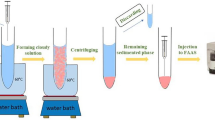
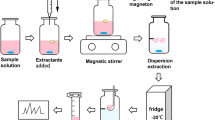
Abstract
Cherry stems have been used in traditional medicine mostly for the treatment of urinary tract infections. Extraction with subcritical water, according to its selectivity, efficiency and other aspects, differs substantially from conventional extraction techniques. The complexity of plant subcritical water extracts is due to the ability of subcritical water to extract different chemical classes of different physico-chemical properties and polarities in a single run. In this paper, dispersive liquid-liquid microextraction (DLLME) with simultaneous derivatisation was optimised for the analysis of complex subcritical water extracts of cherry stems to allow simple and rapid preparation prior to gas chromatography-mass spectrometry (GC-MS). After defining optimal extracting and dispersive solvents, the optimised method was used for the identification of compounds belonging to different chemical classes in a single analytical run. The developed sample preparation protocol enabled simultaneous extraction and derivatisation, as well as convenient coupling with GC-MS analysis, reducing the analysis time and number of steps. The applied analytical protocol allowed simple and rapid chemical screening of subcritical water extracts and was used for the comparison of subcritical water extracts of sweet and sour cherry stems.

DLLME GC MS analysis of cherry stem extracts obtained by subcritical water



Similar content being viewed by others
References
Švarc-Gajić J. Sampling and sample preparation in analytical chemistry. 1st ed. New York: Nova Science Publishers; 2010.
Bursal E, Köksal E, Gülçin İ, Bilsel G, Gören AC. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC–MS/MS. Food Res Int. 2013;51:66–74.
Bastos C, Barros L, Dueñas M, Calhelha RC, Queiroz MJRP, Santos-Buelga C, et al. Chemical characterization and bioactive properties of Prunus avium L.: the widely studied fruits and the unexplored stems. Food Chem. 2015;173:1045–53.
Chen Z, Landman P, Colmer TD, Adams MA. Simultaneous analysis of amino and organic acids in extracts of plant leaves as tert-butyldimethylsilyl derivatives by capillary gas chromatography. Anal Biochem. 1998;2:203–11.
Pereira C, Barros L, Carvalho AM, Ferreira I. Use of UFLC-PDA for the analysis of organic acids in thirty five species of food and medicinal plants. Food Anal Methods. 2013;5:1337–44.
Huang T, Wang S, Xiuming L, Wu LH, Xuqiang L. Rapid determination of eight organic acids in plant tissue by sequential extraction and high performance liquid chromatography. Se Pu. 2014;12:1356–61.
Proestos C, Sereli D, Komaitis M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006;95:44–52.
Saim N, Osman R, Yasin WAH, Hamid RD. Subcritical water extraction of essential oil from coriander (Coriandrum sativum l.) seeds. The Malaysian J Anal Sci. 2008;1:22–4.
Jimenez-Carmona MM, Luque de Castro MD. Isolation of eucalyptus essential oil for GC-MS analysis by extraction with subcritical water. Chromatographia. 1999;9/10:578–82.
Miller DJ, Hawthorne SB. Method for determining the solubilities of hydrophobic organics in subcritical water. Anal Chem. 1998;70:1618–21.
Gonzales A, Avivar J, Cerda V. Determination of priority phenolic pollutans exploiting an in-siringe dispersive liquid-liquid microextraction-multisyringe chromatographic system. Anal Bioanal Chem. 2015;407:2013–22.
Clavijo S, Avivar J, Suarez R, Cerdà V. Analytical strategies for coupling separation and flow-injection, techniques. Trends Anal Chem. 2015;67:26–33.
Hageman KJ, Mazeas L, Grabanski CB, Miller DJ, Hawthorne SB. Coupled subcritical water extraction with solid-phase microextraction for determining semivolatile organics in environmental solids. Anal Chem. 1996;68:3892–8.
Beichert A, Padberg S, Wenclawiak BW. Selective determination of alkylmercury compounds in solid matrices after subcritical water extraction, followed by solid-phase microextraction and GC-MS. Appl Organometal Chem. 2000;14:493–8.
Wang X, Lin L, Luan T, Yang L, Tam NFY. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in sediment samples by combining subcritical water extraction and dispersive liquid–liquid microextraction with derivatization. Anal Chim Acta. 2012;753:57–63.
Yuan K, Kang H, Yue Z, Yang L, Lin L, Wang X, et al. Determination of 13 endocrine disrupting chemicals in sediments by gas chromatography-mass spectrometry using subcritical water extraction coupled with dispersed liquid-liquid microextraction and derivatization. Anal Chim Acta. 2015;866:41–7.
Švarc-Gajić J, Cvetanović A, Segura-Carretero A, Borrás Linares I, Mašković P. Characterisation of ginger extracts obtained by subcritical water. J Supercrit Fluids. 2017;123:92–100.
Stroo HF, Ward CH. In situ remediation of chlorinated solvent plumes. Springer Science; 2010.
Wahl GH. Microscale experiments in organic chemistry. 2nd ed. New Jersey: Practice Hall PTR; 2003.
Menon S, Nayeem N, Ranganath MK. Method development and validation of caproic acid from Vanilla planifolia pods by HPLC. Pharmacophore. 2014;5:388–95.
Mero AA, Ojala T, Hulmi JJ, Puurtinen R, Karila TAM, Seppälä T. Effects of alfa-hydroxy-isocaproic acid on body composition, DOMS and performance in athletes. J Int Soc Sports Nutr. 2010;7:1–8.
Moussa TAA, Almaghrabi OA. Fatty acid constituents of Peganum harmala plant using gas chromatography–mass spectroscopy. Saudi J Biol Sci. 2016;23:397–403.
Ayamaé OC, Benjamain Kassi AB, Adjé AF, Adima AA, Parfait Kouadio EJ. Obtaining a concentrated fresh product of capsicum annuum by reverse osmosis process and analysis of its bioactive constituents and mineral composition. Int J Food Pro Techn. 2015;2:1–9.
Kim KJ, Kim MA, Jung JH. Antitumor and antioxidant activity of protocatechualdehyde produced from Streptomyces lincolnensis M-20. Arch Pharm Res. 2008;31:1572–7.
Zhao X, Zhai S, An MS, Wang YH, Yang YF, Ge HQ, et al. Neuroprotective effects of protocatechuic aldehyde against neurotoxin-induced cellular and animal models of Parkinson’s disease. PLoS One. 2013;8:e78220.
Deisinger PJ, Hill TS, English JC. Human exposure to naturally occurring hydroquinone. J Toxicol Environ Health Sci. 1996;47:31–46.
McDonald TA, Holland NT, Skibola C, Duramad P, Smith MT. Hypothesis: phenol and hydroquinone derived mainly from diet and gastrointestinal flora activity are causal factors in leukemia. Leukemia. 2001;15:10–20.
Hameed IH, Altameme HJ, Idan SA. Artemisia annua: biochemical products analysis of methanolic aerial parts extract and anti-microbial capacity. Res J Pharm Biol Chem Sci. 2016;7:1843–68.
Münzenberger B, Heilemann J, Strack D, Kottke I, Oberwinkler F. Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce. Planta. 1990;182:142–8.
HSDB. Acetophenone, CASRN: 98-86-2. Hazardous substances databank number: 969. Last Revision Date: 2003/10/15. https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~oq8jJp. 2010.
Wahyudiono S, Machmudah S, Goto M. Utilization of sub and supercritical water reactions in resource recovery of biomass wastes. Eng J. 2013;17:1–12.
Huang CH, Chen MF, Chung HH, Cheng JT. Antihyperglycemic effect of syringaldehyde in streptozotocin-induced diabetic rats. J Nat Prod. 2012;75:1465–8.
Del Pozo-Insfran D, Percival SS, Talcott ST. Açai (Euterpe oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J Agric Food Chem. 2006;54:1222–9.
Borrega M, Niemelä K, Sixta H. Effect of hydrothermal treatment intensity on the formation of degradation products from birchwood. Holzforschung. 2013;67:871–9.
Charrouf Z, Guillaume D. Phenols and polyphenols from Argania spinose. Am J Food Technol. 2007;2:679–83.
Capasso R, Cristinzio G, Evidente A, Visca C, Iannini C. Oleuropein and other polyphenols from olive (Olea europea) for protecting the plant against Pseudomonas syringae subsp. Savastanoi. In: Rudolph K, Burr TJ, Mansfield JW, Stead D, Vivian A, von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. Netherlands: Springer; 1997. p. 133–7.
Onwudili JA, Williams PT. Catalytic depolymerization of alkali lignin in subcritical water: influence of formic acid and Pd/C catalyst on the yields of liquid monomeric aromatic products. Green Chem. 2014;16:4740–8.
KL M. Antiseptic lubricants and guaiacol preparations in prevention of tracheobronchial reactions after bronchoscopy in pulmonary tuberculosis. Monatsschr Ohrenheilkd Laryngorhinol J. 1956;90:182–3.
Wen KC, Huang CY, Liu FS. Determination of cinnamic acid and paeoniflorin in traditional Chinese medicinal preparations by high-performance liquid chromatography. J Chromatogr. 1992;593:191–9.
Jung J, Lee JH, Bae KH, Jeong CS. Anti-gastric actions of eugenol and cinnamic acid isolated from Cinnamomi ramulus. Yakugaku Zasshi. 2011;131:1103–10.
Fujii S, Aoki H, Komoto M, Munakata K. Production of melilotic acid by action of taplzrina studies on hypertrophic disease of cherry (Genus Prunus), so-called “witch’s broom” caused by taphrina wiesneri. Part III. Agric Biol Chem. 1971;35:1133–8.
Roberts WL, Link KP. A precise method for the determination of coumarin, melilotic acid, and coumaric acid in plant tissue. J Biol Chem. 1937;119:269–81.
Švarc-Gajić J. Biological activity of natural products. 1st ed. New York: Nova Science Publishers; 2013.
Muller-Schwarze D. The beaver: its life and impact. 2nd ed. Ithaca, New York: Cornell University Press; 2003.
Jannu VG, Sanjenbam P, Kannabiran K. Preclinical evaluation and molecular docking of 2,5-di-tert-butyl-1,4-benzoquinone (DTBBQ) from Streptomyces sp. VITVSK1 as a potent antibacterial agent. Int J Bioinforma Res Appl. 2015;11:142–52.
Dodd MC, Stillman WB, Roys M, Crosby C. The in vitro bacteriostatic action of some simple furan derivatives. J Pharmacol Exp Ther. 1944;82:11–8.
Hall IH, Wong OT, Reynolds DJ, Chang J. The hypolipidemic effects of 2-furoic acid in Sprague-Dawley rats. Arch Pharm. 2006;3:15–23.
Siegel U, Mues R, Dӧnig R, Eicher T, Blechsmidt M, Becker H. Ten azulenes from Plagiochila longispina and Calypogeia azurea. Phytochemistry. 1992;31:1671–8.
Funding
The authors are grateful to the Serbian Ministry of Education, Science and Technological Development and Provincial Secretariat for Science and Technological Development. Financial support from the ‘Ministerio de Economía y Competitividad’ (Spanish Government) through the grant CTQ2013-47461-R is also gratefully acknowledged. R. Suárez thanks the Conselleria d’Educació, Cultura i Universitats from the Government of the Balearic Islands for a PhD stipend co-financed by Fondo Social Europeo (FPI/1444/2012). S. Clavijo acknowledges the Torres Quevedo Program of the MINECO co-financed with European Funds for the financial support through the PTQ-2015-08038.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Švarc-Gajić, J., Clavijo, S., Suárez, R. et al. Simultaneous dispersive liquid-liquid microextraction derivatisation and gas chromatography mass spectrometry analysis of subcritical water extracts of sweet and sour cherry stems. Anal Bioanal Chem 410, 1943–1953 (2018). https://doi.org/10.1007/s00216-018-0858-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0858-4