Abstract
A candidate reference measurement procedure (RMP) for measurement of unconjugated estriol in human serum has been developed and validated. The proposed method is highly reliable and uses isotope dilution coupled with liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) and requires no derivatization. An appropriate amount of serum was accurately weighed and spiked with an isotopically labeled internal standard. Unconjugated estriol and its internal standard were extracted from serum matrix using liquid-liquid extraction prior to reversed-phase LC-MS/MS. Calibrator bracketing was used to give higher specificity and accuracy for assigning serum level. The accuracy of the candidate RMP was validated by split-sample comparison to established RMPs. The lowest limit of detection (LLoD) and lowest limit of quantification (LLoQ) for developed RMP was estimated to be 0.14 nmol/L and 0.35 nmol/L, respectively. Both intra- and inter-assay imprecisions were ≤2.19% at 1.39, 17.34 and 69.35 nmol/L, respectively. Recoveries were 98.54% to 100.34% and linear response ranged from 0.35 to 173.38 nmol/L. No interference was observed. Biases were 5.6% and 2.8% against the targets of RELA2015A (3.87 nmol/L) and RELA2015B (40.62 nmol/L), respectively. Moreover, the candidate RMP was successfully applied to measure level of unconjugated estriol in serum samples of pregnant women (n = 3) and compared with two immunoassays in clinical laboratory. Our developed method is simple, accurate, and can be used as a candidate RMP to determine total unconjugated estriol level in human serum. Further improvement of certain immunoassays in accuracy and precision is needed.

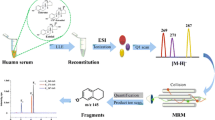
Selected ion chromatograms by LC-MS/MS using a C18 column for uE3 from a serum sample





Similar content being viewed by others
Abbreviations
- uE3 :
-
unconjugated estriol
- LC-MS/MS:
-
liquid chromatography/tandem mass spectrometry
- MRM:
-
multiple reaction monitoring
- RMP:
-
reference measurement procedure
- JCTLM:
-
Joint Committee for Traceability in Laboratory Medicine
- PT:
-
proficiency testing
- RELA:
-
Reference Laboratories in Laboratory Medicine
- IFCC:
-
International Federation of Clinical Chemistry and Laboratory Medicine
References
Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16(5):608–48.
Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and auto-immunity. Clin Exp Rheumatol. 1995;13(2):217–26.
Fuhrman BJ, Schairer C, Gail MH, Ziegler RG. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer I. 2012;104(4):326–39.
Fishman J, Brown JB, Hellman L, Zumoff B, Gallagher TF. Estrogen metabolism in normal and pregnant women. J Biol Chem. 1962;237(237):1489–94.
Touchstone JC, Greene JW Jr, McElroy RC, Murawec T. Blood estriol conjugation during human pregnancy. Biochemisty. 1963;2(4):653–7.
Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Canick JA, Haddow JE. Maternal serum unconjugated estriol as an antenatal screening test for Down syndrome. Br J Obstet Gynaecol. 1988;95(4):334–41.
Haddow JE, Palomaki GE, Knight GJ. Prenatal screening for Down syndrome with use of maternal serum markers. N Engl J Med. 1992;327(9):588–93.
Kellner LH, Weiss RR, Weiner Z, Schulman H. The advantages of using triple-marker screening for chromosomal abnormalities. Am J Obstet Gynecol. 1995;172(3):831–6.
Bestwick JP, Huttly WJ, Wald NJ. Unconjugated estriol values between 14 and 22 weeks of gestation in relation to prenatal screening for Down’s syndrome. Prenat Diagn. 2012;32(7):299–43.
Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010;19(1):292–300.
Huang X, Spink DC, Schneider E, Ling H, Rai AJ, Rosano TG, et al. Measurement of unconjugated estriol in serum by liquid chromatography-tandem mass spectrometry and assessment of the accuracy of chemiluminescent immunoassays. Clin Chem. 2014;60(1):262–8.
Benn PA, Makowski GS, Egan JF, Wright D. Reproducibility of risk figures in 2nd-trimester maternal serum screening for Down’s syndrome: comparison of two laboratories. Clin Chem. 2006;52(11):2087–94.
Reynolds T, John R. Comparison of assay kits for unconjugated estriol shows that expressing results as multiples of the median causes unacceptable variation in calculated risk factors for syndrome. Clin Chem. 1992;38(9):1888–93.
Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16(9):1713–9.
Weissbach A, Freymann E, Hubl W, Seefried W, Neef B, Thiele HJ. Enzyme immunoassay of unconjugated estriol in serum and saliva during pregnancy. Exp Clin Endocrinol. 1985;86(5):178–84.
Ghosh M, Dhar TK, Ali E, Bachhawat BK. Homogeneous enzyme immunoassay of estriol in pictogram amounts. Clin Chim Acta. 1983;128(2):223–31.
College of Pathologists (CAP) Ligand (Special) Y-B 2016 Participant Summary.
Siekmann L. Determination of steroid hormones by the use of isotope dilution-mass spec-trometry: a definitive method in clinical chemistry. J Steroid Biochem. 1979;11(1):117–23.
Gust M, Vulliet E, Giroud B, Garnier F, Couturier S, Garric J, et al. Development, validation, and comparison of LC-MS/MS and RIA methods for quantification of vertebrates-like sex steroids in prosobranch mollusks. J Chromatogr B. 2010;878(19):1487–92.
Remane D, Wetzel D, Peters FT. Development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) procedure for screening of urine specimens for 100 analytes relevant in drug-facilitated crime (DFC). Anal Bioanal Chem. 2014;406(18):4411–24.
Zhang Q, Han L, Wang J, Lin H, Ke P, Zhuang J, et al. Simultaneous quantitation of endogenous estrone, 17beta-estradiol, and estriol in human serum by isotope-dilution liquid chromatography-tandem mass spectrometry for clinical laboratory applications. Anal Bioanal Chem. 2017;409(10):2627–38.
Xu X, Roman JM, Issaq HJ, Keefer LK, Ziegier RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(20):7813–21.
Nelson RE, Grebe SK, O'Kane DJ, Singh RJ. Liquid chromatography tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–84.
Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am.J Clin Pathol. 2008;129(4):530–9.
Tai SSC, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of estradiol-17β in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77(19):6359–63.
Fiers T, Casetta B, Bernaert B, Vandersypt E, Debock M, Kaufman JM. Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. J Chromatogr B. 2012;893/894:57–62.
Guo T, Gu J, Soldin OP, Singh RJ, Soldin SJ. Rapid measurement of estrogens and their metabolites in human serum by liquid chromatography-tandem mass spectrometry without derivatization. Clin Biochem. 2008;41(9):736–41.
International Organization for Standardization (ISO). In vitro diagnostic medical devices: measurement of quantities in samples of biological origin–requirements for content and presentation of reference measurement procedures. Geneva: ISO; 2009. ISO15193.
International Organization for Standardization (ISO). In vitro diagnostic medical devices: metrological traceability of values assigned to calibrators and control materials. Geneva: ISO; 2003. ISO 17511.
Botelho JC, Shacklady C, Cooper HC, Tai SS, Van Uytfanghe KV, Thienpont LM, Vesper HW (2013) Isotope-dilution liquid chromatography-tandem mass spectrometry candidate reference method for total testosterone in human serum. Clin Chem 59(2):372–380
Tai SS, Xu B, Welch MJ, Phinney KW. Development and evaluation of a candidate reference measurement procedure for the determination of testosterone in human serum using isotope dilution liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2007;388(5/6):1087–94.
Clinical and Laboratory Standards Institute (CLSI). Liquid Chromatography-Mass Spectrometry Methods; Approved Guideline. William C: CLSI, 2014. CLSI document C62-A
International Organization for Standardization (ISO)/IEC Guide 98-3:2008. Uncertainty of measurement –Part 3: Guide to the expression of uncertainty in measurement (GUM:1995). Geneva: ISO; 2008.
Clinical and Laboratory Standards Institute (CLSI). Preliminary evaluation of quantitative clinical laboratory measurement procedures; Approved guideline -3rd edition. Wayne: CLSI, 2014. CLSI document EP10-A3-AMD.
International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). RELA-IFCC external quality assessment scheme for Reference Laboratories in Laboratory Medicine. Available at: http://www.dgkl-rfb.de:81/4Daction/g_search_RELA/RELA2015. Accessed Jan 2017.
International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). RELA-IFCC external quality assessment scheme for Reference Laboratories in Laboratory Medicine. Available at: http://www.dgkl-rfb.de:81/4Daction/g_search_RELA/RELA2014. Accessed Sep 2015.
Clinical and Laboratory Standards Institute (CLSI). Procedures for the handling and pro- cessing of blood specimens for common laboratory tests; Approved guideline, 4th edition. Wayne: CLSI, 2010. CLSI document H18-A4.
Jung PG, Kim B, Park SR, So HY, Shi LH, Kim Y. Determination of serum cortisol using isotope dilution–liquid chromatography–mass spectrometry as a candidate reference method. Anal Bioanal Chem. 2004;380(5–6):782–8.
Sargent M, Harrington C, Harte R. Guidelines for achieving high accuracy in isotope dilution mass spectrometry (IDMS). LGC Limited: Royal Society of Chemistry, 2002.
Botelho JC, Ribera A, Cooper HC, Vesper HW. Evaluation of an Isotope Dilution HPLC Tandem Mass Spectrometry Candidate Reference Measurement Procedure for Total 17-β Estradiol in Human Serum. Anal. Chem.,2016; 88 (22):11123–9.
Kushnir MM, Komaromy-Hiller G, Shushan B, Urry FM, Roberts WL. Analysis of dicar- boxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin Chem. 2001; 47(11): 1993–2002.
Stöckl D, Sluss PM, Thienpont LM. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta. 2009;408(1):8–13.
Fraser CG, Kallner A, Kenny D, Petersen PH. Introduction: Strategies to set global quality specifications in laboratory medicine. Scand J Clin Lab Invest. 1999; 59(7):477–8.
LM. Thienpont. Quality specifications for reference methods. Scand J Clin Lab Invest 1999; 59:535–538.
StoÈckl D, Reinauer H. Development of criteria for the evaluation of reference method values. Scand J Clin Lab Invest 1993; 53 Suppl 212:16–8.
Clinical and Laboratory Standards Institute (CLSI). Measurement procedure comparison and bias estimation using patient samples; Approved guideline, 3rd edition. Wayne: CLSI, 2013. CLSI document EP09-A3.
Acknowledgements
The authors thank Prof. Zhiguo Wang for sharing information on the uE3 results of the PT event 2016 conducted by China National Center for Clinical Laboratory.
Financial support
This work was supported by the National Natural Science Foundation of China (81572088), the Natural Science Foundation of Guangdong Province (2015A030313340), the Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (YN2014ZHR204 and YN2016QJ15), and Administration of Traditional Chinese Medicine Bureau of Guangdong Province (20172063).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
The research involved human participants and/or animals
The research involves serum samples taken from pregnant women and this study is approved by Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (the other name: Second Affiliated Hospital of Guangzhou University of Chinese Medicine). The number of this Review is: B2016-159-01.
Informed consent
Ethics committee approved the exemption of informed consent.
Additional information
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official views or positions of the Wadsworth Center of New York State Department of Health. Use of trade names and commercial sources is for identification only and does not constitute endorsement by New York State Department of Health.
Electronic supplementary material
ESM 1
(PDF 16 kb)
Rights and permissions
About this article
Cite this article
Huang, X., Zhang, Q., Zheng, S. et al. Measurement of human serum unconjugated estriol without derivatization using liquid chromatography-tandem mass spectrometry candidate reference method and compared with two immunoassays. Anal Bioanal Chem 410, 6257–6267 (2018). https://doi.org/10.1007/s00216-018-1236-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1236-y