Abstract
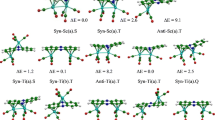
Structures and relative energies of binuclear iron-manganese complexes with the phosphine ligand L, which exist in vinylidene Cp(CO)(L)MnFe(μ-C=CHPh)(CO)4 (2) and benzylidene ketene η4-{C[Mn(CO)(L)Cp]∙ ∙(CO)CHPh}Fe(CO)3 (3) forms are calculated by the B3LYP density functional method. Four isomers with different positions of ligand L relative to the phenyl ring (conformers a and b) and the substituent Ph relative to the С=С bond (conformers E and Z) are considered for each form and their relative stability is determined. It is shown that all isomers of 2 have approximately the same energy (within 4 kcal/mol) whereas the energies of isomers of 3 differ within 21 kcal/mol. Isomer 3Ea in which the PPh3 ligand contacts with the phenyl substituent of the vinylidene group is most energetically favorable. It is found that with an increase in the L ligand size in the order PH3 < PH2Ph < PHPh2 < PPh3 the Mn–P bond length increases to 2.37 Å in the most stable isomer of form 3 and to 2.43 Å in the isomers of 2 and three conformers of 3. A more substantial increase in the Mn–P bond length in complexes 2 and 3 correlates with their lower stability as compared to isomer Ea of 3, which is consistent with experimental data on the presence of only one conformer 3Ea in solution.
Similar content being viewed by others
References
A. B. Antonova, Coord. Chem. Rev., 251, 1521 (2007).
N. E. Kolobova, L. L. Ivanov, O. S. Zhvanko, et al., J. Organomet. Chem., 228, 265 (1982).
H. Werner, F. J. Garcia Alonso, H. Otto, et al., Chem. Ber., 121, 1565 (1988).
V. G. Andrianov, Yu. T. Struchkov, N. E. Kolobova, et al., J. Organomet. Chem., 122, 33 (1976).
M. R. Churchill and K. Gold, Inorg. Chem., 8, 401 (1969).
R. Birk, H. Berke, G. Huttner, and L. Zsolnai, Chem. Ber., 121, 471 (1988).
I. Ara, J. R. Berenguer, J. Fornies, E. Lalinde, and M. Tomas, Organometallics, 15, 1014 (1996).
A. B. Antonova, O. S. Chudin, A. D. Vasiliev, et al., J. Organomet. Chem., 696, 963 (2011).
E. A. Ivanova-Shor, V. A. Nasluzov, A. M. Shor, et al., J. Organomet. Chem., 696, 3445 (2011).
L. Cavallo and M. Solà, J. Am. Chem. Soc., 123, 12294 (2001).
L. Gonsalvi, H. Adams, G. J. Sunley, et al., J. Am. Chem. Soc., 124, 13597 (2002).
C. H. Suresh, Inorg. Chem., 45, 4982 (2006).
J. Mathew, T. Thomas, and H. Suresh, Inorg. Chem., 46, 10800 (2007).
L. W. Chung, W. M. C. Sameera, R. Ramozzi, et al., Chem. Rev., 115, 5678 (2015).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785 (1988).
S. Vosko, L. Wilk, and M. Nussair, Can. J. Phys., 58, 1200 (1980).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Revision D.01, Gaussian Inc., Wallingford CT (2013).
A. Schaefer, C. Huber, and R. Ahlrichs, J. Chem. Phys., 100, 5829 (1994).
A. A. Johansson, A. B. Antonova, N. I. Pavlenko, et al., J. Mol. Struct., 408, 329 (1997).
B. Cordero, V. Gómez, A. E. Platero-Platz, et al., Dalton Trans., 2832 (2008).
Ch. Tolman, Chem. Rev., 77, 313 (1977).
Ch. Tolman, J. Am. Chem. Soc., 92, 2953 (1970).
E. A. Shor, A. M. Shor, V. A. Nasluzov, et al., J. Struct. Chem., 46, 220 (2005).
B. J. Dunne, R. B. Morris, and A. G. Orpen, J. Chem. Soc., Dalton Trans., 653 (1991).
A. L. Morris and J. T. York, J. Chem. Educ., 86, 1408 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Devoted to the 80th anniversary of Professor S. P. Gabuda
Translated from Zhurnal Strukturnoi Khimii, Vol. 57, No. 2, pp. 283-291, March-April, 2016.
Rights and permissions
About this article
Cite this article
Ivanova-Shor, E.A., Shor, A.M., Nasluzov, V.A. et al. A quantum chemical study of the effect of phosphine ligand on the structure of the Mn and Fe vinylidene binuclear complex. J Struct Chem 57, 267–274 (2016). https://doi.org/10.1134/S0022476616020049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476616020049