Abstract
Based on microwave-assisted extraction (MAE) and headspace solid-phase microextraction (HS-SPME) followed by gas chromatography mass spectrometry (GC–MS) was developed for the fast determination of volatile chemical compositions of Polygala furcata Royle, the experimental parameters of the MAE–HS-SPME were optimized by orthogonal experimental design. The results indicated that the optimum condition of the determination of the volatile compounds in P. furcata Royle. was achieved with the experimental parameters including microwave power of 400 W and irradiation time of 4 min, sample size 2.0 g. Under the optimal conditions, for the first time, 52 volatile compounds were separated and identified from the fresh plants of P. furcata Royle. The highest content component of the 52 compounds was 2-hydroxybenzoic acid methyl ester (21.65%). The relative standard deviation values <7% showed that the method of MAE–HS-SPME followed by GC–MS has good precision. The experimental results demonstrated that it is a simple, time-saving and solvent-free method, and it is a potential analytic tool for the determination of the volatile compounds of the vegetations materials and other materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polygala furcata Royle, belongs to the family Polygalaceae and Polygala, the total amount of the Polygalaceae in the world is about 13–17 genera and about 1,000 species, wildly distributed in worldwide, especially in tropical and subtropical regions of both hemispheres, and there are 5 genera and 53 species (24 endemic) in China. Chinese genera of economic importance include Polygala (medicinal), Salomonia (medicinal), Securidaca (medicinal), and Xanthophyllum (fine wood) [1], and the Polygala about 500 species, 44 species (21 endemic) in China. P. furcata Royle is one of the species, as a variation of Polygala is mainly wild and native to China mainly in Duyun of Guizhou province, Wenshan and Mengzi of Yunnan Province and Zhenkang of Guangxi Province. In addition, a small quantity of the plant distributed in India and Myanmar [2, 3]. Annual herbs, 5–15 cm tall, the flowering period of P. furcata Royle begins in late October to early November with primrose yellow color and four petals.
In recent years, studies on focused mainly on P. tenuifolia Willd, P. tatarinowii Regel, P. sibirca Linn and P. japonica Houtt, the literature reported that various types of activity constituents were separated from P. tenuifolia Willd, P. tatarinowii Regel, P. sibirca Linn and P. japonica Houtt, they are mainly saponins, ketones and sugar esters, and so on [4–9]. However, so far, the essential oils components in P. furcata Royle have not received much attention. Therefore, in the current work, for the first time, microwave-assisted extraction (MAE) [10–16] followed by HS-SPME [17–30] and GC–MS was developed for the fast determination of volatile chemical compositions in the fresh aerial parts of P. furcata Royle. The experimental parameters were studied by orthogonal array design, and the precision of the method was also investigated.
Experimental
Plant material, SPME fibers and microwave oven
Fresh aerial parts of P. furcata Royle were collected at full flowering in Duyun City in Guizhou (Southern China) in October 2008. They were identified by associate professor Zhiyou Guo (Department of Life Sciences, Qiannan Normal University for Nationalities, city of Duyun in Guizhou, China). After fresh aerial parts of the plants were cut in small pieces, the plants samples of P. furcata Royle were extracted using the MAE–HS-SPME. The fiber coatings 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) were purchased from Supelco (Bellefonte, USA). The microwave oven with a maximum delivered power of 700 W was purchased from Qingdao Haier Microwave Products Co., Ltd. (Qingdao, China).
The procedure of MAE–HS-SPME
The homemade MAE–HS-SPME apparatus is used in this study; 1.50 g of vegetation samples were cut into small pieces and followed was introduced into a 25 mL glass bottle. To absorb enough microwave energy, 1.50 g of water was used to moisten the vegetation samples. Then, the bottle was put into the microwave oven where the vegetation samples were heated at the power of 200, 400 and 700 W for 2–6 min, respectively, and a condenser with a continuous flow of freezing water was used to condense the vapors, so that the water could be used in the extraction process repeatedly. Then, in the process of heating, the volatile compounds were extracted from P. furcata Royle by fiber coating. All of the volatile compounds absorbed on the SPME fiber were desorbed at GC injector (250 °C for 3 min), and then analyzed by GC–MS, respectively.
GC–MS analysis
HP 6890N GC system, coupled with HP 5973 MSD quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) were used to analyze volatile compounds. The extracted compounds were separated on an HP-5MS capillary column (30 m length × 0.25 mm I.D., 0.25-µm film thickness). Splitless injection was employed. The column oven temperature was programmed to rise from an initial temperature of 50 °C (3 min) to 120 °C at 3 °C min−1, then to 210 °C at 4 °C min−1,at which the column was maintained for 10 min. The injection temperature and ion-source temperature were 250 and 230 °C, respectively. Helium (99.999%) was used as the carrier gas, with a flow rate of 1 mL min−1. The ionizing energy was 70 eV. A scan time of 0.5 s and a mass range of m/z 33–450. The percentages of compounds were calculated by the area normalization method without considering response factors. The components of the oil were identified by comparison of their mass spectra with those of the spectrometer database using the NIST147 mass spectral database. The identification was confirmed by comparison of Kovats indices (KI) [19, 20, 22, 31] with those reported in the literature [24, 31–36]. The KI was set-up a standard mixture of C8–C26 compounds under chromatographic conditions, consistent with those of the chromatographic conditions of the samples analyzed.
The precision of MAE–HS-SPME
The precision of the MAE–HS-SPME was studied with six replicate analyses of the essential oils in P. furcata Royle under the optimum conditions. The precision was expressed as the relative standard deviation (RSD%) of the peak areas. The peak areas of the volatile compounds in the vegetation obtained by replicate analyses were used for the calculation of their RSD values.
Results and discussion
Optimization of the MAE–HS-SPME parameters
Optimization of the experimental conditions represents a critical step in the development of an MAE–HS-SPME method because various parameters will potentially affect the extraction process. In fact, the sample size, the microwave power and irradiation time are generally considered to be the most important factors. Optimization of the method can be carried out step-by-step or using an experimental design. Table 1 shows the results of MAE–HS-SPME extractions of P. furcata Royle carried out under different conditions according to the Taguchi experimental design [37]. The selected factors were examined using a three-level orthogonal array design with an L9 (34) matrix (Table 1). The peak area sum of P. furcata Royle obtained under orthogonal conditions are also shown in Table 1, the sum of peak area was 8.10 × 107–18.53 × 107.
The total peak area obtained at different sample size, microwave power and irradiation time are shown in Table 1, and the optimal conditions that the sum of the peak area achieves to the high level is at the microwave power of 400 W and irradiation time of 4 min, sample size of 2.0 g, respectively. Evidently, the best extraction efficiency was achieved at 400 W for 4 min and 2.0 g sample. The results of variance analysis showed that there are three significant factors in Table 2. Table 2 indicates that the three factors all have a significant impact on volatile compounds extracted in P. furcata Royle, and the most important is the microwave power, followed by irradiation time and sample size. Therefore, we should pay special attention to the control of microwave power in the experiment that is why, based on the experimental results above, the optimal MAE–HS-SPME conditions are microwave power of 400 W and irradiation time of 4 min, sample size of 2.0 g.
Precision of MAE–HS-SPME
To obtain the precision of the method, six replicate analyses of the volatile compounds in P. furcata Royle were performed by MAE–HS-SPME at the optimum conditions. The RSD values were calculated by the peak areas obtained by replicate analyses. As shown in Table 3, the calculated RSD values <7% showed that MAE–HS-SPME followed by GC–MS had an accepted precision.
Analysis of the volatile compounds in P. furcata Royle
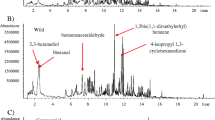
Under the optimal MAE–HS-SPME conditions, the volatile compounds in P. furcata Royle were extracted and concentrated by MAE–HS-SPME, followed by analyzing with GC–MS. The total-ion chromatogram of volatile compounds in P. furcata Royle was shown in Fig. 1. Fifty-two volatile compounds were identified by mass spectra library and KIs, and listed in Table 3. The total peak area of the 52 identified compounds was more than 97% of the total chromatographic area. As shown in Table 3, the 52 compounds were mainly ester and alkane compounds. They were all identified for the first time and mainly included 2-hydroxybenzoic acid methyl ester (21.65%), pentadecane (2.23%), hexadecane (5.27%), 5-propyl-tridecane (4.78%), 2-methyl-hexadecane (1.44%), heptadecane (4.53%), 2,6,10, 14-tetramethyl-pentadecane (7.78%), octadecane (1.20%), 2,6,10,14-tetramethyl-hexadecane (3.18%), diisobutyl phthalate (4.07%), eicosane (1.34%), heneicosane (1.58%), docosane (1.49%), 2,6,10,14-tetramethyl-heptadecane (2.75%) and tetracosane (1.01%)etc. In addition, there were several kinds of low concentration terpenes oxygen derivatives, alcohols and carbonyl compounds in the volatile compounds. When compared with the conventional extraction and concentration methods, most of which involve several tedious and time-consuming sample preparation procedures, MAE–HS-SPME is indeed a simple, time-saving and solvent-free, and so on [20, 21, 38, 39].
Conclusions
In this work, MAE–HS-SPME followed by GC–MS was successfully performed for a fast determination of volatile compounds in P. furcata Royle. Using the proposed method, 52 compounds were identified for the first time. A time effort of only 4 min without solvent was needed in the experiment. The experimental results indicated that MAE–HS-SPME followed by GC–MS was a simple, time-saving, solvent-free and powerful method for the determination of volatile compounds in plant materials. Therefore, it can be concluded that this is a potential analytic tool for the determination of the volatile compounds of the vegetations materials and other materials.
References
Chinese Academy of Sciences (1997) Flora of China Editorial Committee. Science Press, Beijing
Trease GE (1978) Pharmacognosy (Elerenth Edition). Macmillan Publishers Co, New York, p 491
Chen SK (1991) Acta Phytotaxonomica Sin 6:193–229
Jiang Y, Tu PF (2002) Chin Tradit Her Dru 10:874–877
Wang H, Tong YX, Ye WC et al (2003) China J Chin Mat Med 9:828–830
Yukinobu Y, Ko S, Mosao (1994) Chem Pharm Bull 42:2305
Wu F-E, Koike K, Ohmoto T et al (1989) Chem Pharm Bull 37:2445
Selichi S, Jumo S (1981) Chem Pharm Bull 29:2431
Wu JF, Chen SB, Chen SL et al (2007) Acta Pharmaceutica Sin 42:757–761
Bilia AR, Flamini G, Taglioli V, Morelli I, Vincieri FF (2002) Food Chem 76:307–310
Deng CH, Yao N, Wang B, Zhang XM (2006) J Chromatogr A 1103:15–21
Jain MP, Sama JK, Jain SM, Sharma VK, Singh B (2003) Ind J Chem Tech 10:370–374
Silva MGD, Dos Santos RN, Matos FJA, Machado MIL (2003) Flavour Fragr J 18:303–304
Silva MGDV, Matos FJA, Machado MIL, Silva FDO (2004) J Essent Oil Res 16:189–190
Stashenko EE, Jaramillo BE, Martinez JR (2004) J Chromatogr A 1025:93–103
Lucchesi ME, Chemat F, Smadja J (2004) J Chromatogr A 1043:323–327
Sartoratto A, Augusto E (2003) Chromatographia 57:351–356
Deng CH, Song GX, Hu YM et al (2003) Chromatographia 58:289–294
Díaz-Maroto MC, Pérez-Coello MS, Cabezudo MD (2002) Chromatographia 55:723–728
Ye H, Ji J, Deng CH (2006) Chromatographia 63:591–594
Deng CH, Xu XQ, Zhang XM (2006) Anal Chim Acta 556:289–294
Krist Sabine, Unterweger Heidrun, Bandion Franz et al (2004) Eur Food Res Technol 219:470–473
Wu Q, Deng CH, Shen S et al (2004) Chromatographia 59:763–767
Liu L, Song GX, Hu YM (2007) Chromatographia 66:785–790
Ngassoum MB, Jirovetz L, Buchbauer G (2001) Eur Food Res Technol 213:18–21
Porto CarlaDa, Pizzale Lorena, Bravin Michela et al (2003) Flavour Fragr J 18:66–72
Bertoli A, Menichini F, Mazzetti M et al (2003) Flavour Fragr J 18:91–94
Miller ME, Stuart JD (1999) Anal Chem 71:23–27
Dungan RobertS (2005) Anal Lett 38:2393–2405
Lidia S de Oliveira, Frederico de M Rodrigues, Fabio S de Oliveira et al (2008) J Chromatogr B 875:392–398
Chyaui CC, Chen S, Wu M (1992) J Agric Food Chem 40:846–849
Bhuiyan MNI, Chowdhury JU, Begum J (2008) Bangladesh J Pharmacol 3:69–73
Pellati F, Benvenuti S, Yoshizaki F, Bertelli D, Rossi MC (2005) J Chromatogr A 1087:265–273
Onyenekwe PC, Hashimoto S (1999) Eur Food Res Technol 209:407–410
Jaroslava S, Piet AL (1999) J Essent Oil Res 11:441–445
Perazzo FF, Carvalho JCT, Carvalho JE, Rehder VLG (2003) Pharmacol Res 48:497–502
Wei YH, Lai CC, Chang JS (2007) Process Biochem 42:40–45
Deng CH, Mao Y, Yao N, Zhang XM (2006) Anal Chim Acta 575:120–125
Deng CH, Ji J, Li N, Yu YJ, Duan GL, Zhang XM (2006) J Chromatogr A 1117:115–120
Acknowledgments
The authors are very grateful to the Institute of Natural Products, Henan University for the use of the mass spectrometer. This work was supported by the social development projects in Guizhou Province Qiannan Buyi and Miao Autonomous Prefecture of China [No. 2008(1)] and the Fund of Qiannan Normal University for Nationalities (No. 2007Z16, 2008Y15, 2008Y16).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Yang, Z., Mao, H., Long, C. et al. Rapid determination of volatile composition from Polygala furcata Royle by MAE–HS-SPME followed by GC–MS. Eur Food Res Technol 230, 779–784 (2010). https://doi.org/10.1007/s00217-010-1214-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1214-x