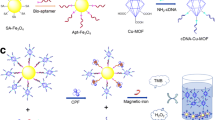
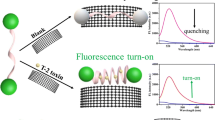
Abstract
Acetamiprid (ACE) is a highly effective broad-spectrum insecticide, and its widespread use is potentially harmful to human health and environmental safety. In this study, magnetic Fe3O4/carbon (Fe3O4/C), a derivative of metal–organic framework MIL-101 (Fe), was synthesized by a two-step calcination method. And a fluorescent sensing strategy was developed for the efficient and sensitive detection of ACE using Fe3O4/C and multiple complementary single-stranded DNA (ssDNA). By using aptamer with multiple complementary ssDNA, the immunity of interference of the aptasensor was improved, and the aptasensor showed high selectivity and sensitivity. When ACE was present, the aptamer (Apt) combined with ACE. The complementary strand of Apt (Cs1) combined with two short complementary strands of Cs1, fluorophore 6-carboxyfluorescein-labeled complementary strand (Cs2-FAM) and the other strand Cs3. The three strands formed a double-stranded structure, and fluorescence would not be quenched by Fe3O4/C. In the absence of ACE, Cs2-FAM would be in a single-chain state and would be adsorbed by Fe3O4/C, and the fluorescence of FAM would be quenched by Fe3O4/C via photoelectron transfer. This aptasensor sensitively detected ACE over a linear concentration range of 10–1000 nM with a limit of detection of 3.41 nM. The recoveries of ACE spiked in cabbage and celery samples ranged from 89.49% to 110.76% with high accuracy.
Graphical abstract







Similar content being viewed by others
References
Phogat A, Singh J, Kumar V, Malik V. Toxicity of the acetamiprid insecticide for mammals: a review. Environ Chem Lett. 2022;20:1453–78.
Thompson DA, Lehmler HJ, Kolpin DW, Hladik ML, Vargo JD, Schilling KE, et al. A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environ Sci Process Impacts. 2020;22(6):1315–46.
Tudi M, Ruan HD, Wang L, Lyu J, Sadler R, Connell D, et al. Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health. 2021;18(3):1112.
Tartaglia A, Locatelli M, Samanidou V. Trends in the analysis of biopharmaceuticals by HPLC. Curr Anal Chem. 2020;16(1):52–8.
Lawal A, Wong RCS, Tan GH, Abdulra’uf LB, Alsharif AMA. Recent modifications and validation of QuEChERS-dSPE coupled to LC–MS and GC–MS instruments for determination of pesticide/agrochemical residues in fruits and vegetables. J Chromatogr Sci. 2018;56(7):656–69.
Elmastas A, Umaz A, Pirinc V, Aydin F. Quantitative determination and removal of pesticide residues in fresh vegetables and fruit products by LC–MS/MS and GC–MS/MS. Environ Monit Assess. 2023;195(2):277.
Stachniuk A, Szmagara A, Czeczko R, Fornal E. LC-MS/MS determination of pesticide residues in fruits and vegetables. J Environ Sci Health B. 2017;52(7):446–57.
Walorczyk S, Gnusowski B. Fast and sensitive determination of pesticide residues in vegetables using low-pressure gas chromatography with a triple quadrupole mass spectrometer. J Chromatogr A. 2006;1128(1–2):236–43.
Pundir CS, Malik A, Preety. Bio-sensing of organophosphorus pesticides: a review. Biosens Bioelectron. 2019;140:111348.
Wang K, Wang M, Ma T, Li W, Zhang H. Review on the selection of aptamers and application in paper-based sensors. Biosensors. 2022;13(1):39.
Xie M, Zhao F, Zhang Y, Xiong Y, Han S. Recent advances in aptamer-based optical and electrochemical biosensors for detection of pesticides and veterinary drugs. Food Control. 2022;131:108399.
Saberi Z, Rezaei B, Ensafi AA. Fluorometric label-free aptasensor for detection of the pesticide acetamiprid by using cationic carbon dots prepared with cetrimonium bromide. Microchim Acta. 2019;186:1–7.
Liang C, Wang Y, Zhang T, Nie H, Han Y, Bai J. Aptamer-functionalised metal-organic frameworks as an ‘on–off–on’ fluorescent sensor for bisphenol S detection. Talanta. 2023;253:123942.
Wu L, Wang Y, Xu X, Liu Y, Zhang M, Zhang J, et al. Aptamer-based detection of circulating targets for precision medicine. Chem Rev. 2021;121(19):12035–105.
Deore PS, Manderville RA. Ratiometric fluorescent sensing of the parallel G-quadruplex produced by PS2.M: implications for K+ detection. Analyst. 2020;145:1288–93.
Chen J, Liu J, Wang J, Zhang Y, Wang X, Zhou N. Fluorescent biosensor based on FRET and catalytic hairpin assembly for sensitive detection of polysialic acid by using a new screened DNA aptamer. Talanta. 2022;242:123282.
Geng W, Feng Y, Chen Y, Zhang X, Zhang H, Yang F, et al. Interactions of amino group functionalized tetraphenylvinyl and DNA: a label-free “on-off-on” fluorescent aptamer sensor toward ampicillin. Biosensors. 2023;13(5):504.
Podder A, Lee HJ, Kim BH. Fluorescent nucleic acid systems for biosensors. Bull Chem Soc Jpn. 2021;94(3):1010–35.
Liu M, Khan A, Wang Z, Liu Y, Yang G, Deng Y, et al. Aptasensors for pesticide detection. Biosens Bioelectron. 2019;130:174–84.
Wu L, Wang Z, Zhao S, Meng X, Song X, Feng J, et al. A metal–organic framework/DNA hybrid system as a novel fluorescent biosensor for mercury (II) ion detection. Chem Eur J. 2016;22(2):477–80.
Liu X, Zhao Y, Li F. Nucleic acid-functionalized metal-organic framework for ultrasensitive immobilization-free photoelectrochemical biosensing. Biosens Bioelectron. 2021;173:112832.
Cheng W, Tang X, Zhang Y, Wu D, Yang W. Applications of metal-organic framework (MOF)-based sensors for food safety: enhancing mechanisms and recent advances. Trends Food Sci Technol. 2021;112:268–82.
Qi H, Wang Z, Li H, Li F. Directionally in situ self-assembled iridium(III)-polyimine complex-encapsulated metal–organic framework two-dimensional nanosheet electrode to boost electrochemiluminescence sensing. Anal Chem. 2023;95:12024–31.
Li H, Su C, Liu N, Lu Q, Zhang N, Sun C, et al. Zeolitic imidazolate framework/aptamer-based fluorescence assay for the facile and high-sensitivity detection of acetamiprid. Anal Chim Acta. 2023;1276:341641.
Tavassoli M, Khezerlou A, Khalilzadeh B, Ehsani A, Kazemian H. Aptamer-modified metal organic frameworks for measurement of food contaminants: a review. Microchim Acta. 2023;190(9):371.
Zhang Z, Lou Y, Guo C, Jia Q, Song Y, Tian J, et al. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food Sci Technol. 2021;118:569–88.
Mishra G, Sharma V, Mishra R. Electrochemical aptasensors for food and environmental safeguarding: a review. Biosensors. 2018;8(2):28.
Marimuthu M, Arumugam SS, Sabarinathan D, Li H, Chen Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci Technol. 2021;116:1002–28.
Zhang S, Rong F, Guo C, Duan F, He L, Zhang Z, et al. Metal–organic frameworks (MOFs) based electrochemical biosensors for early cancer diagnosis in vitro. Coord Chem Rev. 2021;439:213948.
Li W, Wu X, Li S, Tang W, Chen Y. Magnetic porous Fe3O4/carbon octahedra derived from iron-based metal-organic framework as heterogeneous Fenton-like catalyst. Appl Surf Sci. 2018;436:252–62.
Sun Y, Zhang Y, Wang Z. A “turn-on” FRET aptasensor based on the metal-organic framework-derived porous carbon and silver nanoclusters for zearalenone determination. Sens Actuators B Chem. 2021;347:130661.
Xu Y, Zhang W, Shi J, Li Z, Huang X, Zou X, et al. Impedimetric aptasensor based on highly porous gold for sensitive detection of acetamiprid in fruits and vegetables. Food Chem. 2020;322:126762.
Shen Z, Xu D, Wang G, Geng L, Xu R, Wang G, et al. Novel colorimetric aptasensor based on MOF-derived materials and its applications for organophosphorus pesticides determination. J Hazard Mater. 2022;440:129707.
Su Z, Ye F, He K, Yang T, Li W, Ren J. Determination of acetamiprid by fluorescence monitoring of a glycine-l-histidine copper-organic framework aptasensor. Anal Lett. 2022;55(4):529–38.
He K, Dong S, Yang J, Shi Q, Guan L, Sun L, et al. Efficient and switchable aptamer “fluorescence off/on” method based on UiO-66@ Cu for ultrasensitive detection of acetamiprid. J Environ Chem Eng. 2022;10(4):108178.
Bahreyni A, Robati RY, Ramezani M, Abnous K, Taghdisi SM. Fluorometric aptasensing of the neonicotinoid insecticide acetamiprid by using multiple complementary strands and gold nanoparticles. Microchim Acta. 2018;185:1–7.
Xu C, Lin M, Song C, Chen D, Bian C. A gold nanoparticle-based visual aptasensor for rapid detection of acetamiprid residues in agricultural products using a smartphone. RSC Adv. 2022;12(9):5540–5.
Wei W, Huang Q. Preparation of cellophane-based substrate and its SERS performance on the detection of CV and acetamiprid. Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;193:8–13.
Funding
This work was supported by the National Natural Science Foundation of China (No.32372438, 31772068), the Central Finance Guidance Local Science and Technology Development Fund Project (YDZX2022163), the Natural Science Foundation of Shandong Province (ZR2023MC088), the Shandong Province Major Applied Technology Innovation Project (SD2019NJ007), the Technological Innovation Guidance Project of the Department of Science &Technology of Gansu Province (22CX8NA023), and the Weifang Science and Technology Development Project (2021ZJ1103).
Author information
Authors and Affiliations
Contributions
B.L.: conceptualization, methodology, formal analysis, and writing, original draft. H.W.: investigation, formal analysis, and validation. M.L.: writing, review and editing, and investigation. L.G.: investigation, formal analysis, and validation. S.D.: investigation and resources. S.Z.: investigation and resources. J.L.: investigation and resources. J.S.: investigation and resources. W.Z.: writing, review and editing. Y.G.: writing, review and editing, supervision, project administration, and funding acquisition. X.S.: writing, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, B., Wang, H., Liu, M. et al. Fluorescent aptasensor mediated with multiple ssDNA for sensitive detection of acetamiprid in vegetables based on magnetic Fe3O4/C-assisted separation. Anal Bioanal Chem 416, 1105–1115 (2024). https://doi.org/10.1007/s00216-023-05104-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05104-9