. Dry matter content was determined by drying beet brei at 105 °C. After cold digestion with 0.3% Al-sulphate solution the sugar content of the filtrates was analyzed polarimetrically (ICUMSA 1994), and amino N was determined using the blue number method (ICUMSA 2007) in an automated line (Anton Paar OptoTec GmbH, Seelze, Germany). Because of the intense red color of the filtrates it was not possible to perform these optical analyses for beetroot.
Mechanical beet properties
Five representative beets were selected from each plot to analyze their mechanical properties by using the texture analyzer “TA.XT Plus” (Stable Microsystems, Surrey, UK) according to the methods described by Kleuker and Hoffmann [9].
The puncture test was conducted with a 2 mm cylindrical probe (P/2) and a crosshead speed of 60 mm min−1 to a depth of 5 mm. The maximum force that is needed to penetrate the periderm of the beet is the puncture resistance (Fmax), whereas the average force from 0.5 mm after rupture until 5 mm represents the tissue firmness (Fmean). Root groove and crown tissue were omitted for the six measurements around the widest part of each beet.
The compression test was performed with three cylindrical samples per beet (Ø: 18 mm, 20 mm) taken from the centre to the outermost part of the beet. The cylinders were compressed with a 75 mm compression platen (P/75) until rupture with a crosshead speed of 60 mm min−1. The maximum pressure at rupture was measured as compressive strength and is presented as mean of the three cylinders.
Preparation of alcohol insoluble residue (AIR)
Further analyses were conducted with representative beet material of one field replicate, as beets from the replicates showed comparable properties (see low standard deviation of Figs. 1, 2). A fourfold amount of ethanol (99%) (based on the water content of the sample) was added to the fresh samples, and alcohol insoluble residue (AIR) was precipitated overnight in 80% ethanol. The suspension was centrifuged (10 min, 4696×g), and the residue was washed twice with 80% ethanol (50 °C), and dried at 60 °C in a vacuum oven. To remove residual sugars, washing steps were repeated four times (80% ethanol, 50 °C) (number of total washing steps using 80% ethanol: six). Finally, AIR was dried at 60 °C in a vacuum oven.
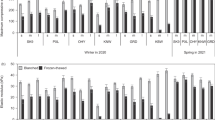
Root yield (a), dry matter content (b), sugar content (c) and content of amino nitrogen (d) in different Beta varieties grown without (N0) and with (N300) additional nitrogen fertilization. Field trial, 4 replicates, Sieboldshausen 2017. Different letters indicate significant differences between varieties, Tukey test α = 0.05; **p ≤ 0.01 and ***p ≤ 0.001. FM fresh weight, var varieties, N appl nitrogen application, V×N varieties × nitrogen
Puncture resistance (a), tissue firmness (b), and compressive strength (c) in different Beta varieties grown without (N0) and with (N300) additional nitrogen fertilization. Field trial, 4 replicates, Sieboldshausen 2017. Different letters indicate significant differences between varieties, Tukey test α = 0.05; *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001, ns not significant. FM fresh weight, var varieties, N appl nitrogen application, V×N varieties × nitrogen
Determination of protein content and amino acid composition
Nitrogen contents of AIR were determined after Kjeldahl digestion. AIR (100 mg) suspended in 5 ml of concentrated sulfuric acid was heated with 500 mg of Kjeldahl catalyst for 1.5 h. Liberated ammonium-N was analyzed using an ammonia selective electrode (Thermo Scientific, Waltham, USA) [10]. Protein contents were calculated using the general conversion factor of 6.25.
Amino acid composition of AIR proteins was determined after hydrolysis of 50 mg of AIR with 2.5 ml of 6 M hydrochloric acid at 120 °C for 20 h [10]. Following centrifugation, the supernatant was filtered (PTFE, 0.45 µm), 180 µl of borate buffer (0.06 M, pH 8.8), 50 µl of 6-aminoquinoline N-succinimidyl ester solution (0.01 M in acetonitrile), and 20 µl of 0.1 mM norleucine (internal standard) were added, and liberated amino acids were derivatized at 55 °C for 10 min. Amino acid profiles were determined by using HPLC with fluorescence detection. The following parameters were applied: Luna C18 (2) column (250 × 4.6 mm, 5 µm, Phenomenex, Torrance, USA); column temperature: 35 °C; eluent A: sodium acetate buffer (60 mM, pH 5.1), eluent B: acetonitrile, eluent C: H2O; excitation wavelength: 250 nm, emission wavelength: 395 nm. The following elution gradient was used: initial eluent composition: 94% A, 6% B, and 0% C; linear over 30 min to 87% A, 13% B, and 0% C; linear over 0.5 min to 80% A, 20% B, and 0% C; linear over 7.5 min to 76% A, 24% B, and 0% C; linear over 10 min to 73.7% A, 26.3% B, and 0% C; linear over 7 min to 0% A, 50% B, and 50% C; equilibration to initial conditions for 10 min. Asparagine and glutamine are largely converted to aspartic and glutamic acid because of the acid hydrolysis. In addition, tryptophan cannot be determined correctly using this method. Arginine and threonine are not quantifiable due to coelution.
Characterization of cell wall polysaccharides
Monomer composition of cell wall polysaccharides was determined after both H2SO4 hydrolysis [11] and methanolysis [12]. H2SO4 hydrolysis was performed by adding 1.5 ml of H2SO4 (12 M) to AIR (100 mg) and incubation at room temperature for 2.5 h. The suspension was diluted with H2O (to 1.25 M H2SO4) and heated at 100 °C for 3 h. Methanolysis was performed by hydrolyzing AIR (10 mg) with 1.25 M methanolic hydrochloric acid at 80 °C for 16 h. Liberated monosaccharides were analyzed by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) [13].
Methylation analysis was performed according to Nunes et al. [14] with minor modifications [13]. The methylation step in DMSO/NaOH using methyl iodide was performed twice. The methylated polysaccharides were hydrolyzed using 2 M trifluoroacetic acid at 121 °C for 1.5 h, followed by reduction with NaBD4, and acetylation with 1-methylimidazole/acetic anhydride. The partially methylated alditol acetates were identified by using gas chromatography (GC) coupled with mass spectrometric detection, and semiquantitatively determined by using GC coupled with flame ionization detection using molar response factors [13, 15].
Neutral pectic sidechains (arabinans and (arabino)galactans of rhamnogalacturonan I) were further characterized by enzymatic hydrolysis of AIR using endo-arabinanase (2 U/100 mg AIR) and endo-galactanase (10 U/mg AIR). Incubation was performed at 40 °C for 24 h. Liberated arabino- and galacto-oligosaccharides were determined by HPAEC-PAD analysis using raffinose as internal standard as described previously [16].
Determination of methylation and acetylation degree of pectins
Uronic acid contents were determined spectrophotometrically [17]. AIR (75 mg) was suspended in 1.5 ml of 12 M H2SO4 at room temperature for 2.5 h, followed by dilution with 9.75 ml of H2O. The diluted solution (aliquot of 600 µl) was mixed with 3.6 ml of 0.0125 M sodium tetraborate (in 18 M H2SO4). Sixty µl of 0.15% m-hydroxydiphenyl (in 0.5% NaOH solution) was added, and absorbance at 520 nm was read after 20 min. The blank was performed by adding 60 µl of 0.5% NaOH solution instead of 0.15% m-hydroxydiphenyl solution.
Quantitative 1H NMR analysis was performed to determine ester-bound methanol and acetic acid contents of AIR to calculate esterification degree of pectins [18]. Methyl and acetyl esters were hydrolyzed for 2 h in an ultrasonic bath by adding 1 ml of 2 M NaOH (in D2O) to 15 mg of AIR. Additionally, trimethylsilylpropanoic acid-d4 (20 µg) was added as internal standard. Following centrifugation, the supernatant was filtered (PTFE, 0.45 µm), and 1H NMR spectra were acquired on a Bruker Avance 500 MHz instrument (Rheinstetten, Germany). A standard 1H NMR pulse program (zg30) from Bruker was used, and 32 scans were acquired with an acquisition time of 3.28 s, a spectral width of 10,000 Hz, and a relaxation delay of 35 s. Processing of the acquired spectra was done by applying baseline and phase correction, zero filling (factor 2), and by multiplication of the FID with an exponential weighting function (LB: 0.3). Quantitative determination of acetic acid and methanol was done by integration of the corresponding signals (δ = 1.93 ppm for acetic acid and δ = 3.33 ppm for methanol). The degree of acetylation and methylation was calculated related to uronic acid contents.
Determination of cell wall bound phenolic compounds
Cell wall bound phenolic monomers and dehydrodi- and triferulic acids (DFAs, TFAs) were analyzed after alkaline saponification of AIR (50 mg) with 5 ml of 2 M NaOH at room temperature for 18 h [19]. Following acidification with HCl, trans-o-coumaric acid (150 µg) and 5-5(monomethyl)-DFA (6 µg) were added as internal standards, and phenolic compounds were extracted into diethyl ether. Cell wall bound phenolic components were analyzed by HPLC–DAD on a 250 mm × 4.6 mm, 5 µm Luna phenylhexyl column (Phenomenex, Torrance, USA) [19]. Quantitation of trans- and cis-ferulic acid was performed at 322 and 309 nm by applying an external calibration. cis-Ferulic acid for external calibration was prepared by irradiation of a trans-ferulic acid stock solution in MeOH with UV-light at 254 nm for 4 h. The cis-ferulic acid content in the solution after irradiation was calculated from trans-ferulic acid contents before and after irradiation. DFA and TFA were analyzed at 280 nm, and contents were calculated using correction factors determined by Dobberstein and Bunzel [19].
Determination of acetyl bromide soluble lignin (ABSL)
Acetyl bromide soluble lignin (ABSL) contents [20] were determined after enzymatic digestion of AIR using the carbohydrase mixture Driselase (1 g AIR, 60 mg Driselase, 100 ml H2O, 24 h at 37 °C) as detailed in Bunzel et al. [21]. Enzyme-digested sample material (10 mg) was suspended in 4 ml of 25% acetyl bromide in glacial acetic acid and heated at 50 °C for 2 h. The solution was diluted with 12 mL of glacial acetic acid, and 2 ml of the diluted solution was mixed with 2.5 ml of glacial acetic acid, 1.5 ml of 0.3 M NaOH, and 0.5 ml of 0.5 M hydroxylamine hydrochloride solution. The solution was made up to 10 ml with glacial acetic acid, and the absorbance was read at 280 nm using a Jasco V-550 spectrophotometer (Jasco, Groß-Umstadt, Germany). Lignin contents were calculated by using an absorption coefficient of 20 ml/(cm*mg) [20].
Statistics
For the field trial data (yield, beet quality, mechanical properties), a statistical analysis was carried out with the program SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The data were checked for normal distribution and homogeneity of variance and subsequently analysed with the PROC GLM function followed by Tukey test (α = 0.05). The factors variety and nitrogen application were taken as fixed. Mean and standard deviation are presented in the figures. Significant effects are indicated with * for p ≤ 0.05, ** for p ≤ 0.01 and *** for p ≤ 0.001. Not significant is abbreviated as ns. Different letters indicate significant differences between varieties.
Samples from one field replicate were analyzed chemically. Statistical analyses were performed for AIR contents, galacturonic acid contents, and cell wall bound phenolic components by using the software Origin 2019 9.6.0.172. Differences among varieties were tested for statistical significance by using ANOVA, followed by Tukey test (α = 0.05). Differences between N0 and N300 samples were tested for each variety for statistical significance by using the t test (α = 0.05).
Chemicals
Endo-1,4-β-galactanase (EC 3.2.1.89 from Aspergillus niger) and endo-1,5-α-arabinanase (EC 3.2.1.99 from Aspergillus niger) were obtained from Megazyme (Bray, Ireland). Commonly used chemicals and high performance liquid chromatography (HPLC) solvents were obtained from Sigma-Aldrich (St. Louis, MO), VWR, part of Avantor (Radnor, USA), Roth (Karlsruhe, Germany), Merck (Darmstadt, Germany), or Alfa Aesar (Ward Hill, USA). 6-Aminoquinoline N-succinimidyl ester was purchased from Chemodex (St. Gallen, Switzerland). Sodium borodeuteride-d4 and trimethylsilylpropanoic acid-d4 were from Deutero (Kastellaun, Germany), and D2O was obtained from Sigma.
Results
Beet characteristics and mechanical beet properties
The four beet varieties studied here (sugar beet with either a sugar content type (high-sugar) or a yield type (high-yield), fodder beet, beetroot) differed significantly in root yield and the contents of dry matter, sugar, and amino nitrogen (Fig. 1a–d). Nitrogen application resulted in a considerable increase of amino nitrogen contents in the beets (Fig. 1d). Mechanical beet properties are shown in Fig. 2. Varieties differed only slightly in puncture resistance (A), where the sugar beet (high-yield) showed a significantly lower force needed to penetrate the periderm compared to the other three Beta varieties. Significant differences between varieties occurred in tissue firmness (B) and compressive strength (C) characterizing the more internal tissue. Sugar beet (high-sugar) had the highest strength, followed by sugar beet (high-yield), and fodder beet, while beetroot showed by far the lowest tissue firmness and compressive strength as also reported by Kleuker and Hoffmann [9]. Nitrogen application hardly affected beet mechanical properties.
Alcohol insoluble residue and protein content
AIR contents of different beet (Beta vulgaris L.) varieties were determined to estimate the amount of cell wall material in beet samples. As shown in Fig. 3, beetroot had a higher amount of cell wall material (dry weight basis, A) compared to the other three Beta varieties, whereas the AIR content of sugar beet (high-yield) was lowest. When related to fresh weight (Fig. 3b), the variety effect changes and the sugar beet (high-sugar) had the highest AIR content. Additional nitrogen input had no distinct effect on the portion of cell wall material. Protein corrected AIR contents based on dry weight and fresh weight are shown in Table S1 (supporting information).
Alcohol insoluble residue (AIR) contents (based on dry weight (DM) (a), based on fresh weight (FM) (b)), and protein contents of AIR (c) of different Beta varieties grown without (N0) and with additional nitrogen fertilization (N300) (n = 3). Different letters indicate significant differences between varieties (Tukey test, α = 0.05; N0 and N300 samples were tested separately). Significant differences between N0 and N300 are indicated with asterisk. Field trial, Sieboldshausen 2017
Protein content of AIR (Fig. 3c) of beetroot (without additional nitrogen fertilization) was significantly higher compared to fodder beet and sugar beet (high-sugar) (without additional nitrogen fertilization). Protein contents of the two sugar beet samples and the fodder beet did not differ. Protein contents slightly increased with additional nitrogen fertilization (significantly only for fodder beet) resulting in similar protein contents for all Beta varieties. Amino acid profiles of AIR proteins (Fig. 4) were comparable among fodder beet and the two sugar beet varieties. Only slight differences were observed for the amino acid composition of beetroot AIR compared to the other three beet samples. The main difference was observed for hydroxyproline, which was more abundant in beetroot AIR protein (about 10% compared to 5–6% for the other beet samples), suggesting higher contents of hydroxyproline rich structural proteins. Amino acid profiles of the beet samples were not affected by nitrogen fertilization.
Amino acid profiles of proteins from alcohol insoluble residues obtained from different Beta varieties grown without (N0) and with (N300) additional nitrogen fertilization (n = 3). Asparagine, glutamine, tryptophan, arginine, and threonine cannot be determined correctly using this method. Field trial, Sieboldshausen 2017
Polysaccharide composition
Monosaccharide composition of cell wall polysaccharides was determined after both H2SO4 hydrolysis and methanolysis (Fig. 5). The procedures differ in their hydrolysis efficiency for different cell wall polysaccharides. Hardly any crystalline cellulose is hydrolyzed during methanolysis, whereas uronic acid portions (most notably galacturonic acid) are usually underestimated using H2SO4 hydrolysis. Linkage positions of the monosaccharides within the polysaccharides were characterized by methylation analysis (Table 1). Interestingly, the results showed that the structural composition of cell wall polysaccharides hardly varied among the different Beta varieties and was not affected by additional nitrogen fertilization. The main monosaccharides of AIR polysaccharides from all beet varieties (liberated after H2SO4 hydrolysis) were glucose and arabinose (36–41 mol%) followed by galactose (9–10 mol%), xylose, mannose, and rhamnose (< 5 mol%). The main neutral monosaccharide obtained from methanolysis was arabinose (51–57 mol%), followed by galactose (14–15 mol%), rhamnose (6–7 mol%), and xylose and glucose (< 5 mol%). In addition, galacturonic acid portions of 16–19 mol% were determined after methanolysis. Glucose was mainly 1,4-linked, suggesting large amounts of cellulose. However, the backbone of xyloglucans partially consists of 1,4-linked glucopyranose units, too. The comparably low glucose portions (< 4 mol%) liberated by methanolysis may result from xyloglucans, which is confirmed by identification of the PMAA resulting from 1,4,6-linked glucopyranose units. In addition, 1,4-linked manno- and xylopyranose units were identified, suggesting linear (gluco-)mannans and xylans as hemicellulosic polysaccharides.
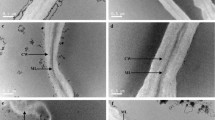
The pectic fraction was composed of almost similar portions (based on the backbone) of homogalacturonan and rhamnogalacturonan I, which can be concluded from galacturonic acid/rhamnose ratios of about 3 (methanolysis data). The dominant neutral sidechains of rhamnogalacturonan I were arabinans as indicated by higher portions of arabinose liberated after H2SO4 hydrolysis and methanolysis as compared to galactose. Arabinans, which consist of a backbone of 1,5-linked arabinofuranose units, were highly branched (ratio of ∑ of 1,3,5- and 1,2,5-linked arabinofuranose units to 1,5-linked arabinofuranose units of about 1). This is also indicated by high portions of terminal arabinofuranose units. Substitution of the arabinose backbone was predominantly in position O-3 (ratios of 1,3,5- to 1,2,5-linked arabinofuranose units of about 11–16). The preferential substitution of sugar beet arabinans in position O-3 was also described by Bertin et al. [22] and Oosterveld et al. [23]. Neutral sidechains of pectins were further characterized by an enzymatic arabinan and galactan profiling method. By using this approach, substitution in position O-3 was confirmed as being dominant over substitution in position O-2 due to the identification of branched arabino-oligosaccharides that were mainly substituted in position O-3 (Fig. 6A-4a, A-6a, A-7b). In addition, arabino-oligosaccharides with neighboring or close-by sidechains were detected (Fig. 6A-6a, A-7b), showing that arabinans of the different beet varieties contain highly branched areas.
PMAAs representing 1,2,3,5-arabinofuranose units were determined by methylation analysis, which may result from fully substituted arabinofuranose units and/or from undermethylation. However, arabino-oligosaccharides resulting from fully branched arabinans were not detected using the enzymatic arabinan profiling method. Therefore, the PMAA representing 1,2,3,5-arabinofuranose units resulted more likely from undermethylation and was therefore not included into the calculation of linkage type profiles (Table 1).
The presence of predominantly linear galactans was confirmed by the identification of 1,4-linked galactopyranose units (methylation analysis), and by liberating 1,4-linked galactose dimers using the enzymatic galactan profiling method. In addition, lower portions of 1,6- and 1,3,6-linked galactopyranose units indicate the presence of arabinogalactan type II in lower amounts. However, the PMAA resulting from 1,3-linked galactopyranose units, another characteristic linkage type of arabinogalactan type II, was not detected.
Pectins were further characterized by determination of their degree of acetylation and methylation (Table 2). In general, both degree of acetylation and degree of methylation were in a comparably narrow range across samples (about 42–69%, depending on beet variety and fertilization), which is in accordance with literature data for sugar beet pectin [22, 24]. Fodder beet pectins were slightly lower methylated and acetylated (60% each) than the other Beta variety pectins (65–69%). Additional nitrogen fertilization results in decreased esterification/acylation (decrease of about 20–30%, relative) for all Beta varieties, which was mainly due to lower contents of liberated methanol and acetic acid after saponification because galacturonic acid contents did not change (Table 2, Table S2).
Cell wall bound phenolic components
Total ferulic acid contents, DFA contents, and TFA contents of AIRs from different Beta varieties grown without and with additional nitrogen fertilization are shown in Fig. 7 (Table S3, contents calculated on protein corrected AIR are shown in Table S4). Both trans- and cis-ferulic acid were identified as cell wall bound phenolic monomers in all samples, with the trans-isomer being dominant (Fig. 7a), which is also described in literature [25,26,27]. Total (monomeric) ferulic acid contents, DFA contents, and TFA contents varied among the different Beta varieties grown without additional nitrogen fertilization (Fig. 7a–c). Beetroot contained the highest ferulic acid content, followed by sugar beet (high-yield), fodder beet, and sugar beet (high-sugar). Waldron et al. [25] described higher ferulic acid contents in beetroot compared to sugar beet, too. The highest DFA content was analyzed for sugar beet (high-sugar), followed by fodder beet, beetroot, and sugar beet (high-yield). For all varieties, the same DFAs were identified: 8-5-coupled DFAs (31–49% of the total DFA content) were the main DFAs, followed by 5-5- (21–30% of the total DFA content), 8-O-4- (19–21% of the total DFA content), and 8-8-non cyclic-DFA (9–18% of the total DFA content). The identified DFAs were described in literature as sugar beet and beetroot components earlier, with 8-5- and 8-O-4-DFAs being the main DFAs [25,26,27,28]. The contents of 8-5-coupled DFA are given as sum of 8-5-non-cyclic- and 8-5-cyclic-DFA because 8-5-non-cyclic-DFA is formed during sample preparation from the native 8-5-cyclic-DFA [29]. The content of 5-5-DFA may be (slightly) overestimated because 8-5-non-cyclic/5-5-TFA coelutes under the HPLC conditions used. The presence of this trimer was confirmed by using LC–MS/MS analysis based on its mass transition (parent ion: m/z = 577; daughter ion: m/z = 489). 5-5/8-O-4-TFA was the only trimer that was quantitated in all samples, with sugar beet (high-sugar) containing the highest amount compared to the other three Beta varieties. Beetroot contained the lowest 5-5/8-O-4-TFA content. In general, higher contents of ferulic acid oligomers suggest a higher extent of cell wall cross-linking, which may positively affect cell wall stability. Therefore, cell wall polymers of sugar beet (high-sugar) may be cross-linked to a greater extent (ferulic acid oligomer content: 90.5 ± 1.7 mg/100 g AIR) compared to cell wall cross-linking of fodder beet (ferulic acid oligomer content: 78.7 ± 3.1 mg/100 g AIR), beetroot (ferulic acid oligomer content: 66.1 ± 2.1 mg/100 g AIR), and sugar beet (high-yield) (ferulic acid oligomer content: 60.5 ± 2.6 mg/100 g AIR) cell wall components.
Contents of cell wall bound phenolic monomers (a), and ferulic acid dehydrodimers (DFA) (b) and –trimers (TFA) (c) analyzed after alkaline saponification of alcohol insoluble residues (AIR) from different Beta samples (n = 3). Significant differences in ferulic acid (FA), and total DFA and TFA contents among Beta varieties are indicated by different letters (Tukey test, α = 0.05; samples from Beta varieties grown without (N0) and with additional nitrogen fertilization (N300) were tested separately). Significant differences between N0 and N300 samples are indicated with *(tested for each variety, t test, α = 0.05)
Nitrogen fertilization affected contents of cell wall bound ferulic acid and ferulic acid oligomers, too. Monomeric ferulic acid contents decreased in beetroot and sugar beet (high-yield), but increased in fodder beet with nitrogen fertilization. The different extent of changes in ferulic acid contents as a result of additional fertilization resulted in similar contents for fodder beet and sugar beet (high-yield), whereas sugar beet (high-sugar) still contained the lowest and beetroot the highest contents of cell wall bound ferulic acid.
In addition, total DFA and 5-5/8-O-4-TFA contents of sugar beet (high-sugar) and fodder beet decreased by applying additional nitrogen fertilizer, but increased slightly for beetroot. Again, changes were observed to a different extent depending on beet variety resulting in similar DFA contents for sugar beet (high-yield) and fodder beet, and for sugar beet (high-sugar) and beetroot. However, the profile of quantified DFAs was rarely affected by nitrogen fertilization.
Lignin contents
Lignin contents were determined as ABSL. The principle of this method is based on the UV-spectrophotometric analysis (quantitation at 280 nm) of lignin that is soluble in glacial acetic acid/acetyl bromide. In order to reduce the influence of polysaccharide bound phenolic components that interfere with the measurement pretending to be actual lignin, cell wall material was enzyme-digested using the carbohydrase mixture Driselase. Resulting ABSL contents of all samples were about 0.1% of AIR (data not shown). Taking into account that residual cell wall bound ferulic acid and/or monosaccharide degradation products may affect the calculated ABSL contents, the low amounts of ABSL are unlikely to play a major role in beet cell wall stability. Additional nitrogen fertilization did not affect ABSL contents.
Discussion
The Beta varieties included in this study represent the extremes within Beta vulgaris subspecies with regard to processing quality and composition. These Beta varieties were shown to differ distinctly in tissue firmness and compressive strength, pointing to distinct differences in their tissue strength and mechanical properties. These differences between the Beta varieties were particularly pronounced in the inner tissue of the beets (tissue firmness, compressive strength), whereas the force required to penetrate the periderm (puncture resistance) was not affected to the same extent. Apparently, there are Beta genotypes such as beetroot where the surface peel (periderm) is relatively stronger as compared to the inner tissue [30].
It was hypothesized that these differences in tissue strength can be attributed to compositional variations of the structural root tissue and cell walls. However, a detailed characterization of cell wall components revealed comparably minor differences among the Beta varieties; the effect of nitrogen fertilization appears to be minor, too. Interestingly, the cell wall polysaccharide composition of the four beet varieties, which was comparable to the reported polysaccharide structures for sugar beet [22,23,24], appears to be highly comparable. Slight changes were observed for AIR and protein contents, amino acid profiles of the proteins, and esterification degree of pectins. Analysis of AIR contents indicated that sugar beet (high-yield) has the lowest amount of polymeric cell wall material, and beetroot the highest (calculated on dry weight). Therefore, the AIR content (on dry weight) did not provide an explanation for the observed differences in tissue strength. However, dry matter contents and vice versa water contents of all Beta varieties differed significantly, as beetroot had the highest water content, followed by fodder beet, sugar beet (high-yield), and sugar beet (high-sugar). The ranking of varieties was thus more comparable to tissue strength when water content was considered. It seems that apart from possible compositional features the water content of the tissue has a decisive impact on mechanical properties as a decreasing genotypic water content was associated with an increase in compressive strength.
The highest protein content was analyzed for AIR of beetroot. Also, AIR protein from beetroot was characterized by a higher portion of hydroxyproline compared to the other beets. In general, this might be interesting in terms of cell wall stability because cell wall structural proteins are defined via specific repeated sequence motifs: glycine-rich proteins, proline-rich proteins, and hydroxyproline-rich glycoproteins. Especially the structure of hydroxyproline-rich glycoproteins was described to be dominated by stiff, rod-like domains [31]. However, tissue firmness and compressive strength of beetroot was lowest compared to the other Beta varieties, suggesting that for these samples the higher portions of hydroxyproline cannot be directly correlated with (positive) changes in mechanical properties. Esterification degree of fodder beet pectin was lower compared to the other Beta varieties, but had no obvious relationship with varying mechanical properties.
The main structural differences among the various Beta samples were observed for cell wall bound phenolic components. Data gathered here were largely in agreement with published data [25,26,27,28], but added considerably more details because we differentiated high-yield vs. high sugar beet varieties and added more data about TFA, for example. The highest content of ferulic acid oligomers was analyzed for sugar beet (high-sugar), followed by fodder beet, beetroot, and sugar-beet (high-yield). High ferulic acid oligomer contents may suggest a higher extent of cell wall cross-links, which may improve cell wall stability. Although sugar-beet (high-sugar) showed the highest compressive strength and tissue firmness, a clear correlation between ferulic acid oligomer contents and mechanical properties was not demonstrated in this study.
Additional nitrogen fertilization resulted in slight changes of cell wall components, too, with the extent being different depending on Beta variety. Esterification degree, protein contents, and amount of cell wall cross-links were mainly affected by applying nitrogen fertilizer. However, negligible differences in mechanical properties were observed depending on nitrogen fertilization.
In summary, the results suggest that compositional differences of the cell wall do not provide convincing explanations for differences in mechanical properties/tissue strength of different Beta beets, mostly because these differences are often minor. The only correlation observed in this study was that compressive strength increases with decreasing water content of the samples (increasing dry matter content). However, water content as the only factor influencing cell wall stability seems to be unlikely. Possibly, the overall cell wall composition (instead of a single structural feature) and interactions of individual cell wall polymers contribute to differences in mechanical properties.
Abbreviations
- ABSL:
-
Acetyl bromide soluble lignin
- AIR:
-
Alcohol insoluble residue
- DFA:
-
Dehydrodiferulic acid
- GC:
-
Gas chromatography
- HPAEC-PAD:
-
High-performance anion exchange chromatography with pulsed amperometric detection
- HPLC:
-
High performance liquid chromatography
- PMAA:
-
Partially methylated alditol acetate
- TFA:
-
Dehydrotriferulic acid
References
Mumford DL, Wyse RE (1976) Effect of fungus infection on respiration and reducing sugar accumulation of sugarbeet roots and use of fungicides to reduce infection. J Am Soc Sugar Beet Technol 19:157–162
van Swaaij ACPM, van der Linden JP, Vandergeten JP (2003) Effect of growth conditions and variety on damage susceptibility of sugarbeet. Sugar Ind 128:888–891
Hoffmann CM, Schnepel K (2016) Susceptibility to root tip breakage increases storage losses of sugar beet genotypes. Sugar Ind 141:625–632
Schnepel K, Hoffmann CM (2016) Genotypic differences in storage losses of sugar beet—causes and indirect criteria for selection. Plant Breed 135:130–137
Nedomova S, Kumbar V, Pytel R, Buchar J (2017) Mechanical properties of sugar beet root during storage. Int Agrophys 31:507–513
Sila DN, Smout C, Vu ST, Loey A, Hendrickx M (2005) Influence of pretreatment conditions on the texture and cell wall components of carrots during thermal processing. J Food Sci 70:E85–E91
Herppich WB, Huyskens-Keil S, Kadau R (2005) Effects of short-term low-temperature storage on mechanical and chemical properties of white Asparagus cell walls. J Appl Bot Food Qual 79:63–71
Rodriguez R, Jimenez A, Guillen R, Heredia A, Fernandez-Bolanos J (1999) Postharvest changes in white asparagus cell wall during refrigerated storage. J Agric Food Chem 47:3551–3557
Kleuker G, Hoffmann CM (2019) Method development for the determination of textural properties of sugar beet roots. Sugar Ind 144:392–400
Urbat F, Müller P, Hildebrand A, Wefers D, Bunzel M (2019) Comparison and optimization of different protein nitrogen quantitation and residual protein characterization methods in dietary fiber preparations. Front Nutr 6:127 (8 pages)
Saeman JF, Bubl JL, Harris EE (1945) Quantitative saccharification of wood and cellulose. Ind Eng Chem Anal Ed 17:35–37
De Ruiter GA, Schols HA, Voragen AGJ, Rombouts FM (1992) Carbohydrate analysis of water-soluble uronic acid-containing polysaccharides with high-performance anion-exchange chromatography using methanolysis combined with TFA hydrolysis is superior to four other methods. Anal Biochem 207:176–185
Wefers D, Gmeiner BM, Tyl CE, Bunzel M (2015) Characterization of diferuloylated pectic polysaccharides from quinoa (Chenopodium quinoa WILLD.). Phytochemistry 116:320–328
Nunes FM, Reis A, Silva AMS, Domingues MRM, Coimbra MA (2008) Rhamnoarabinosyl and rhamnoarabinoarabinosyl side chains as structural features of coffee arabinogalactans. Phytochemistry 69:1573–1585
Sweet DP, Shapiro RH, Albersheim P (1975) Quantitative analysis by various GLC response factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr Res 40:217–225
Wefers D, Bunzel M (2016) Arabinan and galactan oligosaccharide profiling by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). J Agric Food Chem 64:4656–4664
Blumenkrantz N, Asboe-Hanseb G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Müller-Maatsch J, Caligiani A, Tedeschi T, Elst K, Sforza S (2014) Simple and validated quantitative 1H NMR method for the determination of methylation, acetylation, and feruloylation degree of pectin. J Agric Food Chem 62:9081–9087
Dobberstein D, Bunzel M (2010) Separation and detection of cell wall-bound ferulic acid dehydrodimers and dehydrotrimers in cereals and other plant materials by reversed phase high-performance liquid chromatography with ultraviolet detection. J Agric Food Chem 58:8927–8935
Iiyama K, Wallis AFA (1990) Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agric 51:145–161
Bunzel M, Seiler A, Steinhart H (2005) Characterization of dietary fiber lignins from fruits and vegetables using the DFRC method. J Agric Food Chem 53:9553–9559
Bertin C, Rouau X, Thibault JF (1988) Structure and properties of sugar beet fibres. J Sci Food Agric 44:15–29
Oosterveld A, Beldman G, Schols HA, Voragen AGJ (2000) Characterization of arabinose and ferulic acid rich pectic polysaccharides and hemicelluloses from sugar beet pulp. Carbohydr Res 328:185–197
Levigne S, Ralet MC, Thibault JF (2002) Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr Polym 49:145–153
Waldron KW, Ng A, Parker ML, Parr AJ (1997) Ferulic acid dehydrodimers in the cell walls of Beta vulgaris and their possible role in texture. J Sci Food Agric 74:221–228
Oosterveld A, Grabber JH, Beldman G, Ralph J, Voragen AGJ (1997) Formation of ferulic acid dehydrodimers through oxidative cross-linking of sugar beet pectin. Carbohydr Res 300:179–181
Ng A, Harvey AJ, Parker ML, Smith AC, Waldron KW (1998) Effect of oxidative coupling on the thermal stability of texture and cell wall chemistry of beet root (Beta vulgaris). J Agric Food Chem 46:3365–3370
Micard V, Grabber JH, Ralph J, Renard CMGC, Thibault JF (1997) Dehydrodiferulic acids from sugar-beet pulp. Phytochemistry 44:1365–1368
Bunzel M (2010) Chemistry and occurrence of hydroxycinnamate oligomers. Phytochem Rev 9:47–64
Kleuker G, Hoffmann CM (2019) Einfluss der Festigkeit der Rübe auf Beschädigung und Lagerungsverluste von Zuckerrüben. Sugar Ind 144:89–97
Cassab GI (1998) Plant cell walls protein. Annu Rev Plant Physiol Plant Mol Biol 49:281–309
Acknowledgements
Open Access funding provided by Projekt DEAL. Data of texture analysis and plant material were collected in the frame of the IGF Project of the FEI (AiF 19836 BG) supported via AiF within the program for promoting the Industrial Collective Research (IGF) of German Ministry of Economic Affairs and Energy (BMWi), based on a resolution of German Parliament. Thanks are expressed to Facundo Ramón Ispizua Yamati for the determination of beet texture.
Author information
Authors and Affiliations
Contributions
Christa M. Hoffmann and Mirko Bunzel conceptualized the study, all authors contributed to its design. Field trials and measurement of mechanical properties were performed by Christa M. Hoffmann. Chemical analyses were performed by Julia Hale. Judith Schäfer and Christa M. Hoffmann wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read an approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
217_2020_3562_MOESM1_ESM.docx
Tables S1-4: Contents of protein corrected AIR based on dry weight and fresh weight (Table S1), contents of spectrophotometrically detected galacturonic acid of protein corrected AIR (Table S2), and contents of cell wall bound ferulic acid, DFA and TFA of AIR (Table S3) and of protein corrected AIR (Table S4) from different Beta varieties grown without and with nitrogen fertilization; field trial Sieboldshausen 2017 (DOCX 29 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schäfer, J., Hale, J., Hoffmann, C.M. et al. Mechanical properties and compositional characteristics of beet (Beta vulgaris L.) varieties and their response to nitrogen application. Eur Food Res Technol 246, 2135–2146 (2020). https://doi.org/10.1007/s00217-020-03562-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03562-4