Abstract
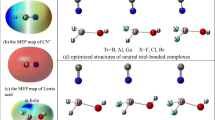
Triel bond, a recent emerging noncovalent interaction, has attracted extensive attention from physical and chemical scientists. Some triel atoms, such as Ga and In atoms, theirs valence, can be bivalent, trivalent, and even tetravalent. What is the influence of the valence of triel atom on the triel-bonding formation? This issue generates our initial research interest. Then, the influence of valence of triel atom on the triel bond has been theoretically investigated in thirty-two complexes composed by Lewis acids TrHX/TrH2X (Tr = Ga, In; X = F, Cl, Br, I) and Lewis base H2Y (Y = O, S) molecules at MP2/aug‐cc‐pVTZ(PP) theory level. MEP analyses show that both TrHX and TrH2X molecules possess π-holes on Tr atoms, which can be used as Lewis acids, and interact with Lewis bases to form triel bonds. AIM and Non-covalent interaction (NCI) analyses demonstrate that triel bond exists in all the studied thirty-two complexes. Hydrogen bond coexists with triel bond only in six of these complexes. In the process of triel bond formation, TrH2X molecule has stronger structural distortion than TrHX molecule. And TrH2X molecule can form a stronger triel bond than that of TrHX molecule by analyzing multiple factors that affect triel-bonding formation including substituents, electron donors, and nature of triel atoms. In addition, NBO analyses show that triel bond in TrH2X–H2Y complexes has stronger orbital interaction than that in TrHX–H2Y complexes.






Similar content being viewed by others
References
Grabowski SJ (2019) Bifurcated triel bonds-hydrides and halides of 1,2-bis(dichloroboryl)benzene and 1,8-bis(dichloroboryl)naphthalene. Curr Comput Aided Drug Des 9:503
Chval Z, Dvorackova O, Chvalova D, Burda JV (2019) Square-planar Pt(II) and Ir(I) complexes as the lewis bases: donor–acceptor adducts with group 13 trihalides and trihydrides. Inorg Chem 58:3616–3626
Escudero-Adan EC, Bauza A, Lecomte C, Frontera A, Ballester P (2018) Boron triel bonding: a weak electrostatic interaction lacking electron-density descriptors. Phys Chem Chem Phys 20:24192–24200
Frontera A, Bauza A (2017) Concurrent aerogen bonding and lone pair/anion-pi interactions in the stability of organoxenon derivatives: a combined CSD and ab initio study. Phys Chem Chem Phys 19:30063–30068
Echeverria J (2017) Alkyl groups as electron density donors in pi-hole bonding. CrystEngComm 19:6289–6296
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc Chem Res 38:386–395
Schneider HJ (2009) Binding mechanisms in supramolecular complexes. Angew Chem Int Ed 48:3924–3977
Politzer P, Lane P, Concha MC, Ma YG, Murray JS (2007) An overview of halogen bonding. J Mol Model 13:305–311
Murray JS, Lane P, Politzer P (2009) Expansion of the sigma-hole concept. J Mol Model 15:723–729
Politzer P, Murray JS, Clark T (2010) Halogen bonding: an electrostatically-driven highly directional noncovalent interaction. Phys Chem Chem Phys 12:7748–7757
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) sigma-Holes, pi-holes and electrostatically-driven interactions. J Mol Model 18:541–548
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other sigma-hole interactions: a perspective. Phys Chem Chem Phys 15:11178–11189
Guo X, Liu YW, Li QZ, Li WZ, Cheng JB (2015) Competition and cooperativity between tetrel bond and chalcogen bond in complexes involving F2CX (X = Se and Te). Chem Phys Lett 620:7–12
Wei YX, Li QZ, Scheiner S (2018) The pi-Tetrel bond and its influence on hydrogen bonding and proton transfer. Chem Phys Chem 19:736–743
Grabowski SJ (2014) Boron and other triel lewis acid centers: from hypovalency to hypervalency. Chem Phys Chem 15:2985–2993
Wang WZ, Ji BM, Zhang Y (2009) Chalcogen Bond: A Sister Noncovalent Bond to Halogen Bond. J Phys Chem A 113:8132–8135
Azofra LM, Alkorta I, Scheiner S (2014) Noncovalent interactions in dimers and trimers of SO3 and CO. Theor Chem Acc 133:1586
Pascoe DJ, Ling KB, Cockroft SL (2017) The origin of chalcogen-bonding interactions. J Am Chem Soc 139:15160–15167
Zahn S, Frank R, Hey-Hawkins E, Kirchner B (2011) Pnicogen bonds: A new molecular linker? Chem Eur J 17:6034–6038
Bauza A, Ramis R, Frontera A (2014) A combined theoretical and cambridge structural database study of pi-Hole Pnicogen bonding complexes between electron rich molecules and both nitro compounds and inorganic bromides (YO2Br, Y = N, P and As). J Phys Chem A 118:2827–2834
Bauza A, Mooibroek TJ, Frontera A (2015) Directionality of pi-holes in nitro compounds. ChemComm 51:1491–1493
Bauza A, Mooibroek TJ, Frontera A (2013) Tetrel-bonding interaction: rediscovered supramolecular force? Angew Chem Int Ed 52:12317–12321
Bauza A, Mooibroek TJ, Frontera A (2016) Tetrel bonding interactions. Chem Rec 16:473–487
Scheiner S (2017) Systematic elucidation of factors that influence the strength of tetrel bonds. J Phys Chem A 121:5561–5568
Alkorta I, Elguero J, Frontera A (2020) Not only hydrogen bonds: other noncovalent interactions. Curr Comput-Aided Drug Des 10:180
Grabowski SJ (2017) Hydrogen bonds, and sigma-hole and pi-hole bonds—mechanisms protecting doublet and octet electron structures. Phys Chem Chem Phys 19:29742–29759
Grabowski SJ (2015) pi-Hole bonds: boron and aluminum lewis acid centers. Chem Phys Chem 16:1470–1479
Gao L, Zeng YL, Zhang XY, Meng LP (2016) Comparative studies on group iii sigma-hole and pi-hole interactions. J Comput Chem 37:1321–1327
Xu HL, Cheng JB, Yang X, Liu ZB, Li WZ, Li QZ (2017) Comparison of sigma-hole and -hole tetrel bonds formed by pyrazine and 1,4-dicyanobenzene: the interplay between anion- and tetrel bonds. Chem Phys Chem 18:2442–2450
Dong WB, Yang X, Cheng JB, Li WZ, Li QZ (2018) Comparison for sigma-hole and pi-hole tetrel-bonded complexes involving F2C=CFTF3 (T=C, Si, and Ge): substitution, hybridization, and solvation effects. J Fluor Chem 207:38–44
Zierkiewicz W, Michalczyk M, Scheiner S (2018) Comparison between tetrel bonded complexes stabilized by sigma and pi hole interactions. Molecules 23:1416
Nziko VDN, Scheiner S (2016) Comparison of pi-hole tetrel bonding with sigma-hole halogen bonds in complexes of XCN (X = F, Cl, Br, I) and NH3. Phys Chem Chem Phys 18:3581–3590
T.A. Ford (2020) The structural, vibrational and electronic properties of the triel-bonded complexes of boron trifluoride with some cyclic ethers—an ab initio study. J Mol Struct 1210:128020
Zierkiewicz W, Michalczyk M, Scheiner S (2020) Competition between intra and intermolecular triel bonds. Complexes between naphthalene derivatives and neutral or anionic Lewis bases. Molecules 25:635
Brinck T, Murray JS, Politzer P (1993) A computational analysis of the bonding in boron-trifluoride and boron-trichloride and their complexes with ammonia. Inorg Chem 32:2622–2625
Rowsell BD, Gillespie FJ, Heard GL (1999) Ligand close-packing and the Lewis acidity of BF3 and BCl3. Inorg Chem 38:4659–4662
Chi ZQ, Li QZ, Li HB (2019) Comparison of triel bonds with different chalcogen electron donors: Its dependence on triel donor and methyl substitution. Int J Quantum Chem 120:26046
Jablonski M (2018) Hydride-triel bonds. J Comput Chem 39:1177–1191
Zhang JR, Wei YX, Li WZ, Cheng JB, Li QZ (2018) Triel-hydride triel bond between ZX(3) (Z = B and Al; X = H and Me) and THMe3 (T = Si, Ge and Sn). Appl Organomet Chem 32:4367
Grabowski SJ (2015) Triel bonds, pi-Hole-pi-electrons interactions in complexes of boron and aluminium trihalides and trihydrides with acetylene and ethylene. Molecules 20:11297–11316
Esrafili MD, Mohammadian-Sabet F (2016) Theoretical insights into nature of pi-hole interactions between triel centers (B and Al) and radical methyl as a potential electron donor: Do single-electron triel bonds exist? Struct Chem 27:1157–1164
Chi ZQ, Dong WB, Li QZ, Yang X, Scheiner S, Liu SF (2019) Carbene triel bonds between TrR3 (Tr = B, Al) and N-heterocyclic carbenes. Int J Quantum Chem 119:25867
Fiacco DL, Leopold KR (2003) Partially bound systems as sensitive probes of microsolvation: a microwave and ab initio study of HCN center dot center dot center dot HCN-BF3. J Phys Chem A 107:2808–2814
Zhang JR, Li WZ, Cheng JB, Liu ZB, Li QZ (2018) Cooperative effects between pi-hole triel and pi-hole chalcogen bonds. Rsc Adv 8:26580–26588
Tang QJ, Li QZ (2015) Abnormal synergistic effects between Lewis acid- base interaction and halogen bond in F3B center dot center dot center dot NCX center dot center dot center dot NCM. Mol Phys 113:3809–3814
Yourdkhani S, Korona T, Hadipour NL (2015) Interplay between tetrel and triel bonds in RC6H4CN center dot center dot center dot MF3CN center dot center dot center dot BX3 complexes: a combined symmetry-adapted perturbation theory, moller-plesset, and quantum theory of atoms-in-molecules study. J Comput Chem 36:2412–2428
Zhang JR, Wang ZX, Liu SF, Cheng JB, Li WZ, Li QZ (2019) Synergistic and diminutive effects between triel bond and regium bond: Attractive interactions between pi-hole and sigma-hole. Appl Organomet Chem 33:4806
Liu MX, Zhuo HY, Li QZ, Li WZ, Cheng JB (2016) Theoretical study of the cooperative effects between the triel bond and the pnicogen bond in BF3 center dot center dot center dot NCXH2 center dot center dot center dot Y (X = P, As, Sb; Y = H2O, NH3) complexes. J Mol Model 22:10
Esrafili MD, Mousavian P (2018) The triel bond: a potential force for tuning anion-pi interactions. Mol Phys 116:388–398
Esrafili MD, Mousavian P (2017) Mutual influence between triel bond and cation-pi interactions: an ab initio study. Mol Phys 115:2999–3010
Michalczyk M, Zierkiewicz W, Scheiner S (2018) Triel-bonded complexes between TrR3 (Tr=B, Al, Ga; R=H, F, Cl, Br, CH3) and Pyrazine. Chem Phys Chem 19:3122–3133
Xu Z, Li Y (2019) Triel bonds in RZH2…NH3: hybridization, solvation, and substitution. J Mol Model 25:219
Grabowski SJ (2018) Two faces of triel bonds in boron trihalide complexes. J Comput Chem 39:472–480
Grabowski SJ (2017) Triel bonds-complexes of boron and aluminum trihalides and trihydrides with benzene. Struct Chem 28:1163–1171
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622
Woon DE, Dunning TH (1993) Gaussian-basis sets for use in correlated molecular calculations. 3. The atoms aluminum through argon. J Chem Phys 98:1358–1371
Boys SF, Bernardi F (2002) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors (Reprinted from Molecular Physics, vol 19, pg 553–566, 1970). Mol Phys 100:65–73
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, D.J. Fox Gaussian 09 Revision D.01, Gaussian Inc. Wallingford CT (2013)
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev 88:899–926
Arnold WD, Oldfield E (2000) The chemical nature of hydrogen bonding in proteins via NMR: J-couplings, chemical shifts, and AIM theory. J Am Chem Soc 122:12835–12841
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (Grant Nos. 11404268, 11774294).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Li, Y., Wang, H. et al. Which triel bond is stronger? TrHX⋯H2Y versus TrH2X⋯H2Y (Tr = Ga, In; X = F, Cl, Br, I; Y = O, S). Theor Chem Acc 140, 80 (2021). https://doi.org/10.1007/s00214-021-02790-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02790-5