Abstract
Lupin seeds are already widely used as an ingredient in different food products. Their attractiveness is related mainly to their high protein content that is characterised by a favourable amino acid composition, as well as the desired technological properties. However, with the increase of lupin seeds usage in food manufacture, their potential allergic properties have been demonstrated. The aim of this work was to study the immunoreactivity changes taking place during the enzymatic hydrolysis of the major seed proteins of narrow-leafed (Lupinus angustifolius, varieties Zeus and Bojar) and yellow (L. luteus, var. Lord and Parys) lupin species. Two digestion systems were used, namely the in vitro model simulating digestion taking place in digestive track, and specific hydrolysis carried out by trypsin. The obtained hydrolysates were analysed by means of one-dimensional electrophoresis, and their immunoreactivity was assessed with the use of a sera pool from patients with lupin-specific IgE. An important reduction in allergenicity of lupin seed proteins was observed when trypsin digestion was applied. The digestion in the in vitro model revealed the possibility of formation of neoallergens which were identified on the basis of mass spectrometry results as β-conglutin fraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lupin seeds can be successfully used in food industry as a source of high quality protein, and they can be a good substitute for soybean [1]. Unfortunately, with the increase in food use of lupin seeds, their potential allergenic properties were revealed. The formation of allergies caused by food allergens contribute to reducing the health safety of food produced using them. Therefore, lupin was declared as a new food allergen, and was officially added to the EU list of known allergens, obliging food producers to label its presence even in traces [2].
Allergenic properties of lupin proteins occurring after ingestion of the seeds were first described by Romano et al. [3]. Since that time, there have also been reports of allergic reaction during inhalation and prolonged exposure to this plant [4]. Hypersensitivity to the lupin proteins may take different forms such as: urticaria, rhinitis, redness of mucous membranes, swelling of a face, cough and difficulty in breathing [4,5,6]. In extreme cases, response was observed as systemic anaphylaxis [7]. Unfortunately, precise doses which cause adverse symptoms were not established. Fæste [8] estimated that this dose may be in the range from 265 to 1000 mg of lupin proteins.
The main lupin proteins able to bind IgE antibodies of allergic individuals are characterised by molecular weights in the range of 43–45 kDa [6, 9, 10]. However, similar effects may also be caused by other lupin proteins characterised by different molecular weight, i.e., 13 kDa [6], 29 kDa [11], 34 kDa [12], 38 kDa [8], and 66 kDa [13]. The large heterogeneity of lupin seed proteins that exhibits antigenic properties makes diagnostic of lupin allergy very complicated and, therefore, requires intensive studies to define molecular epitopes of different lupin proteins. So far, no detailed amino acid sequences of lupin seed proteins (antigenic determinant) that interact with lupin-IgE antibodies have been identified.
A serious problem in the case of food allergies are cross-reactions. In the case of lupin the most frequent reactions occurred in peanut sensitive individuals [5, 6, 14]. Fractions of α- and β-conglutin are considered to be the main proteins that cause allergic reactions in patients with known allergies to peanuts [15,16,17]. Recent studies in this subject were carried out by Ballabio et al. [18]. These authors analysed the allergic reaction in twelve patients with confirmed allergy to peanuts, and found that seven of them reacted positively with lupin β-conglutin. Other conglutin fractions caused weaker response: γ-conglutin (in four out of twelve patients), α-conglutin (in five of twelve patients), and δ-conglutin (in three out of twelve patients) [18].
After insertion into digestive track products containing lupin seeds are subjected to complex digestion processes, during which part of components undergo hydrolysis, while others remain unchanged [19]. So far, there has been little information concerning susceptibility of lupin seed proteins to enzymatic hydrolysis. The presence of non-hydrolysed proteins, and peptides released during digestion may result in the occurrence of allergic reaction. Therefore, the aim of this study was to investigate the processes which might occur during digestion using in vitro model simulating digestive track, as well as specific hydrolysis carried out by trypsin. Immunoreactivity of the resulted products and the efficiency of hydrolysis in reduction of allergenicity of lupin seed storage proteins were assessed.
Materials and methods
Reagents
Pepsin from the porcine gastric mucosa (P7000, activity ≥250 units/mg protein), pancreatin from the porcine pancreas (P1750, activity equivalent to 4 × USP specifications), trypsin from the porcine pancreas, which was treated by N-tosyl-l-phenylalanyl chloromethyl ketone (TPCK) to prevent any chymotrypsin activity (T1426, ≥10,000 units/mg protein), bile (B8381), Tris(hydroxymethyl) aminomethane, sodium dodecyl sulphate (SDS), N,N,N′,N′-tetramethylethylenediamine (TEMED), trifluoroacetic acid (TFA), glycine, acrylamide, nitroblue tetrazolium (NBT), 5-bromo-4-chloro-3-indolyl phosphate (BCIP), bovine serum albumin (BSA), monoclonal anti-human IgE-alkaline phosphatase antibodies produced in mouse (A3076), protease inhibitors mixture (P9599) and Coomassie Brilliant Blue G-250 were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used in this study were of analytical grade.
Patient sera
Sera were obtained from individuals having allergic reaction upon contact with lupin seed storage proteins or other legumes. Additionally, sera were also taken from patients classified as a control group consisted of individuals: (1) having regular contact with lupin, but not showing allergic reaction to lupin, and (2) not having contact with lupin and not showing allergic reaction to lupin. All procedures related with sera obtaining and preparation were carried out in the centre of diagnosis and treatment of allergies Allergology Plus NZOZ under appropriate permission of the Bioethics Committee of Poznan University of Medical Sciences (decision number 533/10). Skin prick tests (SPTs) were performed using freshly prepared lupin seed proteins extracts as well as commercially available allergens purchased from Stallergenes-Greer (Nuway Circle, NE, USA), i.e., soybean—cat. no. F209 and peanut—cat. no. F171. The lupin seed proteins extracts used in SPTs were prepared accordingly to the previously published procedure [1, 19] and standardised using Bradford method [20]. The allergies profiles of patients suffering from hypersensitivity to lupin seed proteins are shown in Table 1.
Materials
Narrow-leafed (L. angustifolius, var. Zeus and Bojar) and yellow (L. luteus, var. Lord and Parys) low-alkaloid lupin seeds were obtained from the Plant Breeding Station Smolice, Przebedowo branch, Poland (harvested in August 2012). All samples were milled using an IKA M20 universal laboratory mill (IKA, Staufen, Germany), sieved to obtain a fraction below 0.6 mm and stored in closed polyethylene bags at −18 °C. Prior analyses all samples were defatted for 2 h using an automatic Soxhlet Bühi Extraction System B-811 (Bühi Labortechnik, Flawil, Switzerland) with n-hexane as a solvent.
Trypsin digestion of lupin seed proteins
Isolation and digestion of lupin seed proteins were carried out applying the method described previously [1, 19].
Enzymatic digestion of lupin seed proteins in the model system
Different models simulating the conditions of a gastrointestinal tract are used to assess the digestibility of food products. These models differ in terms of a number of steps included in digestion and composition of digestive fluids. The in vitro digestion model used in the present study was developed on the basis of method describing digestion of food products characterised by high protein content, and was successfully used in our previous studies of lupin seed proteins digestibility [1, 19,20,21,22]. Lupin seed proteins were isolated from 10 g of defatted lupin flour, which roughly corresponds to two slices of bread made with 5% addition of lupin seeds. The proteins isolation procedure was in accordance with previously used [1, 19].
SDS-PAGE
SDS-PAGE separation of lupin seeds proteins, and their enzymatic hydrolysates, was carried out according to the method proposed by Laemmli [23] using 12.5% polyacrylamide. Polypeptide bands were stained in Coomassie Brilliant Blue G-250. Relative molecular masses of protein were determined by a comparison with molecular weight markers (Precision Plus Protein™ Dual Xtra Prestained Protein Standards; Bio-Rad, Irvine, CA, USA).
Immunoblotting
Immunoblotting analyses were carried out accordingly to previously described procedure [24]. Firstly, SDS-PAGE separated samples were transferred from the gel onto a 0.45 μm PVDF membrane Immobilon-P (Millipore, Bedford, MA, USA) by a semi-dry electrophoretic transfer cell TRP-77 (GE Healthcare, Uppssala, Sweden). The membrane was pre-activated by sequentially immersing in: methanol (1 min), distilled water (5 min) and 20 mM Tris, pH 8.3 buffer containing 192 mM glycine and 20% methanol (10 min). The transfer was conducted at the constant current of 0.6 mA/cm2 of the membrane for 1.5 h. Secondly, the membrane was incubated overnight at 4 °C in Tris buffer (10 mM Tris–HCl pH 7.5, 150 mM NaCl) containing 2% BSA. After blocking, the membrane was washed three times with 10 mM Tris-HCl pH 7.5 containing 150 mM NaCl and 0.05% Tween 20 (v/v). The membrane was then soaked for 3 h with patient sera in a ratio of 100:1 (v/v). The antigen-IgE complexes were detected by 2-h incubation with the secondary antibody (monoclonal anti-human IgE; A3076; Sigma-Aldrich, St. Louis, MO, USA). The secondary antibody was diluted 3000:1. Due to the fact that the secondary antibody was conjugated with alkaline phosphatase, the membrane before detection was transferred to 100 mM Tris-HCl pH 9.5 buffer containing 100 mM NaCl and 5 mM MgCl2. Finally, the membrane was incubated with alkaline phosphatase substrate consisted of NBT (3 mg/ml) and BCIP (1.5 mg/ml). The reaction was stopped by rinsing the membrane with distilled water. The dried membranes were scanned and compared with the electrophoretic gels.
Mass spectrometry protein identification
The protein bands showing immunoreactive features during the immunoblotting analyses were identified using mass spectrometry accordingly to previously described procedure [25]. The analysis was performed in the Environmental Mass Spectrometry Laboratory (Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw). The results were analysed in Mascot Distiller program (Matrix Science, Boston, MA, USA) and then screened for sequence identity using the software Mascot search engine (Matrix Science, Boston, MA, USA) with the defined trypsin specificity. Parameters set up for the search of sequence identity in the NCBI database were as follows: the parent ion mass tolerance of 20 ppm and MS/MS mass accuracy of 0.2 Da. The identification included the possibility of optional modifications: oxidation of methionine, N-glycosylation, methylation, and carboxymethylation of a cysteine.
Results and discussion
Enzymatic digestion of lupin seed proteins
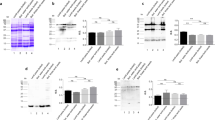
Initial characterisation of crude lupin seed proteins extracts were done based on SDS-PAGE separations (Fig. 1). This characterisation is essential to determine which proteins were digested. The observed profiles were characteristic for lupin seed proteins, and were similar within the varieties of the studied species. The proteins profiles were characterised by the presence of proteins with wide molecular weight range. However, most of proteins were in the range of 37–75, and 45–75 kDa for L. angustifolius and L. luteus samples, respectively (Fig. 1).
In the present study lupin seed proteins were digested by trypsin, which is a highly specific protease, i.e., this enzyme shows specificity to hydrolyse peptide bonds only between positively charged amino acids, such as arginine or lysine [1]. The obtained hydrolysates were analysed using SDS-PAGE (Fig. 1). Based on the obtained separations, a high hydrolytic efficiency of trypsin against the major storage proteins present in the lupin seeds (α- and β-conglutin fractions) was determined. The band pattern of proteins not hydrolysed by trypsin was similar, both in the studied varieties and species (Fig. 1). The main proteins resistant to trypsin action were characterised by molecular weights of 18, 27–29, 36, 50 and 55 kDa (Fig. 1, lanes 1–4). These results confirm our previous findings showing the trypsin resistance of L. angustifolius γ-conglutin faction (molecular weights: 18 and 27 kDa) [1, 19].
The lupin samples obtained after digestion in the model system were also characterised by SDS-PAGE (Fig. 1). The proteolysis resulted in digestion of the main proteins characterised by molecular weight from 37 to 75 kDa and from 45 to 75 kDa for L. angustifolius and L. luteus, respectively. Not hydrolysed proteins were characterised by the molecular weight 25–28 and 50–52 kDa, and were present in all the digested samples (Fig. 1). Additionally, the samples hydrolysed with the use of trypsin contain a number of low molecular proteins <25 kDa where the predominant band was characterised by molecular weight of 18 kDa.
Enzymatic hydrolysis is one of the most recognised method used to reduce allergenicity of food proteins [24]. Best to our knowledge, there is limited data concerning changes of allergic properties of lupin seed proteins as a result of an enzymatic digestion. Only hydrolysis of one protein fraction (γ-conglutin) of L. albus has been studied by Capraro et al. [26]. A considerable effort was put by our group to explain hydrolysis phenomena which take place during digestion of proteins isolated from of L. angustifolius [1, 19, 24]. Our results indicate that γ-conglutin remains undigested when trypsin or pancreatin are used for hydrolysis.
Antigenic properties of lupin seed proteins and their hydrolysates
The SDS-PAGE separated proteins were transferred onto a PVDF Immobilon-P membrane, and subjected to immunochemical identification with the use of the sera of the patients with determined allergy to lupin, as well as not-allergic (the control group).
In case of the group of patients not sensitised to lupin, which have or not have contact with this plant, similar images during immunodetection were obtained (Fig. 2). Interestingly, only in not hydrolysed samples of L. angustifolius the protein band characterised by the molecular weight of ~28 kDa was identified to bind IgE antibodies. This protein might correspond to α-subunit of γ-conglutin which is able to bind IgE antibodies [27]. In the literature there can be found contradictory opinion where this interaction is specific or not [17]. The not specific interaction theory can be explained by the fact that γ-conglutin is glycoprotein and can easily interact with glycans moiety of immunoglobulins present in the patients sera [25, 27]. Surprisingly, this band was not observed in case of crude L. luteus samples, which may indicate that there are differences of glycosylation patterns of γ-conglutin from this particular lupin species.
Immunoblotting of lupin seed proteins and their hydrolysis products. Immunoblots were obtained with the sera from not sensitised patients who have (a), and do not have (b) contact with lupin. Lane numbers correspond to L. angustifolius (1 var. Bojar, 2 var. Zeus), and L. luteus (3 var. Lord, 4 var. Parys). MW molecular weight marker. Arrows indicate the immunoreactive protein bands
The proposed glycosylation of γ-conglutin is presented in Fig. 3. Recently, the structure of L. angustifolius N-glycan core was determined by X-ray crystallography [25] (Fig. 3a, b). Moreover, a glycoproteomic characterisation of γ-conglutin from L. albus has been assessed by Schiarea et al. [28] (Fig. 3 c). These results provided detailed information about the main micro-heterogeneous variants of a glycan attached to γ-conglutin. Among the N-glycans bounded to γ-conglutin two mannosidic-type variants are dominant, while two complex-type N-glycans are less abundant. The most abundant glycans variants had both core β1,2-xylose and core α1-3-fucose, and are generally considered as cross-reactive carbohydrate determinants (CCDs). These glycans can interact with antibodies of the IgE class; however, do not elicit clinical symptoms and, therefore, such N-glycosylated proteins can give false positive results in allergy diagnosis [29]. This is a particularly important issue during lupin seed proteins immunoreactivity studies due to the fact that these proteins undergo very complex post translational modifications, among which glycosylation is one of the most abounded one [25].
Glycosylation of γ-conglutin. Structure of N-glycan core of γ-conglutin isolated from L. angustifolius (PDB accession number: 4pph; Czubinski et al. [25]), the α1,3-fucose core is typical for all cross-reactive carbohydrate determinants (CCDs) (a). Glycosylation diagram prepared based on the available N-glycan core (b). Possible N-glycan structures determined in γ-conglutin from L. albus [28] (c). The structures are drawn using the Consortium for Functional Glycomics Symbol Nomenclature
Figure 4 represents results of immunochemical analyses with sera obtained from individuals who demonstrated allergy to lupin proteins in skin prick tests. Different images of the membranes obtained after immunodetection were observed. In all of the crude lupin seed proteins samples, the presence of a band characterised by a molecular weight of ~28 kDa which interacts with IgE antibodies was determined (Fig. 4). As mentioned in the previous section this particular protein band can correspond to α-subunit of γ-conglutin and bind IgE antibodies. Additionally, in the case of one patient (patient M.60) the additional narrow protein band with the molecular weight of 50 kDa was present in all of the crude lupin samples (Fig. 4).
Immunoblotting of lupin seed proteins and their hydrolysis products. Panels represent the membranes obtained with the sera from lupin-allergic individuals (Table 1). Lanes numbers correspond to L. angustifolius (1 var. Bojar, 2 var. Zeus), and L. luteus (3 var. Lord, 4 var. Parys). MW – molecular weight marker. Arrows indicate the immunoreactive protein bands
Immunoreactivity analysis of lupin seed proteins hydrolysates revealed the presence of antigenic protein band in the case of two patients (patient F.31 and F.41). This protein band was detected only in lupin samples after the in vitro hydrolysis (Fig. 4), and was characterised by molecular weight in the range of 52–54 kDa (Fig. 4). The presence of such band in hydrolysed samples can indicate the formation of neoallergens. During digestion peptide bonds are cleavaged, which results in protein structure changes. Such changes may cause the formation of new epitopes or uncovering antigenic determinants, initially hidden inside protein structures [11]. The trypsin activity resulted in complete loss of immunoreactivity of lupin seed proteins (Fig. 4).
The initial identification of proteins which specifically bind to IgE, was based on the determination of their molecular weight. This was done on the basis of comparison of the membranes after immunoblotting with the results obtained from the SDS-PAGE separation of lupin seed proteins (Fig. 1). Particular attention was paid to the fact that great heterogeneity of lupin seed proteins may result in co-migration of proteins characterised by the same molecular weight, thus, on the immunoblots, one band can correspond to more than one protein. Therefore, the proteins specific binding to IgE antibodies of lupin-sensitised individuals has been identified as: γ-conglutin (α subunits), and the proteins belonging to the β-conglutin fraction (medium molecular weight proteins) which are both characterised by the molecular weight of ~28 kDa [17]. This might be an additional explanation to the fact that in the control group only 28 kDa protein isolated from L. angustifolius interacts with patient sera (Fig. 2). The band detected in the immunoblotting probed with F.31 and F.41 patient sera corresponded to two protein bands on the SDS-PAGE separations (Fig. 1). These two proteins bands were characterised by molecular weight in the range of 52–54 kDa. The detailed identification of these protein bands was made on the basis of mass spectrometry results (Table 2). The results indicate that both proteins belong to L. angustifolius β-conglutin fraction (Table 2). Currently, there are no amino acid sequences of storage proteins from L. luteus available in the database, therefore, the corresponding protein bands from this lupin seed species were also classified as β-conglutin. This particular conglutin fraction was identified as the first lupin allergen by Goggin et al. [17].
Serious health risk associated with food allergy is the presence of cross-reactions, which, in the case of lupin, usually occurs mainly with other legumes [14, 15]. The reason for this phenomenon is the presence of identical or similar determinants (epitopes) within different proteins, in terms of conformation (three-dimensional structure) or sequence (primary structure). Hefle et al. [5] reported the occurrence of urticaria and swollen lymph nodes in patients who are allergic to peanuts after eating pasta enriched with lupin seed flour. Using the serum obtained from these individuals, the authors found that the major proteins responsible for these reactions were characterised by molecular weights of 21, 35 and 55 kDa [5]. Additionally, Leduc et al. [30] showed that the presence of cross-reactions corresponded to the presence of proteins with molecular weights in the range 43–45 kDa (most immunoreactive) and 65 kDa. The observed cross-reactivity may be due to the fact that the lupin β-conglutin fraction, within which the mentioned proteins are present and exhibits high sequence homology with the main peanut allergen Ara h 1 [31]. Magni et al. [16] based on results obtained from 2-DE and immunoblotting stated that lupin proteins reacting with IgE antibodies of lupin sensitive individuals belong to fractions of α- and γ-conglutin. On the other hand, Goggin et al. [17] utilising the same methods showed that major allergen of L. angustifolius are proteins from β-conglutin fraction. On this basis, the Allergen Nomenclature Sub-Committee (IUSI) defined β-conglutin fraction as Lup an 1 allergen. This methodological approach also allowed to confirm the presence of cross-reactions between one of the peanut allergens (Ara h 3) and the basic subunit of α-conglutin isolated from white lupin seed (L. albus) [15].
The present study indicated additional important fact that during digestion proteins classified as β-conglutin might undergo rearrangement, which results in formation of new allergens. This finding is of particular attention due to the fact that during initial allergy testing (e.g., SPTs), individuals might not demonstrate allergic symptoms, while after lupin based products intake such adverse reactions might take place. There is limited information describing change of antigenicity of lupin proteins during technological processing. The available data provide insight into immunoreactivity changes during heat treatment of lupin seed proteins [11]. Álvarez-Álvarez et al. [11] analysed the effect of extrusion cooking, boiling, autoclaving, and microwave heating on the lupin seed proteins stability. Based on the immunoblotting results, the authors found that only the autoclaving process caused a significant change in the amount of proteins that interact with lupin-IgE antibodies. Analysing the samples after autoclaving (20 min) revealed that only proteins having molecular weights of 23 and 29 kDa preserved IgE binding ability. Extending the process time by additional 10 min resulted in the disappearance of the above-mentioned bands on the membrane. However, 30-min autoclaving caused the appearance of a new protein band, characterised by a molecular weight of 70 kDa, which interacted with the sera of lupin sensitive patients. Unfortunately, these authors did not identify to which conglutin fraction this protein belonged.
Conclusions
The introduction of lupin seeds and lupin fortified products to a human diet requires analysis of the potential effects that may occur in the digestive tract. Present studies provided a description of the changes and the nature of the processes taking place as a result of proteolysis of lupin seed proteins, and certainly can contribute to a better understanding of lupine allergy. Moreover, based on the results, it can be concluded that hydrolysis is a very effective way of reduction of immunoreactivity of lupin seed proteins. However, digestion carried out in the in vitro model system revealed the significant problem of formation of new allergens. This phenomenon was not observed when lupin proteins were digested with specific protease—trypsin. The present study indicates necessity of the future research to evaluate the ability of formation of neoallergens in the human gastrointestinal track during digestion of lupin seeds and their hydrolysates.
References
Czubinski J, Dwiecki K, Siger A, Neunert G, Lampart-Szczapa E (2014) Food Chem 143:418–426
Commission Directive 2006/142/EC of 22 December 2006 amending Annex IIIa of Directive 2000/13/EC of the European Parliament and of the Council listing the ingredients which must under all circumstances appear on the labelling of food stuffs
Romano C, Ferrara A, Tarallos S (1997) Allergy 52:113–114
Moreno-Ancillo A, Gil-Adrados AC, Dominguez-Noche C, Cosmes PM (2005) Pediatr Allergy Immunol 16:542–544
Hefle SL, Lemanske RF, Bush RK (1994) J Allergy Clin Immunol 94:167–172
Moneret-Vautrin DA, Guerin L, Kanny G, Flabbee J, Fremont S, Morisset M (1999) J Allergy Clin Immunol 104:883–888
Smith WB, Gillis D, Kette FE (2004) Med J Aust 181:219–220
Fæste CK, Lovik M, Wiker HG, Egaas E (2004) Int Arch Allergy Immunol 135:36–39
Novembre E, Moriondo M, Bernadini R, Azzari C, Rossi ME, Vierucci A (1999) J Allergy Clin Immunol 103:1214–1216
Holden L, Sletten GBG, Lindvik H, Fæste CK, Dooper MMBW (2008) Intern Arch Allergy Immunol 146:267–276
Álvarez-Álvarez J, Guillamon E, Crespo J, Cuadrado C, Burbano C, Rodriguez J, Fernandez C, Muzquiz M (2005) J Agric Food Chem 53:1294–1298
Quaresma RR, Viseu R, Martins LM, Tomaz E, Inacio F (2007) Allergy 62:1473–1474
Peeters KA, Nordlee JA, Penninks AH, Chen L, Goodman RE, Bruijnzeel-Koomen CAFM, Hefle SL, Taylor SL, Knulst AC (2007) J Allergy Clin Immunol 120:647–653
Ballabio C, Magni C, Restani P, Mottini M, Fiocchi A, Tedeschi G, Duranti M (2010) Plant Food Hum Nutr 65:396–402
Magni C, Ballabio C, Restani P, Sironi E, Scarafoni A, Poiesi C, Duranti M (2005) J Agric Food Chem 53:2275–2281
Magni C, Herndl A, Sironi E, Scarafoni A, Ballabio C, Restani P, Bernardini R, Novembre E, Vierucci A, Duranti M (2005) J Agric Food Chem 53:4567–4571
Goggin DE, Mir G, Smith WB, Stuckey M, Smith PMC (2008) J Agric Food Chem 56:6370–6377
Ballabio C, Penas E, Uberti F, Fiocchi A, Duranti M, Magni C, Restatni P (2013) Pediat Allergy Immunol 24:270–275
Czubinski J, Dwiecki K, Siger A, Kachlicki P, Neunert G, Lampart-Szczapa E, Nogala-Kalucka M (2012) J Agric Food Chem 60:1830–1836
Bradford M (1976) Anal Biochem 72:248–254
Aura AM, Harkonen H, Fabritius M, Poutanen K (1999) J Cereal Sci 29:139–152
Hoebler C, Lecannu G, Belleville C, Devaux MF, Popineau Y, Barry JL (2002) Int J Food Sci Nutr 53:389–402
Laemmli UK (1970) Nature 227:680–685
Czubinski J, Siger A, Lampart-Szczapa E (2016) Eur Food Res Technol 242:391–402
Czubinski J, Barciszewski J, Gilski M, Szpotkowski J, Debski J, Lampart-Szczapa E, Jaskolski M (2015) Acta Crys D71:224–238
Capraro J, Magni C, Scarafoni A, Duranti M (2009) J Agric Food Chem 57:8612–8616
Kłos P, Poręba E, Springer E, Lampart-Szczapa E, Józefiak AG (2010) J Food Sci 75:H39–H43
Schiarea S, Arnoldi L, Fanelli R, De Combarieu E, Chiabrando C (2013) PLoS One 8:e73909
Mari A, Iacovacci P, Afferni C, Barletta B, Tinghino R, Di Felice G, Pini C (1999) J Allergy Clin Immunol 103:1005–1011
Leduc V, Moneret-Vautrin DA, Guerin L (2002) Immunol 34:213–217
Guarneri F, Guarneri C, Benvenga S (2005) Internat Arch Allergy Immunol 138:273–277
Acknowledgements
This study was financed by the Ministry of Science and Higher Education of Poland under Project No. N N312 493340, and partially by the National Science Centre, Poland (Project No. 2015/19/D/NZ9/00065). J. C. was supported by the Foundation for Polish Science (FNP) under the FNP Start project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Bioethics Committee of Poznan University of Medical Sciences, Poland, decision number 533/10) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Czubinski, J., Montowska, M., Springer, E. et al. Immunoreactivity changes during lupin seed storage proteins digestion. Eur Food Res Technol 243, 2095–2103 (2017). https://doi.org/10.1007/s00217-017-2910-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2910-6