Abstract
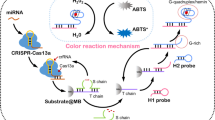
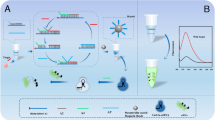
The point-of-care testing (POCT) of miRNA has significant application in medical diagnosis, yet presents challenges due to their characteristics of high homology, low abundance, and short length, which hinders the achievement of quick detection with high specificity and sensitivity. In this study, a lateral flow assay based on the CRISPR/Cas13a system and MnO2 nanozyme was developed for highly sensitive detection of microRNA-21 (miR-21). The CRISPR/Cas13a cleavage system exhibits the ability to recognize the specific oligonucleotide sequence, where two-base mismatches significantly impact the cleavage activity of the Cas13a. Upon binding of the target to crRNA, the cleavage activity of Cas13a is activated, resulting in the unlocking of the sequence and initiating strand displacement, thereby enabling signal amplification to produce a new sequence P1. When applying the reaction solution to the lateral flow test strip, P1 mediates the capture of MnO2 nanosheets (MnO2 NSs) on the T zone, which catalyzes the oxidation of the pre-immobilized colorless substrate 3,3′,5,5′-tetramethylbenzidine (TMB) on the T zone and generates the blue-green product (ox-TMB). The change in gray value is directly proportional to the concentration of miR-21, allowing for qualitative detection through visual inspection and quantitative measurement using ImageJ software. This method achieves the detection of miR-21 within a rapid 10-min timeframe, and the limit of detection (LOD) is 0.33 pM. With the advantages of high specificity, simplicity, and sensitivity, the lateral flow test strip and the design strategy hold great potential for the early diagnosis of related diseases.
Graphical abstract

This work integrates the chromogenic reaction of TMB catalyzed by MnO2 NSs, the high specificity and signal amplification capability of the CRISPR/Cas13a cleavage system, and the simplicity and visual clarity of the lateral flow test strip.






Similar content being viewed by others
References
Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut. 2021;70:784–95. https://doi.org/10.1136/gutjnl-2020-322526.
Jenike AE, Halushka MK. Mir-21: A non-specific biomarker of all maladies. Biomark Res. 2021;9:18. https://doi.org/10.1186/s40364-021-00272-1.
Yin F, Cai R, Gui S, Zhang Y, Wang X, Zhou N. A portable and quantitative detection of microRNA-21 based on cascade enzymatic reactions with dual signal outputs. Talanta. 2021;235: 122802. https://doi.org/10.1016/j.talanta.2021.122802.
Wang Y, Hu Y, Xie R, Zeng Q, Hong Y, Chen X, et al. Ultrasensitive label-free miRNA-21 detection based on MXene-enhanced plasmonic lateral displacement measurement. Nanophotonics-berlin. 2023;12:4055–62. https://doi.org/10.1515/nanoph-2023-0432.
Seo SB, Hwang J-S, Kim E, Kim K, Roh S, Lee G, et al. Isothermal amplification-mediated lateral flow biosensors for in vitro diagnosis of gastric cancer-related microRNAs. Talanta. 2022;246: 123502. https://doi.org/10.1016/j.talanta.2022.123502.
Huang Y, Zhang Y, Hao W, Lu H, Dong H, Zhang X. Sensitive microRNA detection based on bimetallic label photothermal lateral flow locked nucleic acid biosensor with smartphone readout. Sensor Actuat B-chem. 2023;375: 132945. https://doi.org/10.1016/j.snb.2022.132945.
Kor K, Turner AP, Zarei K, Atabati M, Beni V, Mak WC. Structurally responsive oligonucleotide-based single-probe lateral-flow test for detection of miRNA-21 mimics. Anal Bioanal Chem. 2016;408:1475–85. https://doi.org/10.1007/s00216-015-9250-9.
Gao X, Xu H, Baloda M, Gurung AS, Xu L-P, Wang T, et al. Visual detection of microRNA with lateral flow nucleic acid biosensor. Biosens Bioelectron. 2014;54:578–84. https://doi.org/10.1016/j.bios.2013.10.055.
Jin X, Chen L, Zhang Y, Wang X, Zhou N. A lateral flow strip for on-site detection of tobramycin based on dual-functional platinum-decorated gold nanoparticles. Analyst. 2021;146:3608–16. https://doi.org/10.1039/d1an00403d.
Gao X, Xu L-P, Wu T, Wen Y, Ma X, Zhang X. An enzyme-amplified lateral flow strip biosensor for visual detection of microRNA-224. Talanta. 2016;146:648–54. https://doi.org/10.1016/j.talanta.2015.06.060.
Zhang J, Tang L, Yu Q, Qiu W, Li K, Cheng L, et al. Gold-platinum nanoflowers as colored and catalytic labels for ultrasensitive lateral flow microRNA-21 assay. Sensor Actuat B-chem. 2021;344: 130325. https://doi.org/10.1016/j.snb.2021.130325.
Xu C, Zhou Y, Xiao Q, He B, Geng G, Wang Z, et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat Methods. 2021;18:499–506. https://doi.org/10.1038/s41592-021-01124-4.
Zhou H, Bu S, Xu Y, Xue L, Li Z, Hao Z, et al. CRISPR/Cas13a combined with hybridization chain reaction for visual detection of influenza a (H1N1) virus. Anal Bioanal Chem. 2022;414:8437–45. https://doi.org/10.1007/s00216-022-04380-1.
Cui Y, Fan S, Yuan Z, Song M, Hu J, Qian D, et al. Ultrasensitive electrochemical assay for microRNA-21 based on CRISPR/Cas13a-assisted catalytic hairpin assembly. Talanta. 2021;224: 121878. https://doi.org/10.1016/j.talanta.2020.121878.
Liu FX, Cui JQ, Park H, Chan KW, Leung T, Tang BZ, et al. Isothermal background-free nucleic acid quantification by a one-pot Cas13a assay using droplet microfluidics. Anal Chem. 2022;94:5883–92. https://doi.org/10.1021/acs.analchem.2c00067.
Shan Y, Zhou X, Huang R, Xing D. High-fidelity and rapid quantification of miRNA combining crRNA programmability and CRISPR/Cas13a trans-cleavage activity. Anal Chem. 2019;91:5278–85. https://doi.org/10.1021/acs.analchem.9b00073.
Wessels H-H, Méndez-Mancilla A, Guo X, Legut M, Daniloski Z, Sanjana NE. Massively parallel Cas13 screens reveal principles for guide RNA design. Nat Biotechnol. 2020;38:722–7. https://doi.org/10.1038/s41587-020-0456-9.
Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–44. https://doi.org/10.1126/science.aaq0179.
Zhou T, Huang M, Lin J, Huang R, Xing D. High-fidelity CRISPR/Cas13a a trans-cleavage-triggered rolling circle amplified dnazyme for visual profiling of microRNA. Anal Chem. 2021;93:2038–44. https://doi.org/10.1021/acs.analchem.0c03708.
Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–4. https://doi.org/10.1038/nature24049.
Zhou B, Ye Q, Li F, Xiang X, Shang Y, Wang C, et al. CRISPR/Cas12a based fluorescence-enhanced lateral flow biosensor for detection of staphylococcus aureus. Sensor Actuat B-chem. 2022;351: 130906. https://doi.org/10.1016/j.snb.2021.130906.
Arizti-Sanz J, Bradley AD, Zhang YB, Boehm CK, Freije CA, Grunberg ME, et al. Simplified Cas13-based assays for the fast identification of SARS-CoV-2 and its variants. Nat Biomed Eng. 2022;6:932–43. https://doi.org/10.1038/s41551-022-00889-z.
Liu J, Meng L, Fei Z, Dyson PJ, Jing X, Liu X. MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens Bioelectron. 2017;90:69–74. https://doi.org/10.1016/j.bios.2016.11.046.
Hu L, Yuan Y, Zhang L, Zhao J, Majeed S, Xu G. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta. 2013;762:83–6. https://doi.org/10.1016/j.aca.2012.11.056.
Hizir MS, Top M, Balcioglu M, Rana M, Robertson NM, Shen F, et al. Multiplexed activity of perAuxidase: DNA-capped AuNPs act as adjustable peroxidase. Anal Chem. 2015;88:600–5. https://doi.org/10.1021/acs.analchem.5b03926.
Shi W, Wang Q, Long Y, Cheng Z, Chen S, Zheng H, et al. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun. 2011;47:6695. https://doi.org/10.1039/c1cc11943e.
Liu B, Liu J. Surface modification of nanozymes Nano Res. 2017;10:1125–48. https://doi.org/10.1007/s12274-017-1426-5.
Jiao L, Yan H, Wu Y, Gu W, Zhu C, Du D, et al. When nanozymes meet single-atom catalysis. Angew Chem Int Edit. 2019;59:2565–76. https://doi.org/10.1002/anie.201905645.
Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008;80:2250–4. https://doi.org/10.1021/ac702203f.
Jiang T, Song Y, Wei T, Li H, Du D, Zhu M-J, et al. Sensitive detection of Escherichia coli O157:H7 using Pt–Au bimetal nanoparticles with peroxidase-like amplification. Biosens Bioelectron. 2016;77:687–94. https://doi.org/10.1016/j.bios.2015.10.017.
Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22:2206–10. https://doi.org/10.1002/adma.200903783.
Yan X, Song Y, Wu X, Zhu C, Su X, Du D, et al. Oxidase-mimicking activity of ultrathin MnO2 nanosheets in colorimetric assay of acetylcholinesterase activity. Nanoscale. 2017;9:2317–23. https://doi.org/10.1039/c6nr08473g.
Liu Z, Xu K, Sun H, Yin S. One-step synthesis of single-layer MnO2 nanosheets with multi-role sodium dodecyl sulfate for high-performance pseudocapacitors. Small. 2015;11:2182–91. https://doi.org/10.1002/smll.201402222.
Cai X, Liang M, Ma F, Zhang Z, Tang X, Jiang J, et al. Nanozyme-strip based on MnO2 nanosheets as a catalytic label for multi-scale detection of aflatoxin B1 with an ultrabroad working range. Food Chem. 2022;377: 131965. https://doi.org/10.1016/j.foodchem.2021.131965.
Sheng E, Lu Y, Tan Y, Xiao Y, Li Z, Dai Z. Oxidase-mimicking activity of ultrathin MnO2 nanosheets in a colorimetric assay of chlorothalonil in food samples. Food Chem. 2020;331: 127090. https://doi.org/10.1016/j.foodchem.2020.127090.
Shi M, Wang S, Zheng S, Hou P, Dong L, He M, et al. Activatable MRI-monitoring gene delivery for the theranostic of renal carcinoma. Colloid Surface B. 2020;185: 110625. https://doi.org/10.1016/j.colsurfb.2019.110625.
Yao M, Lv X, Deng Y, Rasheed M. Specific and simultaneous detection of microRNA 21 and let-7a by rolling circle amplification combined with lateral flow strip. Anal Chim Acta. 2019;1055:115–25. https://doi.org/10.1016/j.aca.2018.12.040.
Cimmino W, Migliorelli D, Singh S, Miglione A, Generelli S, Cinti S. Design of a printed electrochemical strip towards miRNA-21 detection in urine samples: optimization of the experimental procedures for real sample application. Anal Bioanal Chem. 2023;415:4511–20. https://doi.org/10.1007/s00216-023-04659-x.
Deng K, Zhang Y, Tong X. Sensitive electrochemical detection of microRNA-21 based on propylamine-functionalized mesoporous silica with glucometer readout. Anal Bioanal Chem. 2018;410:1863–71. https://doi.org/10.1007/s00216-018-0859-3.
Zheng W, Yao L, Teng J, Yan C, Qin P, Liu G, et al. Lateral flow test for visual detection of multiple microRNAs. Sensor Actuat B-chem. 2018;264:320–6. https://doi.org/10.1016/j.snb.2018.02.159.
Wei H, Peng Y, Bai Z, Rong Z, Wang S. Duplex-specific nuclease signal amplification-based fluorescent lateral flow assay for the point-of-care detection of microRNAs. Analyst. 2021;146:558–64. https://doi.org/10.1039/d0an01673j.
Funding
This work was financially supported by the National Natural Science Foundation of China (42177212, 62301234, 32371430).
Author information
Authors and Affiliations
Contributions
Mingyuan Wang: ideas, investigation, data curation, writing — original draft. Shixin Cai: investigation, data curation. Yunqing Wu: formal analysis, validation. Qi Li: supervision. Xiaoli Wang: management, funding acquisition. Yuting Zhang: supervision, funding acquisition. Nandi Zhou: writing — review and editing, supervision, funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The experiments were approved by the Ethics Committee of the Wuxi No.2 People’s Hospital in Jiangsu Province, China.
Source of biological material
All of the clinical serum samples were collected from the Wuxi No.2 People’s Hospital in Jiangsu Province, China.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Cai, S., Wu, Y. et al. A lateral flow assay for miRNA-21 based on CRISPR/Cas13a and MnO2 nanosheets-mediated recognition and signal amplification. Anal Bioanal Chem (2024). https://doi.org/10.1007/s00216-024-05290-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-024-05290-0