Abstract
Cultures of Saccharomyces cerevisiae were treated with PEF to improve accumulation of magnesium in the biomass. Under optimized conditions, that is, on 15-min exposure of the 20-h grown culture to PEF of the 2,000 V and 20-μs pulse width, accumulation of magnesium in the yeast biomass reached maximum 3.98 mg/g dm. It constituted 40% of the total magnesium in the medium. That accumulation significantly correlated against magnesium concentration in the medium. Neither multiple exposure of the cultures to PEF nor intermittent supplementation of the cultures with magnesium increased the magnesium accumulation. The intermittent supplementation of the cultures with magnesium could even reduce the accumulation efficiency by 30%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium ions are essential for the growth and metabolism of yeast cells [1–3]. Magnesium ions also stabilize ribosomal nucleic acids, membrane structures and protect yeast cells against environmental stress during either fermentation or high osmotic pressure [4–6]. The high daily requirement of magnesium for the human organism results from the role of the Mg ions as a cofactor for the activity of over 300 enzymes [7, 8]. Magnesium in living cells is bound mostly to phospholipids, proteins and nucleotides, except a small fraction, which resides there in a free form [7, 9–11]. In recent years, there has emerged a growing concern about magnesium deficiency in humans and animals. For that reason, supplementation of the diet with magnesium became an indispensable procedure. Yeast biomass appeared to be a suitable source of deficient elements and vitamins. Thus, enriched in magnesium, such biomass could be an option for pharmacological supplementation with that element.
Organisms better tolerate and assimilate bioelements in the form of metalloproteins than as components of pharmaceuticals. In the future, foodstuffs containing either magnesium-enriched yeast or yeast preparations containing magnesium biocomplexes could become additional sources of that element [12, 13].
Microorganisms bioaccumulate metals in two stages. In the first step, the metal ions adhere to the surface of cell membranes followed by their binding to intramicrofibrilar structures of the cell wall by adsorption and/or complexing. Metals are usually introduced through either ion exchange, precipitation or co-crystallization. The second stage involves an active transport of the metal ions through a cytoplasmatic membrane to the cell interior [1, 14–17].
Pulsed electric fields (PEF) technology offers a non-thermal food preservation that kills most pathogenic and putrefactive microorganisms, inactivates enzymes and limits the loss of taste, color, texture, nutrients, and heat labile functional food components [18–20]. The electric field can affect the cell membranes [21], which can irreversibly be damaged [22–24], influences the transport of ions [25] and structure of enzymes [26]. In a cell subjected to PEF-induced transmembrane tension facilitates the formation of pores in the membrane and leads to an increase in its permeability [27–31]. Electroporation temporarily eliminates integrity of the plasmatic membrane, i.e., produces small pores that close back with a time [31–33].
The aim of this paper was to test whether PEF is a suitable technique for the magnesium enrichment of Saccharomyces cerevisiae.
Materials and methods
Culture maintenance and inoculum preparation
Saccharomyces cerevisiae 11 B1 (industrial strain) from the Yeast Plant (Lublin, Poland) was used.
Medium for agar slants and inoculum growth (g/L): sucrose (20); NH4Cl (3.2); KH2PO4 (2.5); Na2SO4 (2.0) (POCH, Gliwice, Poland); yeast extract (YE) (5.0); agar (15) (DIFCO, Detroit, MI, USA); and unhoped wort (40.0 mL) (Lublin Breweries S.A., Lublin, Poland) had pH 5. Experimental medium for S. cerevisiae contained (g/dm3): peptone (10) (Sigma–Aldrich CO, St. Louis, MI, USA); YE (5); glucose (10) (POCH, Gliwice, Poland) [7].
Biomass cultivation
Yeast was passaged three times for agar slants, grown for 48 h in a thermostat at 30 °C and finally an inoculum was prepared. Cells from a single slant were used to inoculate 150 mL sterile medium in an Erlenmeyer flask. Cultures were grown on a rotary shaker with water bath, at amplitude 4 and at 220 rpm for 48 h at 30 °C. The fermented medium was centrifuged, washed three times with sterile water, and the remaining washed biomass of cells from three Erlenmeyer flasks was diluted with sterile water to a final volume of 300 mL. The inoculum was made of 48-h yeast cultures with OD400 of 2.2 (Spekol 11, Carl Zeiss, Jena, Germany). Biomass inoculum with washing was 12.2 g dw/L. The inoculum prepared in this manner was used for the inoculation of submerged grown in 500-mL Erlenmeyer flasks, each containing 100 mL medium inoculated with 10 mL inoculum. The culturing conditions were identical to those applied for the inoculum preparation. After 42 h culturing, the mycelium was centrifuged, washed several times with distilled water, and then lyophilized in a Labconco freeze dryer (Model 64132, Kansas City, MO, USA).
PEF treatment and enrichment with magnesium
S. cerevisiae cultures grown in flasks were agitated for 20 h then treated with PEF for 15 min (electroporator ECM 830, BTX Harvard Apparatus, USA) at the field frequency of 1 Hz and 20 μs pulse width. Simultaneously, voltage was optimized on the field exposition of 50, 100, 150, 200, 250, 500, 1,000, 1,500, 2,000 and 2,500 V.
Optimization of the PEF exposition time for 20 h was performed culturing the yeast for 5, 10, 15 and 20 min, at optimum voltage of 2,000 V. The PEF pulse width at 10, 20, 50, 75, 100, 125 and 150 μs was selected at 2,000 V on the 15-min exposition.
Optimization of the time interval after which yeast cells were 15 min treated with PEF was performed after 8, 12, 16, 20 and 24 h culturing at 2,000 V and 20 μs pulse width. Concentration of magnesium in the nutrient broth was estimated for PEF-treated cells cultured at given field parameters and time of culturing. Subsequent culturing of S. cerevisiae was carried out in the medium containing 10, 100, 200, 400, 500, 750 and 1,000 μg/mL magnesium.
At optimum PEF parameters and 100 μg/mL magnesium concentration, the medium was additionally enriched with subsequent 25 μg/mL doses of magnesium after 8, 12, 16 and 20 h culturing. Subsequent culturing was performed under optimized conditions, and these cultures were PEF treated several times after 8, 12, 16 and 20 h. The results allowed to check the role of enrichment with magnesium and number of expositions to PEF upon the magnesium accumulation in the yeast cells. Samples not treated with PEF and without magnesium in the medium (K1) or with 100 μg Mg/mL (K2) served as controls.
Determination of the magnesium concentration
Mineralization of yeast for the determination of the magnesium concentration using the method of flame atomic absorption spectrophotometry (FAAS, Solaar 939, Unicam) was conducted as follows: 250 mg samples of lyophilized magnesium-enriched yeast were weighed into glass thimbles, 3 ml HNO3–HClO4 (5:1) was added, the mixtures were left overnight then heated in a heating block at 250 °C for 20 h. After cooling, solutions were transferred to 50 mL measuring flasks and topped up with deionized water.
Determination of the yeast cell viability
Cell viability was determined in the Thoma chamber, dyeing necrotic yeast cells with the 0.01% methylene blue solution. The percentage of necrotic cells was the mean of 16 fields calculated according to the formula: % necrotic cells = (number of necrotic cells/sum of necrotic and living cells) × 100%.
Determination of the crop of yeast biomass
Biomass was estimated from optical density at 400 nm. Dry mass was calculated by referring a standard curve of cell mass versus absorbance. The fermented medium from culturing (2 mL) was centrifuged (3,000 rpm), and supernatant was discarded; cells were rinsed with deionized water and brought to the original volume of 2 mL. Nephelometric measurements were run against pure water in 2-mm measurement cell. Amount of dry residue was calculated using equation for the standard curve: A p = 0.4476c, where A p and c were apparent absorbance and concentration (mg/mL), respectively.
Data analysis
Significant differences between particular groups were found involving the Student’s t-test applied to compare independent samples in pairs, and variance analysis (ANOVA) was used for more than two groups. Statistical processing of results was performed using Statistica 6.0 software.
Results and discussion
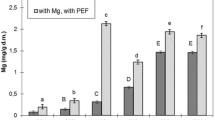
Experiments showed that low voltage in the range of 50–500 V did not influence the magnesium accumulation in the yeast cells maintaining it on the level of 1.9 mg/g dm, which is not significantly different to control sample K2 (Fig. 1). In the control culture K1, magnesium accumulation was 1.2 mg/g dm. Statistically essential changes in accumulation appeared just when voltage above 500 V was applied and that of 2,000 V provided maximum accumulation reaching 3.98 mg/g dm, that is, twice as much as in the K2 sample. The voltage exceeding 2,000 V reduced the magnesium accumulation. Voltage applied had an essential influence on the cell vitality (Fig. 2). An elevation of voltage in the range from 500 to 2,500 V resulted in a gradual increase in the count of necrotic cells. At optimum 2,000 V, there were 9.5% necrotic cells in the culture, and at 2,500 V, the count of necrotic cells was by three times as high as at 500 V.
Prolonged PEF treatment increased the magnesium content in the cell biomass (Fig. 3). The 15-min exposure provided the highest accumulation of magnesium reaching 4 mg/g dm i.e., by 23 and 4% higher concentration in respect to 5- and 20-min exposure, respectively.
The pulse width also appeared to be an essential factor. The 20-μs pulse width provided superior magnesium accumulation of 4 mg/g dm (Fig. 4). This value was by 28% higher then that acquired with the 10-μs pulse width. Pulse width elevated above 20 μs caused essential decrease in the magnesium accumulation. The pulse width affected also the cell vitality (Fig. 5). The 10-μs pulse width did not practically affect their vitality, which was the same as in the control K2 sample not treated with PEF. At optimum magnesium concentration in yeast (4 mg/g dm) and the 20-μs pulse width, the culture contained 9.5% necrotic cells. Increase in the pulse width to 50 μs doubled amount of necrotic cells. Further increase in the pulse width up to 125 μs had no statistically significant effect on the cell vitality; however, at the 150-μs pulse width, the culture contained already 35% necrotic cells. Hülsheger et al. [27] reported over 99.99% inactivation of microorganisms exposed to peak electric fields of 20 kV/cm and pulses of several milliseconds.
Control culture K1 without magnesium supplementation and PEF treatment accumulated magnesium on the level of 1 mg/g dm. The supplementation with magnesium without the PEF treatment (control K2) provided an increase in accumulated magnesium up to 1.86 mg/g dm.
Duration of the culturing after the PEF treatment had no effect upon the magnesium accumulation (Fig. 6). Under applied culturing conditions, the maximum magnesium concentration in the cells reached 4 mg/g dm after 20 h, which was by 34 and 23% higher than that after 8 and 24 h culturing, respectively. Results presented in Figs. 1, 2, 3, 4, 5, 6 reveal that the least and most biomass with washing (7.8 and 9.7 g dw/L, respectively) formed at 50-μs pulse width (Fig. 4), and after 10-min exposure PEF (Fig. 3), respectively.
Accumulation of magnesium in the cells increased with the increase of the magnesium concentration in the nutrient medium and that increase was not proportional (Fig. 7). At the optimum 100 mg/mL magnesium concentration in the medium, the accumulation was doubled when compared to that in the control K2 sample. It reached 4 mg Mg/g dm, that is, about 40% additional magnesium was accumulated. At 1,000 μg Mg/mL medium, cells accumulated only 7.16 mg/g dm magnesium i.e., only 7.16% supplemented magnesium. Increase in the magnesium concentration in the medium from 200 to 750 μg Mg/mL resulted in the 26% increase in the accumulation of that element. However, such increase in the level of magnesium in the medium without PEF treatment had only a slight effect upon the accumulation. It was 1.4 and 1.98 mg/g dm for the 10 and 1,000 μg Mg/mL medium, respectively. Data presented in Fig. 7 show that the lowest biomass with washing was available at the 10 and 1,000 μg/ml, magnesium concentration in the medium, whereas the concentration of 100 μg/ml, provided 7.4 and 9.3 g dw/L biomass, respectively. In the cultures enriched with Mg Cl2 · 6H2O (1.25 g Mg2+/L), after 48 h Błażejak et al. [34] collected 5.77 g dw/L biomass with washing.
In 48 h culturing S. cerevisiae 0.5 g Mg+2/L added, Duszkiewicz and Gniewosz [35] noted 3.13 mg Mg/g dm in the rinsed biomass. That accumulation was by 30% higher than in the control culture, which was not supplemented with magnesium. Varying concentration of supplemented magnesium independently of the source and the dose did not affect magnesium accumulation. Effect upon accumulation of that element. At the supplementation of 0.25 and 1.25 g Mg+2/L, the authors found 2.92 and 3.2 mg Mg/g dm accumulated magnesium, respectively. Amount of accumulated magnesium constituted barely 10% of that supplemented.
Walker and Maynard [8] reached maximum accumulation of magnesium, 450 μM, after 40 h culturing in the medium containing 347 μM magnesium. Higher concentration of magnesium, 496 μM, provided 300 μM of accumulated element after 58 h culturing. After 17 h culturing in the broth with 30 mM magnesium, Blackwell et al. [7] found magnesium accumulation on the level of 4,000 nmol (109 cells)−1.
This study revealed that multiple exposition of the yeast culture to PEF did not increase the accumulation in the yeast cells. Four subsequent 15-min exposures to PEF of 8, 12, 16 and 20 h lasting culturing provided accumulation of 4.18 ± 0.33 mg/g dm magnesium, which was only 5% higher than that after single PEF treatment (3.98 mg/g dm). Supplementation of total magnesium in four subsequent doses to cultures lasting 8, 12, 16 and 20 h had no effect upon the accumulation. A portion wise supplementation provided accumulation of 3.05 ± 0.11 mg/g dm of magnesium in the cells. Thus, it was by 30% lower than that after the single addition of magnesium at the beginning of the culturing.
Conclusions
-
1.
Optimized all voltage (2,000 V), exposure time (15 min), the pulse width (20 μs) and the point of treatment in course of the growth of cultures (20 h) provide the highest level of the magnesium accumulation in Saccharomyces cerevisiae.
-
2.
At 100 mg/mL magnesium concentration in the medium, the 40% accumulation of that element was achieved.
-
3.
That accumulation significantly correlated against magnesium concentration in the medium.
References
Walker GM (1994) The roles of magnesium in biotechnology. Crit Rev Biotechnol 14:311–354
Li LG, Tutone AF, Drummond RSM, Gardner RC, Luan S (2001) A novel family of magnesium transport genes in arabidopsis. Plant Cell 13:2761–2775
Gardner RC (2003) Genes for magnesium transport. Curr Opin Plant Biol 6:263–267
D’Amore T, Panchal CJ, Russell I, Stewart GG (1988) Osmotic pressure effects and intracellular accumulation of ethanol in yeast during fermentation. J Ind Microbiol 2:365–372
Blackwell KJ, Tobin IM, Avery SV (1997) Manganese uptake and toxicity in magnesium- supplemented and unsupplemented Saccharomyces cerevisiae. Appl Microbiol Biotechnol 47:180–184
Walker GM, Maynard AI (1997) Accumulation of magnesium ions during fermentative metabolism in Saccharomyces cerevisiae. J Ind Microbiol Biot 18:1–3
Romani AMP, Maguire ME (2002) Hormonal regulation of Mg2+ transport and homeostasis in eukaryotic cells. Biometals 15:271–283
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15:309–323
Bui DM, Gregan J, Jarosch E, Ragnini A, Schweyen RJ (1999) The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondria membrane. J Biol Chem 274:20438–20443
Graschopf A, Stadler JA, Hoellerer MK, Eder S, Sieghardt M, Kohlwein SD, Schweyen RJ (2001) The yeast plasma membrane protein A1r1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J Biol Chem 276:16216–16222
da Costa BMT, Cornish K, Keasling JD (2007) Manipulation of intracellular magnesium levels in Saccharomyces cerevisiae with deletion of magnesium transporters. Appl Microbiol Biotechnol 77:411–425
Błażejak S, Duszkiewicz-Reinhard W, Gniewosz M, Mazurkiewicz B (2004) Distribution of magnesium in the Candida utilis ATCC 9950 yeast cells enriched in that element (in Polish). Acta Sci Pol Technologia Alimentaria 3:95–110
Gniewosz M, Błażejak S, Roman J, Duszkiewicz-Reinhard W (2006) A study on Saccharomyces cerevisiae and Candida utilis cell Wall capacity for binding magnesium. Eur Food Res Technol 224:49–54
Brady D, Duncan JR (1994) Bioaccumulation of metal cations by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 41:149–154
Blackwell KJ, Singelton I, Tobin JM (1995) Metal cation uptake by yeast: a review. Appl Microbiol Biotechnol 43:579–584
Tuszyński T, Pasternakiewicz A (2000) Bioaccumulation of metal ions by yeast cells of Saccharomyces cerevisiae. Pol J Food Nutr Sci 4:31–39
Liu GJ, Martin DK, Gardner RC, Ryan PR (2002) Large Mg2+—dependent currents are associated with the increased expression of ALR1 in Saccharomyces cerevisiae. Microbiol Lett 213:231–237
Torregrosa F, Esteve MD, Frigola A, Cortes C (2006) Ascorbic acid stability during refrigerated storage of orange-carrot juice treated by high pulsed electric field and comparison with pasteurized juice. J Food Eng 73:339–345
Marselles-Fontanet AR, Martin-Belloso O (2007) Optimization and validation of PEF processing conditions to inactivate oxidative enzymes of grape juice. J Food Eng 83:452–462
Sampedro F, Rivas A, Rodrigo D, Martinez A, Rodrigo M (2007) Pulsed electric fields inactivation of Lactobacillus plantarum in an orange juice-milk based beverage: effect of process parameters. J Food Eng 80:931–938
Barsotti L, Cheftel JC (1999) Food processing by pulsed electric fields. II. Biological aspects. Food Rev Inter 5:181–213
Zimmermann U (1986) Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol 105:175–256
Pothakamury UR, Barbosa-Canovas GV, Swanson BG, Spence KD (1997) Ultrastructural changes in Staphylococcus aureus treated with pulsed electric fields. Food Sci Technol Int 3:113–121
Calderon-Miranda ML, Barbosa-Canovas GV, Swanson BG (1999) Transmission electron microscopy of Listeria innocua treated by pulsed electric fields and nisin in skimmed milk. Inter J Food Microbiol 51:31–39
Kim H, Ye J, Li Y (2001) Inactivation of Listeria monocytogenes in chilling brine using a flow through electrochemical treatment. Institute of Food Technologists, annual meeting. Paper 59H-22
Fernandez-Diaz MD, Barsotti L, Dumay E, Cheftel JC (2000) Effects of pulsed electric fields on ovalbumin solutions and dialyzed egg white. J Agr Food Chem 48:2332–2339
Hülsheger H, Potel J, Niemann EG (1983) Electric field effects on bacteria and yeast cells. Radiat Environ Biophys 22:149–162
Zhang Q, Chang FJ, Barbosa-Canovas GV, Swanson BG (1994) Inactivation of microorganisms in a semisolid model food using high voltage pulsed electric fields. LWT 27:538–543
Evrendilek GA, Zhang HQ, Richter ER (1999) Inactivation of Escherichia coli O157:H7 and Escherichia coli 8739 in apple juice by pulsed electric field. J Food Protect 62:793–796
Wouters PC, Dutreux N, Smelt JPP, Lelieveld HM (1999) Effects of pulsed electric fields on inactivation kinetics of Listeria innocua. Appl Environ Microbiol 62:5364–5371
Aronsson K, Rönner U, Borch E (2005) Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in relation to membrane permeabilization and subsequent leakage of intracellular compounds due to pulsed electric field processing. Int J Food Microbiol 99:19–32
Barbosa-Canovas GV, Gongora-Nieto MM, Pothakamury UR, Swanson BG (1999) Preservation of foods with pulsed electric field. Academic Press, San Diego, CA
Aronsson K, Rönner U (2001) Influence of pH, water activity and temperature on the inactivation of Escherichia coli and Saccharomyces cerevisiae by pulsed electric fields. Innov Food Sci Emerg Technol 2:105–112
Błażejak S, Duszkiewicz-Reinhard W, Gniewosz M, Rostkowska-Demner E, Domurad E (2002) The study of Saccharomyces cerevisiae brewery yeast strain capacity of binding with magnesium in dynamic conditions. EJPAU, Food Sci Technol 5:1–8
Duszkiewicz-Reinhard W, Gniewosz M, Błażejak S, Bańkowski A (2002) Study on the ability of Saccharomyces cerevisiae to bind magnesium in the stationary cultures (in Polish). Acta Sci Pol Technologia Alimentaria 1:17–26
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pankiewicz, U., Jamroz, J. Effect of pulsed electric fields upon accumulation of magnesium in Saccharomyces cerevisiae . Eur Food Res Technol 231, 663–668 (2010). https://doi.org/10.1007/s00217-010-1317-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1317-4