Abstract
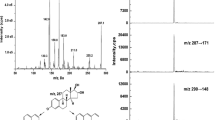
To solve long-term lack of traceability of commercial calibrator kits and standardize clinical routine assays, we developed a human serum matrix-based unconjugated estriol (uE3) reference material (RM) with five concentration gradients. The RMs of uE3 were certified by the National Institute of Metrology (NIM) with the codes of GBW (E) 091048, GBW (E) 091049, GBW (E) 091050, GBW (E) 091051, and GBW (E) 091052. The RMs were determined by isotope dilution liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) reference method which was developed in our group and recommended by the Joint Committee on Traceability on Laboratory Medicine (JCTLM). GBW09224 is intended for use as a primary reference material to enable the SI-traceable measurement of uE3. This study describes the development process of these certified RMs. The candidate material was prepared by collecting from the remaining serum samples after routine clinical testing. Satisfactory homogeneity and stability were shown in these RMs. They are also commutable between the reference method and the three routine clinical immunoassay systems. To improve the accuracy of value assignment, a collaborative study in nine reference laboratories was conducted which was performed according to ISO/WD 15725-1 and all of the reference laboratories have been confirmed by China National Accreditation Service for Conformity Assessment (CNAS). The raw results were statistically analyzed and processed, coupled with uncertainty evaluation, to obtain the certified value: GBW (E) 091048 is 22.1 ± 1.3 nmol/L, GBW (E) 091049 is 33.6 ± 1.6 nmol/L, GBW (E) 091050 is 10.4 ± 0.8 nmol/L, GBW (E) 091051 is 15.5 ± 1.0 nmol/L, GBW (E) 091052 is 47.0 ± 2.0 nmol/L.
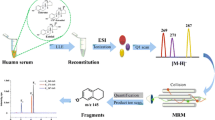
Graphical abstract
The preparation process of human serum matrix-based reference material and the lack of these type of secondary (commutable) reference material of unconjugated estriol lead to the interruption of its traceability chain, which is a problem to be solved in its standardization as mentioned in the metrological traceability in ISO 17511, 2020.



Similar content being viewed by others
Abbreviations
- RM:
-
Reference material
- CRM:
-
Certified reference material
- GUM:
-
Guide to the Expression of Uncertainty in Measurement
- JCTLM:
-
Joint Committee on Traceability on Laboratory Medicine
- IFCC:
-
International Federation of Clinical Chemistry and Laboratory Medicine
References
Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16(5):608–48.
Nekrasova I, Shirshev S. Estriol in regulation of cell-mediated immune reactions in multiple sclerosis. J Neuroimmunol. 2020;349:577421.
Touchstone JC, Greene JW Jr, McElroy RC, Murawec T. Blood estriol conjugation during human pregnancy. Biochemisty. 1963;2(4):653–7.
Bestwick JP, Huttly WJ, Wald NJ. Unconjugated estriol values between 14 and 22 weeks of gestation in relation to prenatal screening for Down’s syndrome. Prenat Diagn. 2012;32(7):299–43.
La Marca-Ghaemmaghami P, Zimmermann R, Haller M, Ehlert U. Cortisol and estriol responses to awakening in the first pregnancy trimester: associations with maternal stress and resilience factors. Psychoneuroendocrinology. 2021;125:105120.
Kumar RS, Goyal N. Estrogens as regulator of hematopoietic stem cell, immune cells and bone biology. Life Sci. 2021;269:119091.
Bornhorst JA, Ramos PA, Sutterer ER, Herrli NM, Figdore DJ, Flieth TL, Ness KM, Fatica EM, Algeciras-Schimnich A. Evaluation of sporadic bovine alkaline phosphatase interference in the Beckman Access unconjugated estriol (uE3) assay affecting maternal serum screening results. Clin Biochem. 2021;87:93–9.
Huang X, Zhang Q, Zheng S, et al. Measurement of human serum unconjugated estriol without derivatization using liquid chromatography-tandem mass spectrometry candidate reference method and compared with two immunoassays. Anal Bioanal Chem. 2018;410(24):6257–67.
Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25:105–20.
Weber TH, Kapyaho KI, Tanner P. Endogenous interference in immunoassays in clinical chemistry. A review. Scand J Clin Lab Invest. 1990;50:77–82.
Sturgeon CM, Viljoen A. Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem. 2011;48(5):418–32.
Huang X, Spink DC, Schneider E, et al. Measurement of unconjugated estriol in serum by liquid chromatography-tandem mass spectrometry and assessment of the accuracy of chemiluminescent immunoassays. Clin Chem. 2014;60(1):260–8.
Bi J, Wu L, Yang B, Yang Y, Wang J. Development of hemoglobin A1c certified reference material by liquid chromatography isotope dilution mass spectrometry. Anal Bioanal Chem. 2012;403(2):549–54.
Liu Y, Song D, Xu B, Li H, Dai X, Chen B. Development of a matrix-based candidate reference material of total homocysteine in human serum. Anal Bioanal Chem. 2017;409(13):3329–35.
Wu L, Takatsu A, Park SR, et al. Development and co-validation of porcine insulin certified reference material by high-performance liquid chromatography-isotope dilution mass spectrometry. Anal Bioanal Chem. 2015;407(11):3125–35.
International Organization for Standardization (ISO). In vitro diagnostic medical devices—measurement of quantities in samples of biological origin—Requirements for certified reference materials and the content of supporting documentation. Genova: ISO; ISO-15194; 2009.
International Organization for Standardization (ISO). Reference materials: guidance for characterization and assessment of homogeneity and stability. Genova: ISO;ISO-Guide 35; 2017.
Clinical and Laboratory Standards Institute (CLSI). Evaluation of matrix effects; Approved Guideline. 2nd ed. Wayne: CLSI; CLSI document EP14-A2; 2008.
International Organization for Standardization (ISO). Accuracy (trueness and precision) of measurement methods and results—Part 1: introduction and basic principles. Genova: ISO; ISO/WD 15725-1; 2011.
Ellison SLR, Williams A. Guide for “quantifying uncertainty in analytical measurement, 3rd Edition”. A Focus for Analytical Chemistry in Europe. EURACHEM, 2012.
Acknowledgements
We greatly appreciate the following reference laboratories which participated in the collaborative study of value assignment of the RM: Beijing Institute of Medical Device Testing Opto-Mechatronics Industrial (ISO/IEC 17025 accreditation CNAS: L6477); Beijing Aerospace General Hospital (ISO/IEC 17025 accreditation CNAS:L5536); Beijing Strong Biotechnologies, Inc. (ISO/IEC 17025 accreditation CNAS:L11846); Shanghai Center For Clinical Laboratory (ISO/IEC 17025 accreditation CNAS: L6730); Center of Laboratory Medicine Affiliated Hospital of Nantong University (ISO/IEC17025 accreditation CNAS: L6260); Maccura Biotechnology (ISO/IEC 17025 accreditation CNAS: L6172); MedicalSystem Biotechnology Co., Ltd.; Reference Laboratory (ISO/IEC 17025 accreditation CNAS: L8377); Autobio Diagnostics Co. Ltd Zhengzhou Eco&Tech Zone (ISO/IEC 17025 accreditation CNAS:L13885).
Funding
This research was supported by The National Key Research and Development Program of China (2021YFC2009300, 2021YFC2009305), the National Natural Science Foundation of China (81572088), the Natural Science Foundation of Guangdong Province (2020A1515010667), the Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (YN2019QL01 and YN2019 MJ04), and the Administration of Traditional Chinese Medicine Bureau of Guangdong Province (20211150).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The research involved plasma samples taken from pregnant women, and this study is approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. The number of this review is ZE2019-149-01.
Informed consent
The ethics committee approved the exemption of informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 93 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Bai, K., Wu, M. et al. Development of human serum matrix-based unconjugated estriol-certified reference material by recommended ID-LC-MS/MS reference method. Anal Bioanal Chem 414, 2523–2531 (2022). https://doi.org/10.1007/s00216-022-03896-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-03896-w