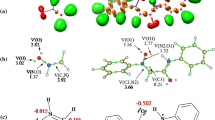
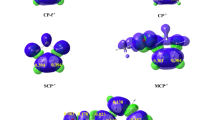
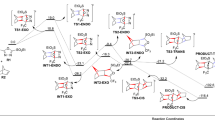
Abstract
The mechanism, regio-, chemo-, diastereo-, stereo- and enantio-selectivity of the [3 + 2] cycloaddition reaction of aryl nitrile oxides with 5-acetoxy-2(5H)-furanone has been performed by means of several computational approaches, namely, activation and reaction energies, global electron density transfer (GEDT), DFT reactivity indices and rate constants. The titled reaction leads to formation of 2-isoxalines which are important intermediates for the construction of many synthetically important cycloadducts. The calculations were performed using hybrid density functionals M06-2X, B3LYP and MPWB1K together with split valence triple zeta basis set 6-311++ G(d,p). Chemo- and regio-selectivity is not affected by the nature of substituent on the aryl nitrile oxides. IRC calculations and activation energies show that this reaction follows an asynchronous concerted mechanism. Analysis of the electrophilic PA+ and nucleophilic PA− Parr functions at the different reaction sites in the 5-acetoxy-2(5H)-furanone indicates that the aryl nitrile oxides add across the atomic centers with the highest Mulliken atomic spin densities. The results reported herein are in good agreement with previous experimental works. The GEDT calculations unravel the low polar character of the [3 + 2] cycloaddition reactions. Overall, the viability of these [3 + 2] cycloaddition reactions depends on the low polar character, which also depends on the electrophilic and nucleophilic character of the reacting 5-acetoxy-2(5H)-furanone and aryl nitrile oxides, respectively.










Similar content being viewed by others
References
Rane D, Sibi M (2011) Recent advances in nitrile oxide cycloadditions. Synthesis of Isoxazolines. Curr Org Synth 8:616–627. https://doi.org/10.2174/157017911796957320
Opoku E, Tia R, Adei E (2019) DFT mechanistic study on tandem sequential [4 + 2]/[3 + 2] addition reaction of cyclooctatetraene with functionalized acetylenes and nitrile imines. J Phys Org Chem 32:e3992. https://doi.org/10.1002/poc.3992
Kissane M, Maguire AR (2010) Asymmetric 1,3-dipolar cycloadditions of acrylamides. Chem Soc Rev 39:845–883. https://doi.org/10.1039/b909358n
Xing Y, Wang N (2012) Organocatalytic and metal-mediated asymmetric [3 + 2 ] cycloaddition reactions. Coord Chem Rev 256:938–952. https://doi.org/10.1016/j.ccr.2012.01.002
Lutz JF (2007) 1,3-Dipolar cycloadditions of azides and alkynes: a universal ligation tool in polymer and materials science. Angew Chemie Int Ed 46:1018–1025. https://doi.org/10.1002/ANIE.200604050
Tron GC, Pirali T, Billington RA et al (2008) Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med Res Rev 28:278–308. https://doi.org/10.1002/MED.20107
Jewett JC, Bertozzi CR (2010) Cu-free click cycloaddition reactions in chemical biology. Chem Soc Rev 39:1272–1279. https://doi.org/10.1039/B901970G
Rayabarapu DK, Cheng CH (2007) New catalytic reactions of oxa-and azabicyclic alkenes. Acc Chem Res 40:971–983. https://doi.org/10.1021/ar600021z
Guillier F, Nivoliers F, Bourguignon J et al (1992) Metalation of pi-deficient heterocycles a facile synthesis of cerpegin. Tetrahedron Lett 33:7355–7356. https://doi.org/10.1016/S0040-4039(00)60186-7
Weidner-Wells MA, Fraga-Spano SA, Turchi IJ (1998) Unusual regioselectivity of the dipolar cycloaddition reactions of nitrile oxides and tertiary cinnamides and crotonamides. J Org Chem 63:6319–6328. https://doi.org/10.1021/jo9807621
Kim JN, Chung KH, Ryu EK (1991) Alkali metal fluoride promoted generation of nitrile oxides from hydroximoyl chlorides. Heterocycles 32:477–480. https://doi.org/10.3987/COM-90-5646
Cossío FP, Morao I, Jiao H, Von Ragué SP (1999) In-plane aromaticity in 1,3-dipolar cycloadditions. solvent effects, selectivity, and nucleus-independent chemical shifts. J Am Chem Soc 121:6737–6746. https://doi.org/10.1021/ja9831397
Taherpour A, Rajaeian E (2008) Computational note on ab initio studies of 1,3-dipolar cycloaddition reactions between 7–10 membered simple cycloalkynes and nitriloxide. J Mol Struct Theo Chem 849:23–24. https://doi.org/10.1016/j.theochem.2007.10.00
Wagner G, Danks TN, Vullo V (2007) Quantum-chemical study of the Lewis acid influence on the cycloaddition of benzonitrile oxide to acetonitrile, propyne and propene. Tetrahedron 63:5251–5260. https://doi.org/10.1016/j.tet.2007.03.169
Abdullah H, Tia R, Adei E (2021) Regio-, stereo-, and site-selectivities of 1,3-dipolar Cycloaddition reaction of benzonitrile oxide with unsymmetrically substituted norbornenes and norbornadienes: a computational study. J Phys Org Chem 34:e4259. https://doi.org/10.1002/poc.4259
Oravec P, Fišera Ľ, Gažo R (1991) Regio- and stereoselective 1,3-dipolar cycloaddition of arylnitrile oxides to 5-acetoxy-2(5 H)-furanone. Monatshefte für Chemie Chem Mon 122:165–170. https://doi.org/10.1007/BF00809361
Hanwell MD, Curtis DE, Lonie DC et al (2012) Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17. https://doi.org/10.1186/1758-2946-4-17
Clark M, Cramer RD, Van Opdenbosch N (1989) Validation of the general purpose tripos 5.2 force field. J Comput Chem 10:982–1012. https://doi.org/10.1002/jcc.540100804
Akuamoah DA, Opoku E, Tia R, Adei E (2020) 1,3-Dipolar cycloaddition reaction of indoles with tosyl azide, subsequent dehydroaromatization and ring-opening cascade: a computational study. Theor Chem Acc 139:1–16. https://doi.org/10.1007/s00214-020-02653-5
Pipim GB, Opoku E (2021) Unveiling the molecular mechanisms of the cycloaddition reactions of aryl hetaryl thioketones and C,N-disubstituted nitrilimines. J Mol Model 27:84. https://doi.org/10.1007/s00894-021-04706-3
Opoku E, Arhin G, Pipim GB et al (2020) Site-, enantio- and stereo-selectivities of the 1,3-dipolar cycloaddition reactions of oxanorbornadiene with C, N-disubstituted nitrones and dimethyl nitrilimines: a DFT mechanistic study. Theor Chem Acc 139:16. https://doi.org/10.1007/s00214-019-2529-8
Opoku E, Tia R, Adei E (2019) Quantum chemical studies on the mechanistic aspects of tandem sequential cycloaddition reactions of cyclooctatetraene with ester and nitrones. J Mol Graph Model 92:17–31. https://doi.org/10.1016/j.jmgm.2019.06.019
Opoku E, Tia R, Adei E (2019) Computational studies on [4 + 2] / [3 + 2] tandem sequential cycloaddition reactions of functionalized acetylenes with cyclopentadiene and diazoalkane for the formation of norbornene pyrazolines. J Mol Model 25:168. https://doi.org/10.1007/s00894-019-4056-x
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218. https://doi.org/10.1002/jcc.540030212
Hratchian HP, Schlegel HB (2004) Accurate reaction paths using a Hessian based predictor–corrector integrator. J Chem Phys 120:9918–9924. https://doi.org/10.1063/1.1724823
Hratchian HP, Schlegel HB (2005) Using Hessian updating to increase the efficiency of a Hessian based predictor-corrector reaction path following method. J Chem Theory Comput 1:61–69. https://doi.org/10.1021/ct0499783
Donkor B, Opoku E, Aniagyei A (2022) Theoretical studies on cycloaddition reactions of N-allyl substituted polycyclic Isoindole-1,3-dione with nitrones and nitrile oxides. Comput Theor Chem 1208:113574. https://doi.org/10.1016/j.comptc.2021.113574
Umar AR, Donkor B, Opoku E (2022) Mechanistic details of domino [3+2] cycloaddition/[3,3] sigmatropic shift reactions of N-vinyl nitrones with isocyanates. Comput Theor Chem 1210:113643. https://doi.org/10.1016/j.comptc.2022.113643
Donkor B, Umar AR (2022) Opoku E (2022) Mechanistic elucidation of the tandem Diels–Alder/(3 + 2) cycloadditions in the design and syntheses of heterosteroids. J Mol Model 283(28):1–16. https://doi.org/10.1007/S00894-022-05063-5
Legault CY (2009) C. Y. CYLview
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts, R, Mennucci, B, Hratchian, HP, Ortiz, JV, Izmaylov, AF, Sonnenberg, JL, Williams-Young, D, Ding, F, Lipparini, F, Egidi, F, Goings, J, Peng, B, Petrone, A, Henderson, T, Ranasinghe, D, Zakrzewski, VG, Gao, J, Rega, N, Zheng, G, Liang, W, Hada, M, Ehara, M, Toyota, K, Fukuda, R, Hasegawa, J, Ishida, M, Nakajima, T, Honda, Y, Kitao, O, Nakai, H, Vreven, T, Throssell, K, Montgomery, JA, Peralta, J., J.E, Ogliaro, F, Bearpark, MJ, Heyd, JJ, Brothers, EN, Kudin, KN, Staroverov, VN, Keith, TA, Kobayashi, R, Normand, J, Raghavachari, K, Rendell, AP, Burant, JC, Iyengar, SS, Tomasi, J, Cossi, M, Millam, JM, Klene, M, Adamo, C, Cammi, R, Ochterski, JW, Martin, RL, Morokuma, K, Farkas, O, Foresman, JB, Fox, DJ (2016) Gaussian 16, Revision B.01, Gaussian, Inc., Wallingford, CT
Ríos-Gutiérrez M, Domingo LR (2019) Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur J Org Chem 2019:267–282. https://doi.org/10.1002/ejoc.201800916
Umar AR, Opoku E (2022) Mechanistic studies on stereoselective domino [4 + 2]/retro[3 + 2]/[3 + 2] cycloaddition reactions of oxadiazoles with strained and unstrained cycloalkenes. Theor Chem Acc 141:1–16. https://doi.org/10.1007/s00214-022-02872-y
Domingo LR, Sáez JA (2009) Understanding the mechanism of polar Diels-Alder reactions. Org Biomol Chem 7:3576–3583. https://doi.org/10.1039/B909611F
Atoms DT, Parr MRG, Yang W (1989) Book review. Density Funct Theory Atoms Mol 47:10101
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748. https://doi.org/10.3390/molecules21060748
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity Index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A Theoretical Study. J Org Chem 73:4615–4624. https://doi.org/10.1021/jo800572a
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J Mol Struct THEOCHEM 865:68–72. https://doi.org/10.1016/j.theochem.2008.06.022
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428. https://doi.org/10.1039/c4ra04280h
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor—acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746. https://doi.org/10.1063/1.449486
Domingo LR, Pe P, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494. https://doi.org/10.1039/c2ra22886f
Ranck JP (2001) Modern physical chemistry: A molecular approach. J Chem Educ 78:1024. https://doi.org/10.1021/ed078p1024
Domingo LR, Ghodsi F, Ríos-Gutiérrez M (2019) A molecular electron density theory study of the synthesis of spirobipyrazolines through the domino reaction of nitrilimines with allenoates. Molecules. https://doi.org/10.3390/molecules24224159
Pipim GB, Opoku E, Tia R, Adei E (2020) Peri-, Chemo-, Regio-, Stereo- and Enantio-Selectivities of 1,3-dipolar cycloaddition reaction of C, N-Disubstituted nitrones with disubstituted 4-methylene-1,3-oxazol-5(4H)- one: A quantum mechanical study. J Mol Graph Model 97:107542. https://doi.org/10.1016/j.jmgm.2020.107542
Baffour Pipim G, Opoku E (2022) Catalyst-free [3 + 2] cycloaddition reaction of oxa-, aza-, and thio-bicyclic alkenes with cyclic and acyclic nitrones: A mechanistic study. Comput Theor Chem 1214:113790. https://doi.org/10.1016/J.COMPTC.2022.113790
Opoku E, Baffour Pipim G, Tia R, Adei E (2020) Mechanistic study of the tandem intramolecular (4 + 2)/intermolecular (3 + 2) cycloaddition reactions for the formation of polyaza- and polyisoxazolidine-steroids. J Heterocycl Chem 57:1748–1758. https://doi.org/10.1002/jhet.3900
Chamorro E, Pérez P, Domingo LR (2013) On the nature of Parr functions to predict the most reactive sites along organic polar reactions. Chem Phys Lett 582:141–143. https://doi.org/10.1016/j.cplett.2013.07.020
Houk KN (1975) Frontier molecular orbital theory of cycloaddition reactions. Acc Chem Res 8:361–369. https://doi.org/10.1021/ar50095a001
Acknowledgements
The authors thank the South Africa’s Centre for High-Performance Computing for a grant of computational resources.
Funding
This work was supported by Nesvard Africa Open Science Project via grant number CHEM-35672021.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests regarding the publication of this manuscript.
Availability of data and material
The data supporting this publication are included in the article and the electronic supporting information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdullah, H., Opoku, E. Quantum chemical study on the mechanism and selectivity of [3 + 2] cycloaddition reactions of aryl nitrile oxides with furanone. Theor Chem Acc 141, 55 (2022). https://doi.org/10.1007/s00214-022-02915-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02915-4