Abstract
Dihydroxyacetone (1,3-dihydroxy-2-propanone, DHA) is applied in the food and cosmetic industries as well as in pharmacy and medicine. It is produced as a result of incomplete oxidation of glycerol by acetic acid bacteria Gluconobacter oxydans. This reaction is catalyzed by PQQ-dependent membrane-bound glycerol dehydrogenase. The research developed a method of obtaining DHA by oxidation of a 3 % aqueous solution of glycerol (pH 7.5) at a temperature of 23 °C, with the only reaction biocatalyst being an immobilized cell preparation obtained from G. oxydans cells. After 5 days of the process, DHA concentration in the solution accounted for 27.2 g/L and the reaction efficiency for 94 %. After 4 days of the reaction run in culture media with pH 5.0, at a temperature of 28 °C, free or immobilized cells of G. oxydans produced on average 25 g of DHA/L at the reaction efficiency of 87 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductory remarks
The dihydroxyacetone (DHA) has various uses in cosmetics [5, 10, 16, 31], medicine [8, 17, 30], pharmaceuticals [12] and food industries [24, 26, 34]. Its production from glycerol is financial interesting due to the overproduction of glycerol by the biodiesel industry.
There are known two methods of dihydroxyacetone production: chemical and microbiological one [16]. The chemical synthesis may proceed via catalytic oxidation of glycerol or its condensation with calcium carbonate [13, 28].
An alternative to the chemical synthesis of DHA is microbiological oxidation of glycerol by microorganisms, acetic acid bacteria in particular, that exhibit a high activity of glycerol dehydrogenase [9, 36].
Glycerol dehydrogenase (GlyDH, EC 1.1.99.22) determines the incomplete oxidation of glycerol to DHA by acetic acid bacteria. This enzyme is strongly bound with the cytoplasmic membrane of acetic acid bacteria [32], and its oxidative activity is independent of NAD presence. The active center of GlyDH is located in the periplasmic space, which enables lesser consumption of energy required for the transport of substrates into and products outside the cell [12, 24, 29]. Due to a strongly hydrophobic character and low stability of the purified fraction of GlyDH, it is difficult to determine the spatial structure of this enzyme [20, 22, 23]. The optimal activity of GlyDH obtaining from cells of acetic acid bacteria of the genus Gluconobacter is observed in the pH range of 7.0–8.0 [4, 20] and in the temperature range of 23–25 °C [1].
Dihydroxyacetone production can be improved by GlyDH overexpression in Gluconobacter oxydans M5AM [21] In this study, the gene-coding membrane-bound alcohol dehydrogenase (ADH) was interrupted. The absence of ADH together with the overexpression of GlyDH gene resulted in an increased GlyDH activity in the strain M5AM/GlyDH, which led to a substantially enhanced production of DHA in the resting cells. In I batch of biotransformation process, G. oxydans M5AM/GlyDH exhibited a 2.4-fold increased DHA productivity of 2.4 g/g CDW/h (CDW-cell dry weight) from 1.0 g/g CDW/h, yielding 96 g/L DHA from 100 g/L glycerol. In four repeated batch runs, 385 g of DHA over a time period of 34 h was achieved from 400 g glycerol with an average productivity of 2.2 g/g CDW/h. This study indicated that new mutant G. oxydans M5AM/GlyDH with high productivity and increased tolerance against product inhibition has potential for DHA production in a industrial bioconversions process [21] Another microbial method of improving production of DHA by G. oxydans consisted of a semi-continuous repeated-fed-batch process by in situ immobilization of G. oxydans cells [15] In this experiment, a novel carrier material was used. Advantages of the new carrier matrix were as follows: ample space for the settling of cells, the protection of the cells from abrasion caused by shear forces or a sufficiently high oxygen supply rate due to the high oxygen permeability of the utilized silicone matrix. The experiment was conducted using packed-bed bubble-column bioreactor. The experimental results indicate that the immobilized biomass amounted to approximately 65 % of the total biomass contained in the bioreactor after 18 days of operation. The space–time yield was approximately 76 % higher compared to a similar process which was performed without an optimized fermentation medium [15]. Other experiments [25] to increase the microbial production of DHA included expression of three various genes which encode GlyDH (e.g., from Hansenula polymorpha). The NAD+-dependent GlyDH of H. polymorpha showed the highest glycerol-oxidizing activity. DHA concentration in shake flask experiments was roughly 100 mg/L from 20 g/L glucose, that is, five times the wild-type level. This level was achieved only when cultures were subjected to osmotic stress known to enhance glycerol production and accumulation in Saccharomyces cerevisiae. Dihydroxyacetone kinase activity was abolished to prevent conversion of DHA to dihydroxyacetone phosphate. The double-deletion mutant overexpressing H. polymorpha GlyDH produced 700 mg DHA/L under the same conditions [25].
One of the main problems in the biotechnological production of DHA is a multistage process of acetic acid bacteria preparation that involves, that is, proliferation of these microorganisms in culture media (diversified in terms of the source of carbon and nutrients) and activation of GlyDH. The course of this reaction may be disturbed by the inhibiting effect of a glycerol substrate or/and the product formed on the metabolic activity of acetic acid bacteria and activity of GlyDH [9, 22, 24]. Apart from DHA, the post-reaction mixture contains other bacterial metabolites that impede purification and crystallization of DHA [6].
Our approach was the advantage that allows the production of DHA in reaction which is independent from the presence and metabolic activity of live cells of G. oxydans. Our method of biotransformation of glycerol to DHA allows to shorten the process of preparation of the biocatalyst (which is cell preparation), and additionally the production of DHA is carried out with the immobilized biocatalyst which can be easily separated from the reaction mixture and reused in the next cycle of reaction. The proposed method is novel due to the replacement of live cells of G. oxydans with an immobilized cell preparation with the activity of GlyDH and production media—with aqueous solutions of glycerol. This method will probably facilitate and accelerate the crystallization of the final product because the post-reaction media contains only DHA and residues of glycerol.
Materials and methods
Strain and culture conditions
The study was carried out with the strain of acetic acid bacteria G. oxydans ATCC 621 from American Type Culture Collection, University Boulevard, Manassas. The strain was passaged every 30 days onto the culture medium containing: yeast extract 5 g/L, peptone 3 g/L, mannitol 25 g/L and agar 15 g/L, and incubated at a temperature of 28 °C for 24 h. For biomass proliferation, a pure culture of G. oxydans was transferred to a 50 cm3 liquid medium containing yeast extract 30 g/L (BTL) and ethyl alcohol 20 cm3/L (POCH), at pH 5.0, and incubated at a temperature of 28 °C for 24 h on a reciprocating shaker (200 cycles/min, Edmund Büchler SM-30 Control). In order to activate GlyDH, 1 % [v/v] of the culture was transferred to an 150 cm3 activating medium that contained the following: yeast extract 5 g/L (BTL), glycerol 20 g/L (POCH) and (NH4)2SO4 5 g/L (POCH), at pH 5.0, and incubated at a temperature of 28 °C for 48 h on a reciprocating shaker (200 cycles/min, Edmund Büchler SM-30 Control).
Obtention of a cell preparation with GlyDH activity
On completion of the GlyDH-activating culture, the centrifuged biomass of G. oxydans (5,000g, 10 min, 4 °C, Eppendorf Centrifuge 5804 R) was double-rinsed with sterile distilled water and suspended in 60 cm3 of water. Afterwards, cells were disintegrated with the ultrasound method (sonication, 210 W, 18 kHz, 4 °C, 5 min, Omni Ruptor 4000, Titanium 3/8′′ Dia Solid OR-T-375), as a result of which a cell preparation with catalytic activity of GlyDH was obtained [19]. The cell sediment (partially purified from water-soluble proteins and small cellular organelles) and the supernatant were obtained after centrifugation (9,500g, 90 min, 4 °C, Eppendorf Centrifuge 5804 R) of disintegrated G. oxydans cells [2, 3].
Immobilization of biocatalysts
The rinsed and centrifuged biomass of G. oxydans cells was suspended in 60 cm3 of a physiological saline solution and mixed with a sterile solution of sodium alginate (40 g/L) (Fluka) at the ratio of 1:1 [v/v]. From bacterial suspension in sodium alginate, gel beads of the same size were formed by direct instilling (using a syringe and a needle 0.9 mm in diameter) to a 0.2 M solution of calcium chloride (II) (POCH). Next, the immobilizates were separated from the CaCl2 solution using a sterile aluminum sieve and rinsed with sterile distilled water (500 cm3). This procedure was applied to immobilize the cell preparation, the sediment and the supernatant.
Biotransformation of glycerol to DHA
Glycerol biotransformation to DHA was conducted using the following biocatalysts:
-
Free or immobilized cells of G. oxydans in production media containing yeast extract 5 g/L, (NH4)2SO4 7.5 g/L, glycerol 30 g/L, at pH 5.0 and a temperature of 28 °C,
-
Immobilized cell preparation, sediment or supernatant in production solutions containing only glycerol at a concentration of 30 g/L at pH 7.5 and a temperature of 23 °C.
Biotransformation was conducted in 150 cm3 of the culture medium or production solution, in flasks with a volume of 500 cm3 by reciprocating shaking (200 cycles/min, Edmund Büchler SM-30 Control).
Determination of glycerol and dihydroxyacetone concentrations
Glycerol concentration was determined with the enzymatic method using a Free Glycerol Reagent test kit (Sigma, F6428) that contained a complex of enzymes catalyzing three reactions. In the first reaction, glycerol was phosphorylated and then glycerol 1-phosphate was oxidized. At the last stage, quinoneimine was produced, the pink-purple color of which was measured spectrophotometrically (Bio-Rad Smart Spec 3000) at the wavelength of 540 nm.
The concentration of DHA was assayed spectrophotometrically according to the method that uses the reducing properties of DHA [7]. To 2 cm3 of the analyzed sample, 2 cm3 of 3,5-DNS acid was added and the sample was incubated at 100 °C for 10 min. After incubation, the sample was immediately cooled. Its absorbance was measured at a wavelength of 550 nm (Bio-Rad Smart Spec 3000) against a blank sample, made of a sterile production media or of a production solution (depending on the biocatalyst applied).
Calculation of reaction efficiency
The efficiency of biotransformation was calculated from the equation of a chemical reaction of glycerol oxidation to DHA.
Statistical analysis of results
All analyses were carried out in three parallel series, with each of the series including three parallel biotransformations. Each measurement was repeated three times. Results achieved were developed statistically with the use of StatGraphicPlus 4.1 software. Multifactor analysis of variance was carried out as well. Significance of differences between mean values was determined with the Tukey’s test at a significance level of α = 0.01.
Results and discussion
Biotransformation of glycerol to DHA with the use of free or immobilized cells of G. oxydans ATCC 621
The first stage of the study involved a series of biotransformations in production media with the use of free or immobilized cells of G. oxydans. The results are shown in Figs. 1 and 2.
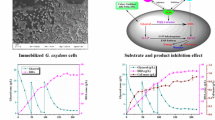
The conducted study indicates that the course of glycerol biotransformation to DHA run with free or immobilized cells of G. oxydans was alike within the first 50 h (Fig. 1). The highest concentration of DHA noted for the free cells accounted for 25.8 ± 0.023 g/L after 4 days, whereas for the immobilized cells, it accounted for 24.6 ± 0.032 g/L after 3 days of the process. Glycerol was completely consumed from the production media (Fig. 1), but the efficiency of biotransformation did not reach 100 % (Fig. 2), which suggests that part of the substrate could have been consumed for other reactions than oxidation to DHA. It pertains, in particular, to G. oxydans bacteria capability for glycerol transformation into sodium glycerate which is undetectable with the applied enzymatic method [14].
Despite achieving similar concentrations of DHA in particular hours of the biotransformation run in the presence of free or immobilized cells of G. oxydans (Fig. 1), this process proceeded with the highest efficiency in the production medium with the free cells (Fig. 2). Probably, oxygen indispensable for glycerol oxidation into DHA was more easily available to the free than to the immobilized cells and the substrate was better utilized. The immobilization of bacterial cells in calcium alginate could, to some extent, limit oxygen and substrate diffusion to the interior and that of the product outside the alginate carrier.
On the second day of biotransformation, a significant increase was observed in DHA concentration in the production medium, irrespective of the biocatalyst applied (Fig. 1). The product’s concentration in the medium with free cells increased from 0.16 ± 0.051 (in the 6th hour) to 10.5 ± 0.027 g/L (in the 24th hour), whereas in the production medium with immobilized cells from 0.19 ± 0.070 (in the 6th hour) to 10.1 ± 0.020 g/L (in the 24th hour). This could be due to an increased activity of GlyDH, which in the case of the G. oxydans strain usually occurs after 20 h of biotransformation [4]. Oxygen concentration in that time could have been high enough to assure the effective course of glycerol oxidation to DHA, which was also pinpointed by Wethmar and Deckwer [35].
Biotransformation was finalized after 4 days because analyses of the production media revealed no presence of glycerol (Fig. 1). Due to a lack of carbon source in the medium, acetic acid bacteria may phosphorylate the earlier-produced dihydroxyacetone, and this leads to a reduction in the efficiency of the process [11].
The multifactor analysis of variance and Tukey’s test conducted for values of DHA concentration achieved at this stage of the experiment demonstrated that reaction time was the only factor having a significant effect on increasing DHA concentration in the production media. The immobilization of G. oxydans cells did not affect the content of produced DHA compared to its concentration achieved with the participation of free cells.
Biotransformation of glycerol to DHA with the use of immobilized catalysts produced from G. oxydans cells
Literature data [20] indicate that GlyDH isolated from cytoplasmic membranes of G. oxydans bacteria showed a lower oxidative activity and stability compared to the fraction in which this enzyme remained bound with the membranes. For this reason, extraction and purification steps of GlyDH from cell organelles or fragments of cell membranes that were obtained upon ultrasound disintegration of G. oxydans cells were omitted deliberately. Figures 3 and 4 show changes in concentrations of DHA and glycerol and in the efficiency of 5-day glycerol biotransformation run in an aqueous solution of this substrate, at a temperature of 23 °C and pH 7.5.
After 24-h reaction run in the solution with the immobilized cell preparation, DHA concentration reached 6.1 ± 0.020 g/L (Fig. 3), whereas 22 ± 0.079 g of glycerol/L was still left in the solution (Fig. 3) and the efficiency of reaction reached 76 % (Fig. 4). On the next day, DHA concentration reached 12.4 ± 0.041 g/L of the production solution (Fig. 3) at the reaction efficiency of 88 % (Fig. 4). After 5 days of the process, the concentration of dihydroxyacetone accounted for 26.1 ± 0.021 g/L of the solution (Fig. 3), the reaction efficiency was at a level of 88 % (Fig. 4), and glycerol was almost completely consumed from the production solution (Fig. 3). As initially expected, the course of changes in concentrations of DHA and glycerol in production solutions with the immobilized sediment was similar to that of changes observed in the process run with the cell preparation (Fig. 3). The supernatant was devoid of the catalytic activity of GlyDH because no DHA was detected in the solution with this biocatalyst (Fig. 3). This experiment confirmed that GlyDH remained bound with fragments of cellular membranes of G. oxydans (present in the cell preparation and in the sediment) and after disintegration was not released to the fraction of water-soluble proteins (present in the supernatant).
In biotransformation run with the cell preparation or the sediment, glycerol was completely consumed from the solutions (Fig. 3), but still the reaction efficiency did not reach 100 % (Fig. 4). It may be speculated that part of the DHA remained trapped in the structure of an alginate carrier and could not be released to the solution and determined with the method applied.
The highest concentrations of DHA achieved in reaction run with the free (25.8 ± 0.023 g/L) or immobilized cells (24.6 ± 0.032 g/L) of G. oxydans (Fig. 1) were similar to concentrations reported in the reactions with the immobilized cell preparation (26.1 ± 0.021 g/L) or the sediment (24.6 ± 0.009 g/L) (Fig. 3). These results enable concluding explicitly that the cell preparation had the catalytic activity of GlyDH that was comparable to the activity exhibited by live G. oxydans cells. The conducted study demonstrated the feasibility of producing DHA via oxidation of an aqueous solution of glycerol in the presence of an immobilized cell preparation from G. oxydans.
Evaluation of the oxidative potential of reused cell preparation
The purpose of successive biotransformation series was to verify whether the immobilized cell preparation applied in one biotransformation cycle maintains its GlyDH activity in the next cycle. On completion of 7-day biotransformation, the immobilized cell preparation was rinsed with sterile distilled water cooled to 4 °C and again transferred into the production solution with glycerol concentration of 30 g/L. Simultaneous biotransformation was run with a cell preparation that was not applied earlier for glycerol oxidation to DHA. Results of these analyses were presented in Figs. 5 and 6.
In the solution with the cell preparation used for the first time, the concentration of DHA accounted for 9.2 ± 0.030 g/L after the first day, for 24.4 ± 0.003 g/L after 4 days and for 27.2 ± 0.001 g/L after 7 days of the process (Fig. 5). The efficiency of reaction in those periods reached 44, 84 and 94 %, respectively (Fig. 6). In the said solution, glycerol was utilized almost completely (Fig. 5). The low final concentration of the substrate (0.09 ± 0.010 g/L) and, simultaneously, a high reaction efficiency reaching 94 % (Fig. 6) enable concluding that glycerol was almost completely oxidized to dihydroxyacetone.
The reused cell preparation was not so efficient in running glycerol biotransformation to DHA. The highest concentration of dihydroxyacetone, noted after 3 days of the reaction, reached 16.1 ± 0.004 g/L (Fig. 5). Although the reaction efficiency accounted for 94 % (Fig. 6), 12.2 ± 0.019 g of glycerol/L was still left in the solution (Fig. 5). The extension of biotransformation time did not cause an increase in product’s concentration (Fig. 5). The content of glycerol in the solution was not changing since the 3rd day till the end of the process.
Glycerol dehydrogenase is an enzyme-dependent PQQ prosthetic group [4, 27]. It is likely that during the first application of the cell preparation, GlyDH contained in it bounded a sufficiently high concentration of PQQ from G. oxydans cell and could run efficient oxidation of the substrate. Probably, the concentration of PQQ in the reused cell preparation was not high enough, which might have had a negative impact on the course and efficiency of glycerol biotransformation to DHA.
Similar investigations with cells of G. oxydans immobilized in polyvinyl alcohol gel were carried out by Wei et al. [33]. The efficient production of DHA from glycerol proceeded at pH 6.0 and a temperature of 30 °C. Such cells displayed the oxidative activity against glycerol even after 14 days of storage; however, the activity was lower by 10 % than that of the cells applied immediately after immobilization. The immobilized cells of G. oxydans bacteria reused in the reaction medium were running the biotransformation process with the efficiency about 86 % [33].
Studies on the oxidation of glycerol to DHA with the use of live G. oxydans cells are mainly focused on reducing production costs, facilitating the process and reaching possibly the highest reaction efficiency [18]. Therefore, the application of the immobilized cell preparation for the biotransformation process may be an interesting alternative to the traditional method of DHA synthesis.
Conclusions
The study was aimed at elaborating a new biotechnological method for the production of dihydroxyacetone via oxidation of a glycerol solution with the use of an immobilized cell preparation produced from acetic acid bacteria G. oxydans ATCC 621. Problems associated with traditional production of DHA based on living cells were supposed to be reduced or eliminated by making biotransformation independent of the metabolic activity of live G. oxydans cells and by replacing microbiological culture media with aqueous solutions of glycerol at a concentration of 30 g/L. The proposed method of glycerol biotransformation to DHA run in a 3 % aqueous solution of substrate with pH 7.5, at a temperature of 23 °C, allowed to obtain 27.2 g DHA/L of the solution with 94 % efficiency of the reaction. The free or immobilized cells of G. oxydans, placed in the production medium that apart from nutrients and minerals contained 3 % of glycerol, at pH 5.0 and a temperature of 28 °C, produced about 25 g DHA/L of the medium at a reaction efficiency of 87 %.
This study proved that efficient biotransformation of glycerol to DHA with the use of a cell preparation could proceed in an aqueous solution of the substrate. Analytical results achieved point to the feasibility of making DHA production independent of the presence of viable G. oxydans cells. The application of the novel method for DHA production will, probably, eliminate the multistage purification of the final product. Immobilization of the cell preparation with GlyDH activity will facilitate the procedure of DHA production, as the biological material entrapped in carrier’s structure may easily be separated from the post-production solution.
Abbreviations
- 3,5-DNS:
-
3,5-dinitrosalicylic acid
- DHA:
-
Dihydroxyacetone
- G.oxydans :
-
Gluconobacter oxydans ATCC 621
- GlyDH:
-
Glycerol dehydrogenase
- PQQ:
-
Pyrroloquinoline quinone
References
Adachi O, Matsushita K (1997) USA Patent nr 56143774
Adlercreutz P, Holst O, Mattiasson B (1985) Appl Microbiol Biotechnol 22:1–7
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P (1999) PWN, Warsaw pp 155–172
Ameyama M, Shinagawa E, Matasushita K, Adachi O (1985) Agric Biol Chem 49:1001–1010
Bicker M, Endres S, Ott L, Vogel H (2005) J Mol Catal A Chem 239:151–157
Charney W, Montclair NJ (1978) USA Patent nr 4076589
Chen J, Chen J, Zhou C (2008) J Chromatogr Sci 46:912–916
Choquenet B, Couteau C, Paparis E, Coiffard LJM (2009) J Dermatol 36:587–591
Claret C, Bories A, Soucaille P (1992) Current Microbiol 25:149–155
Enders D, Voith M, Lenzen A (2005) Angew Chem Int Ed 44:1304–1325
Erni B, Siebold C, Christen S, Srinivas A, Oberholzer A, Baumann U (2006) Cell Mol Life Sci 63:890–900
Gätgens C, Degner U, Bringer-Meyer S, Herrmann U (2007) Appl Microbiol Biotechnol 76:553–559
Gehrer E, Harder W, Vogel H, Knuth B, Ebel K, Groening C (1995) USA Patent nr 5410089
Gupta A, Singh VK, Qazi GN, Kumar A (2001) J Mol Microbiol Biotechnol 3:445–456
Hekmat D, Bauer R, Neff V (2007) Proc Biochem 42:71–76
Hekmat D, Bauer R, Fricke J (2003) Bioproc Biosyst Eng 26:109–113
Henderson PW, Kadouch D, Singh SP, Zawaneh PN, Weiser J, Yazdi S, Weinstein A, Krotscheck U, Wechsler B, Putnam D, Spector JAJ (2010) Biomed Mater Res A 93:776–782
Hu ZC, Zheng YG, Shen YC (2011) Bios Technol 102:7177–7182
Kapucu H, Gülsoy N, Mehmetoğlu Ü (2000) Biochem Eng J 5:57–62
Lapenaite I, Kurtinaitiene B, Razumiene J, Laurinavicius V, Marcinkeviciene L, Bachmatova I, Meskys R, Ramanavicius A (2005) Anal Chem Act 549:140–150
Li M, Wu J, Liu X, Lin J, Wei D, Chen H (2010) Biores Technol 101:8294–8299
Ma L, Lu W, Xia Z, Wen J (2010) Biochem Eng J 49:61–67
Matsushita K, Fujii Y, Ano Y, Toyama H, Shinjoh M, Tomiyama N, Miyazaki T, Sugisawa T, Hoshino T, Adachi O (2003) Appl Environ Microbiol 69:1959–1966
Mishra R, Jain SR, Kumar A (2008) Biotechnol Adv 26:293–303
Nguyen HTT, Nevoigt E (2009) Metabol Eng 11:335–346
Obeid OA, Jamal ZM, Hwalla N, Emery PW (2006) Nutrition 22:794–801
Oubrie A, Dijkstra BW (2000) Protein Sci 9:1265–1273
Painter RM, Pearson DM, Waymonth RM (2010) Angew Chem Int Ed 49:9456–9945
Prust Ch, Hoffmeister M, Liesegang H, Wiezer A, Fricke WF, Ehrenreich A, Gottschalk G, Deppenmeier U (2005) Nat Biotechnol 23:195–200
Rogers CJ (2005) Aesth Surg J 25:413–415
Schmid D, Belser E, Zulli F (2007) Cosmet Toilet 6:55–60
VanLare IJ, Claus GW (2007) Can J Microbiol 53:504–508
Wei S, Song Q, Wei D (2007) Prep Biochem Biotechnol 37:67–76
Weiser JR, Zawaneh PN, Putnam D (2011) Biomacromolecule 12:977–986
Wethmar M, Deckwer WD (1999) Biotechnol Tech 13:283–287
Xu X, Chen X, Jin M, Wu X, Wang X (2009) Chin J Biotechnol 25:903–908
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lidia, SR., Stanisław, B. Production of dihydroxyacetone from an aqueous solution of glycerol in the reaction catalyzed by an immobilized cell preparation of acetic acid bacteria Gluconobacter oxydans ATCC 621. Eur Food Res Technol 235, 1125–1132 (2012). https://doi.org/10.1007/s00217-012-1846-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1846-0