Abstract
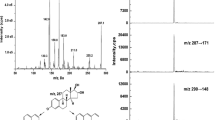
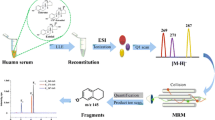
We developed and evaluated two-level, namely 2017011 and 2017012, serum-based reference materials (RMs) for 17 beta-estradiol (17 β-E2) by the reference method of isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) from the remaining serum samples after routine clinical tests, to help improve clinical routine testing and provide the traceability of results. This paper describes the development process of these RMs. The National Metrology Institute of Japan (NMIJ) certified reference material (CRM) 6004-a was used as the primary RM for the measurement of 17 β-E2. These serum-based RMs showed satisfactory homogeneity and stability. They also assessed the commutability between the reference method and the three routine clinical immunoassay systems. Besides, a collaborative study was carried out in five reference laboratories, all of which had been accredited by the China National Accreditation Service for Conformity Assessment (CNAS) in accordance with ISO/WD 15725-1. Statistical analysis of raw results and uncertainty assessment obtained certified values: 2017011 was 445.2 ± 39.0 pmol/L, and 2017012 was 761.9 ± 35.5 pmol/L.
Graphical abstract



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author, QXZ,upon reasonable request.
Change history
29 July 2023
Springer Nature’s version of this paper was updated. The e-mail addresses of the two corresponding authors were swapped.
Abbreviations
- 17 β-E2:
-
17 Beta-estradiol
- RM:
-
Reference material
- CRM:
-
Certified reference material
- ID-LC-MS/MS:
-
Isotope dilution liquid chromatography tandem mass spectrometry
- JCTLM:
-
Joint Committee on Traceability in Laboratory Medicine
References
Liang J, Dong H, Xu F, Li B, Li H, Chen L, et al. Isolation of a monoclonal antibody and its derived immunosensor for rapid and sensitive detection of 17β-Estradiol. Front Bioeng Biotechnol. 2022;10: 818983.
Rosenfeld CS, Wagner JS, Roberts RM, Lubahn DB. Intraovarian actions of oestrogen. Reproduction (Cambridge, England). 2001;122(2):215–26.
Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril. 2012;98(6):1359–62.
Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–18.
Orzołek I, Sobieraj J, Domagała-Kulawik J. Estrogens cancer and immunity. Cancers. 2022;14(9):2265.
Saumande J. Radioimmunoassay of estradiol-17 beta in unextracted ewe plasma. Steroids. 1981;38(4):425–37.
Chiu ML, Tseng TT, Monbouquette HG. A convenient homogeneous enzyme immunoassay for estradiol detection. Biotechnol Appl Biochem. 2011;58(1):75–82.
Ojeda I, López-Montero J, Moreno-Guzmán M, Janegitz BC, González-Cortés A, Yáñez-Sedeño P, et al. Electrochemical immunosensor for rapid and sensitive determination of estradiol. Anal Chim Acta. 2012;743:117–24.
Silva CP, Lima DL, Schneider RJ, Otero M, Esteves VI. Development of ELISA methodologies for the direct determination of 17β-estradiol and 17α-ethinylestradiol in complex aqueous matrices. J Environ Manag. 2013;124:121–7.
Asadi Atoi P, Talebpour Z, Fotouhi L. Introduction of electropolymerization of pyrrole as a coating method for stir bar sorptive extraction of estradiol followed by gas chromatography. J Chromatogr A. 2019;1604: 460478.
Leivo J, Kivimäki L, Juntunen E, Pettersson K, Lamminmäki U. Development of anti-immunocomplex specific antibodies and non-competitive time-resolved fluorescence immunoassay for the detection of estradiol. Anal Bioanal Chem. 2019;411(22):5633–9.
Huang X, Zhang Q, Zheng S, Wang J, Han L, Lin H, et al. Measurement of human serum unconjugated estriol without derivatization using liquid chromatography-tandem mass spectrometry candidate reference method and compared with two immunoassays. Anal Bioanal Chem. 2018;410(24):6257–67.
Zhang Q, Bai K, Wu M, Lin H, Yan J, Zhan M, et al. Development of human serum matrix-based unconjugated estriol-certified reference material by recommended ID-LC-MS/MS reference method. Anal Bioanal Chem. 2022;414(7):2523–31.
Clinical and Laboratory Standards Institute (CLSI). Evaluation of matrix efects; Approved Guideline. 2nd ed. Wayne: CLSI; CLSI document EP14-A2, 2008.
Keski-Rahkonen P, Desai R, Jimenez M, Harwood DT, Handelsman DJ. Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization reagent. Anal Chem. 2015;87(14):7180–6.
Yuan TF, Le J, Cui Y, Peng R, Wang ST, Li Y. An LC-MS/MS analysis for seven sex hormones in serum. J Pharm Biomed Anal. 2019;162:34–40.
Li XS, Li S, Kellermann G. Simultaneous determination of three estrogens in human saliva without derivatization or liquid-liquid extraction for routine testing via miniaturized solid phase extraction with LC-MS/MS detection. Talanta. 2018;178:464–72.
Denver N, Khan S, Homer NZM, MacLean MR, Andrew R. Current strategies for quantification of estrogens in clinical research. J Steroid Biochem Mol Biol. 2019;192: 105373.
Zhang Q, Cai Z, Liu Y, Lin H, Wang Q, Yan J, et al. Comparison of bracketing calibration and classical calibration curve quantification methods in establishing a candidate reference measurement procedure for human serum 17β-estradiol by isotope dilution liquid chromatography tandem mass spectrometry. Microchem J. 2020;152:104270.
International Organization for Standardization (ISO) 15194. In vitro diagnostic medical devices – measurement of quantities in samples of biological origin – requirements for certified reference materials and the content of supporting documentation. Geneva, Switzerland: ISO, 2009.
Technical Committee ISO/TC 334 Reference materials, 2017. ISO Guide 35:2017 Reference Materials — Guidance for Characterization and Assessment of Homogeneity and Stability, 2022. http://insider-h2020.eu/tag/reference-materials/.
Acknowledgements
We greatly appreciate the following reference laboratories which participated in the collaborative study of value assignment of the RM: Maccura Biotechnology (ISO/IEC 17025 accreditation CNAS: L6172); Shanghai Center for Clinical Laboratory (ISO/IEC 17025 accreditation CNAS: L6730); Autobio Diagnostics Co. Ltd. Zhengzhou Eco&Tech Zone (ISO/IEC 17025 accreditation CNAS: L13885); and Shanghai Kehua Bio-engineering Co., Ltd. (ISO/IEC 17025 accreditation CNAS: L13117).
Funding
This research was supported by the National Key Research and Development Program of China (2022YFF0710300, 2022YFF0710302), the "Young Talents Program" of Guangdong Academy of Traditional Chinese Medicine (SZ2022QN09), the State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ30, SZ2021ZZ3003), the Natural Science Foundation of Guangdong Province (2021A1515220099), and the Guangzhou Science and Technology Plan Projects (202201011506).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The research involved plasma samples taken from pregnant women, and this study was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. The number of this study was ZE2019-149-01.
Informed consent
The ethics committee approved the exemption of informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, H., Liu, D., Deng, L. et al. Development of matrix-based reference materials for 17 beta-estradiol by the recommended reference method of ID-LC-MS/MS. Anal Bioanal Chem 415, 5637–5644 (2023). https://doi.org/10.1007/s00216-023-04832-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04832-2