Abstract
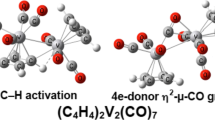
The structures and energetics of the binuclear phospholyl vanadium carbonyls (C4H4P)2V2(CO)n (n = 7, 6, 5, 4, 3, 2, 1) have been investigated using density functional theory. The lowest energy heptacarbonyl (C4H4P)2V2(CO)7 structures resemble those of the dimanganese pentacarbonyl (C4H4P)2Mn2(CO)5 with one seven-electron donor bridging η5,η1-C4H4P ring and no direct vanadium–vanadium bond. Similarly, the lowest energy hexacarbonyl (C4H4P)2V2(CO)6 structure resembles that of the dimanganese tetracarbonyl (C4H4P)2Mn2(CO)4 with two seven-electron donor bridging η5,η1-C4H4P rings and no direct vanadium–vanadium bond. The lowest energy pentacarbonyl (C4H4P)2V2(CO)5 structure has terminal η5-C4H4P phospholyl rings and a formal V≡V triple bond of length ~ 2.45 Å similar to the experimentally known and structurally characterized cyclopentadienyl analogue (η5-C5H5)2V2(CO)5. Various combinations of V=V double bonds or V≡V triple bonds, four-electron donor bridging carbonyl groups and seven-electron donor bridging η5,η1-C4H4P rings are found in the more highly unsaturated derivatives (C4H4P)2V2(CO)n (n = 4, 3, 2) to give 17- and 18-electron vanadium configurations for triplet- and singlet-state structures, respectively. Formal V≣V quadruple bonds are found only in the highly unsaturated lowest energy singlet (C4H4P)2V2(CO)n (n = 2, 1) structures, which, however, lie at somewhat higher energies than isomeric triplet structures.












Similar content being viewed by others
References
Dillon KB, Mathey F, Nixon JF (1998) Phosphorus: the carbon copy: from organophosphorus to phospha-organic chemistry. Wiley, New York
Mathey F (1976) Tetrahedron Lett. 17:4155
Mathey F, Mitschler A, Weiss R (1978) J Am Chem Soc 100:5748
Chen X, Du Q, Jin R, Feng H, Xie Y, King RB (2011) New J Chem 35:1117
Chen X, Yuan L, Ren G, Xi Q, King R, Du Q, Feng H, Xie Y, King RB (2016) Inorg Chim Acta 445:79
Chen X, Jin R, Du Q, Feng H, Xie Y, King RB (2012) J Organomet Chem 701:1
Wang X, Feng C, Ren G, Chen X, Jin R, Du Q, Feng H, Xie Y, King RB (2019) Inorg Chim Acta 494:194
Fortman GC, Kégl T, Li Q-S, Zhang X, Schaefer HF, Xie Y, King RB, Telser J, Hoff CD (2007) J Am Chem Soc 129:14388
Fischer EO, Vigoureux S (1958) Chem Ber 91:2205
Werner RPM, Filbey AH, Manastryskyj SA (1964) Inorg Chem 3:298
Herrmann WA, Plank J (1979) Chem Ber 112:392
Fischer EO, Schneider RJ (1967) Angew Chem Int Ed 6:569
Cotton FA, Kruczynski L (1978) J Organomet Chem 160:93
Huffman JC, Lewis LN, Caulton KG (1980) Inorg Chem 19:2755
Zhang X, Li Q, Xie Y, King RB, Schaefer HF (2007) Eur J Inorg Chem 153:1599
Li Q, Zhang X, Xie Y, King RB, Schaefer HF (2007) J Am Chem Soc 129:3433
Ziegler T, Autschbach J (2005) Chem Rev 105:2695
Bühl M, Kabrede H (2006) J Chem Theory Comput 2:1282
Brynda M, Gagliardi L, Widmark PO, Power PP, Roos BO (2006) Angew Chem Int Ed 45:3804
Sieffert N, Bühl M (2010) J Am Chem Soc 132:8056
Schyman P, Lai W, Chen H, Wang Y, Shaik S (2011) J Am Chem Soc 133:7977
Adams RD, Pearl WC, Wong YO, Zhang Q, Hall MB, Walensky JR (2011) J Am Chem Soc 133:12994
Lonsdale R, Olah J, Mulholland AJ, Harvey JN (2011) J Am Chem Soc 133:15464
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101
Becke AD (1988) Phys Rev A 38:3098
Perdew JP (1986) Phys Rev B 33:8822
Jones V, Thiel W (1995) J Phys Chem 102:8474
Silaghi-Dumitrescu I, Bitterwolf TE, King RB (2006) J Am Chem Soc 128:5432
Assef MK, Dever JL, Brathwaite AD, Mosley JD, Duncan MA (2015) Chem Phys Lett 640:175
Narendrapurapu BS, Richardson NA, Copan AV, Estep ML, Yang Z, Schaefer HF (2013) J Chem Theory Comput 9:2930
Dunning TH (1970) J Chem Phys 53:2823
Huzinaga S (1965) J Chem Phys 42:1293
Wachters AJH (1970) J Chem Phys 52:1033
Hood DM, Pitzer RM, Schaefer HF (1979) J Chem Phys 71:705
Frisch MJ et al (2009) Gaussian, Inc., Wallingford CT. Gaussian 09, Revision A.02
Papas BN, Schaefer HF (2006) J. Mol. Struct. (THEOCHEM) 768:175
Caspar JV, Meyer TJ (1980) J Am Chem Soc 102:7794
Hooker RH, Mahmoud KA, Rest AJ (1983) Chem. Commun. 105:1022
Hepp AF, Blaha JP, Lewis C, Wrighton MS (1984) Organometallics 3:174
Blaha JP, Bursten BE, Dewan JC, Frankel RB, Randolph CL, Wilson BA, Wrighton MS (1985) J Am Chem Soc 107:4561
Sunderlin LS, Wang D, Squires PR (1993) J Am Chem Soc 115:12060
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond order donor–acceptor perspective. Cambridge University Press, Cambridge, pp 32–36
Curtis MD, Butler WM (1978) J Organomet Chem 155:131
King RB, Efraty A, Douglas WM (1973) J Organomet Chem 60:125
Potenza J, Giordano P, Mastropaolo D, Efraty A (1974) Inorg Chem 13:2540
Herrmann WA, Serrano R, Weichmann J (1983) J Organomet Chem 246:C57
Acknowledgements
We are indebted to the Scientific Research Fund of the Key Laboratory of the Education Department of Sichuan Province (Grant No. 10ZX012) for the support of this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, W., Yan, J., Chen, X. et al. The role of the phosphorus lone pair in the low-energy binuclear phospholyl vanadium carbonyl structures: comparison with cyclopentadienyl analogues. Theor Chem Acc 140, 3 (2021). https://doi.org/10.1007/s00214-020-02692-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02692-y