Abstract
Polynuclear superhalogen anion Li12F13 − and its ionic complexes formed by the interaction with selected metal ions (i.e., Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, and Li12F13 −Zn2+) are proposed and investigated on the basis of ab initio calculations. The thermodynamic stability, vertical excess electron detachment energy, and binding energies between ionic components were examined and discussed. The Li12F13 − anion has been proved stable against fragmentation and its vertical electronic stability was found to approach 10 eV. Due to its specific equilibrium structure that resembles a molecular basket, the Li12F13 − anion was found capable of trapping positively charged metal ions inside to form strongly bound ionic complexes. The large values of binding energies predicted for the Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, and Li12F13 −Zn2+ systems and their specific equilibrium structures indicate that the Li12F13 − anion can be useful as a steric shielding system which protect the metal ions from the interaction with the surroundings.
Similar content being viewed by others
1 Introduction
Strong electron acceptors commonly called “superhalogens” were originally proposed and studied by Gutsev and Boldyrev [1]. In general, the superhalogens were defined as a group of compounds matching the MX k+1 formula (where M is a metal atom having the maximal formal valence k, while X corresponds to the halogen atom) and characterized by the electron affinity (EA) higher than that of the chlorine atom (EA = 3.62 eV) [2]. It implies that superhalogens form very strongly bound and thermodynamically stable molecular anions [3–5] which are characterized by the enormous values of vertical electron detachment energies (VDEs) approaching 14 eV in certain cases [6, 7]. The very first experimental evidence of the superhalogen existence was provided in 1999 by the Wang’s group who reported the photoelectron spectra of the selected triatomic MX2 − superhalogen anions (M = Li, Na; X = Cl, Br, I) [8]. Since then, many other superhalogen anions [e.g., Na k Cl − k+1 (k = 1–4) and MX3 − (where M = Be, Mg, Ca; X = Cl, Br)] have also been identified experimentally [9, 10]. As indicated by both theoretical predictions and experimental measurements, the original superhalogen formula (MX k+1) can be extended to include the polynuclear M n X nk+1 neutral superhalogen compounds (containing n central atoms) whose corresponding polynuclear M n X − nk+1 anions exhibit even larger electron binding energies than their mononuclear counterparts [11–17]. In addition to numerous applications of superhalogens, we have recently pointed out their possible usage as strong oxidizing agents [18–21] and Lewis–Brønsted superacid precursors [7, 22, 23]. It should also be mentioned that some recent works revealed novel superhalogen applications in Li-ion batteries, solar cells, and hydrogen storage materials [24–27].
It was also established that one of the important features characterizing superhalogen anions is their tendency to adopt high-symmetry compact structures [10]. Recent report concerning the properties of the Li n F − n+1 (n = 2–5) superhalogen anions confirmed that observation, as the most stable isomers of the Li n F − n+1 anions were found to correspond to relatively high-symmetry structures [28]. Hence, one may anticipate the formation of even larger high-symmetry Li n F − n+1 anions whose stability and properties are yet to be determined. Since the positively and negatively charged (LiF) n clusters and their neutral parents are well-known systems [29–33], the knowledge of their equilibrium structures might be of great help while designing the anions containing an additional fluorine atom. The preliminary considerations of the shape and size that one might expect of the Li n F − n+1 structures for n > 5 led us to the Li12F13 − anion that we initially predicted to be large enough to form a high-symmetry negatively charged molecular basket [34]. If such predictions were confirmed, the Li12F13 − anion could be considered useful as a steric shielding agent (enabling the introducing of selected metal ions into various solutions). Since the steric shielding occurs when molecules or molecular clusters are large enough to protect other reactive groups (or atoms) from contact with the chemical environment, this technique remains very common and useful in chemistry [35, 36], e.g., particularly with reference to certain complexes formed by crown ethers [37–40]. Taking into account both the anticipated cage-like equilibrium structure of the Li12F13 − system and its excess negative charge, one might expect an analogous ability regarding this polynuclear superhalogen anion.
In this contribution, we demonstrate the possible use of the Li12F13 − anion as a steric shielding compound with respect to certain metal cations. We believe that the ability of trapping selected positively charged ions by the polynuclear superhalogen anions will turn out to be a general feature of many other high-symmetry superhalogen clusters and will inspire experimental investigations in this direction.
2 Methods
The equilibrium geometries and the harmonic vibrational frequencies of the Li12F13 − superhalogen anion, its corresponding neutral parent (Li12F13), the ionic complexes (Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, Li12F13 −Zn2+), and Li12F13 − fragmentation products were calculated using the second-order Møller–Plesset ab initio perturbational method (MP2) [41, 42] and the 6-311+G(d) basis set [43, 44]. The fourth-order Møller–Plesset method with single, double, and quadruple substitutions employing analytic gradients (MP4(SDQ) [45–47]) with the same 6-311+G(d) basis set were used to estimate binding energies (BEs) in the Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, and Li12F13 −Zn2+ ionic complexes.
Thermodynamic stability of the superhalogen anion Li12F13 − was verified through the analysis of the Gibbs free energies of the most probable fragmentation reactions. The Gibbs free energies (ΔGs) were evaluated by using electronic energies calculated employing the quadratic configuration interaction method including single and double substitutions (QCISD [48–50]) and the 6-311+G(d) basis set together with the zero-point energy corrections, thermal corrections (at T = 298.15 K), and entropy contributions estimated with the MP2 method and 6-311+G(d) basis set.
To test the reliability of the MP4(SDQ) and QCISD methods in estimating electronic energies of the species investigated in this work, the additional calculations using the coupled-cluster method with single, double, and noniterative triple excitations (CCSD(T) [48, 51]) were performed. It was verified that the reaction energies calculated using the CCSD(T) method for two selected Li12F13 − fragmentation processes leading to relatively structurally simple products (i.e., LiF, Li2F2, and Li4F5 −) differ by less than 2–3 % in comparison with those assessed with either MP4(SDQ) or QCISD approaches. Hence, we assumed that employing both the QCISD and MP4(SDQ) methods should result in reproducing proper electronic energy values for the systems studied in this contribution.
The vertical electron detachment energy (VDE) of the Li12F13 − was calculated by applying the outer valence Green function OVGF method (B approximation) [52–60] together with the 6-311+G(3df) basis set. Analogous methods and basis sets have been used for superhalogen anions before and provided an excellent agreement with the experimentally measured VDE values [10, 61]. Due to a fact that the OVGF remains valid only for outer valence ionization for which the pole strengths (PS) are greater than 0.80–0.85 [62], we verified that the PS value obtained for the Li12F13 − anion (0.93) was sufficiently large to justify the use of the OVGF method.
The partial atomic charges were fitted to the electrostatic potential according to the Merz–Singh–Kollman scheme [63]. All calculations were performed with the GAUSSIAN09 (Rev.A.02) package [64].
3 Results
3.1 Li12F13 − superhalogen anion
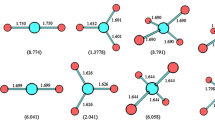
The equilibrium geometry of the Li12F13 − superhalogen anion is presented in Fig. 1. The initial structure for the geometry optimization was assumed to resemble a typical cubic cage (LiF)13 neutral cluster but having one lithium atom detached. The minimum energy structure of the Li12F13 − was found to resemble a C4v-symmetry molecular basket with almost quadratic base (the Li–F–Li valence angles are nearly equal to 90°) and four equivalent walls extended outward (the Li–F–Li valence angles that involve the central F atom are equal to 144.1° and 158.8°). The Li–F bond lengths involving the atoms constituting the base of the molecular basket were found to be in the 1.88–1.90 Å range, while the remaining Li–F bonds span the 1.80–2.07 Å range (see the detailed bond lengths in Fig. 1). Since the Li12F13 − system holds an excess negative charge, one may assume the presence of a negatively charged cavity inside of its structure, which in turn might allow for an effective cation capturing. The cavity size depends on the distance between two central fluorine atoms belonging to the opposite walls of the Li12F13 − equilibrium structure (see the lower part of Fig. 1 where the actual cavity size is shown by presenting the ionic radii of the lithium and fluorine nodes). According to our findings, the mentioned F–F distance is equal to 4.87 Å which indicates that only selected cations (i.e., characterized by the appropriate size) can be placed into the Li12F13 − molecular cavity.
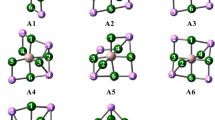
Since any prospective applications involving the Li12F13 − anion require its thermodynamic stability against fragmentation processes, we verified its susceptibility to a few arbitrarily chosen (yet likely most probable) fragmentation reactions. In particular, we chose those routes that lead to the products considered as the most stable and characterized by the highest possible symmetry. The Gibbs free energy (ΔG) values obtained for such selected fragmentation paths span the 57.8–98.1 kcal/mol range which in turn indicates the thermodynamic stability of the Li12F13 − anion against the corresponding reactions; see Table 1. It was also verified that other isomeric structures of the Li12F13 − anion possess higher electronic energies which supports the conclusion that the basket-like structure presented in Fig. 1 indeed corresponds to the global minimum. Namely, the following Li12F13 − isomers were found by testing various initial structures: (1) the quasi-planar layer (consisting of 12 lithium and 13 fluorine atoms) whose electronic energy turned out to be much larger (by 15 kcal/mol) than that of the global minimum structure, (2) the system resembling a regular primitive (simple) crystal structure (with the additional fluorine atom connected to it) whose energy was found to exceed that of the C4v-symmetry molecular basket by ca. 5 kcal/mol (these local minima are presented in Fig. 2). It seems also important to mention that many other attempts of locating different competitive structural isomers of the Li12F13 − anion (performed by starting the geometry optimization process using various initial structures and by using Coalescence Kick (CK) method with the structure-generating algorithm [65]) led to the global minimum structure or to the local minima whose energies exceed that of the global minimum by over 20 kcal/mol.
Even though the evaluation of the electronic stability of the Li12F13 − anion was not the main goal of this work, we provide it here for the reason of completeness. The VDE of the Li12F13 − system was calculated to be 9.85 eV, thus indicating its superhalogen nature. As expected, the vertical electronic stability of Li12F13 − is larger than the VDEs obtained for the Li n F − n+1 (n = 1–4) systems, although slightly smaller than the VDE calculated for the Li5F6 − anion (VDE = 10.18) [28]. One may also notice that the vertical excess electron detachment energy of the Li12F13 − anion is comparable to that of the well-known tetrahedral superhalogen AlF4 − anion (VDE = 9.79 eV, as calculated at the same level of theory) [11]. VDEs of all Li12F13 − minima described in this section are presented in Table 2.
3.2 Ionic complexes with selected metal ions
After the description of the Li12F13 − superhalogen anion we now present and discuss the results of our investigation concerning its potential capability to act as an effective steric shielding agent. As indicated in the preceding section, the size of the cavity formed inside the Li12F13 − structure (given by the separation between the F atoms located in the centers of the opposite walls of the molecular cage) can be estimated as equal to 4.87 Å; however, taking into account the radii of fluorine atoms constituting the structure, the actual size of the cavity should be considered as not exceeding 3 Å; see Fig. 1. Hence, it might be expected that certain metal cations whose radii are small enough to fit, can be embedded into the Li12F13 − system. In order to verify this hypothesis we selected four cations having different ionic radii, i.e., Na+ (r = 1.02 Å), K+ (r = 1.51 Å), Mg2+ (r = 0.72 Å), and Zn2+ (r = 0.74 Å), to examine the steric shielding ability of the Li12F13 − anion.
The equilibrium geometries of the Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, and Li12F13 −Zn2+ ionic complexes are depicted in Fig. 3. Alike the isolated Li12F13 − superhalogen anion, all these minimum energy structures correspond to C4v-symmetry. Each cation is located inside the cavity formed by the Li12F13 − structure; however, the monocations (Na+ and K+) are situated closer to its edge, whereas the dications (Mg2+ and Zn2+) are embedded deeper (see the interatomic distances between metal ions and selected fluorine atoms presented in Fig. 2). The partial atomic charges localized on the Na, K, Mg, and Zn atoms are equal to +0.5, +0.7, +0.7, and +1.3 a.u., respectively, thus indicating that their excess positive charge is preserved. The structure of the Li12F13 − moiety in each complex is only slightly deformed in comparison with the basket-like structure of the Li12F13 − isolated anion. Namely, the lengths of the Li–F bonds change by less than 0.2 Å when the Li12F13 − relaxes its structure while interacting with any of the cations studied (see Figs. 1, 2 for the details). The largest structural effect of the cation insertion is observed for both Mg2+ and Zn2+ dications whose interactions with the Li12F13 − lead to the spatial extension of the anionic basket. This extension is clearly visible when the Li–F bond lengths between the nodes constituting the quasi-square cavity base are concerned; namely, the distance between the central F atom and any of the Li atoms is elongated to 2.02 and 2.03 Å in Li12F13 −Mg2+ and Li12F13 −Zn2+, respectively, whereas the analogous distance is shorter for both Li12F13 −Na+ and Li12F13 −K+ (1.93 Å); see Fig. 3. Clearly, these effects are caused by the deeper location of the Mg2+ and Zn2+ dications in the Li12F13 − cavity in comparison with the Na+ and K+ monocations.
The equilibrium structures of the Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, and Li12F13 −Zn2+ ionic complexes indicate that the Li12F13 − superhalogen anion can bind selected metal ions inside its basket-like structure and form geometrically stable ionic complexes. Hence, the Li12F13 − anion might be expected to play an important role in the steric shielding process. It should be stressed that not only cations from the first and second groups of the periodic table but even the transition metals (e.g., Zn2+) can be inserted inside the Li12F13 cage; see Fig. 3. These conclusions are also supported by the analysis of the calculated binding energies between the ionic complex subunits. The BE values (calculated as the difference between the energy of the complex and those of isolated ions) are in the range of 83.5–426.5 kcal/mol; see Table 3. It needs to be emphasized that all examined complexes are very strongly bound ionic compounds (binding energies exceed 83 kcal/mol), but those involving dications are obviously more strongly bound systems than the complexes formed by trapping either sodium or potassium ions. The difference between binding energies of Li12F13 −Na+ and Li12F13 −K+ systems (ca. 22 kcal/mol) is likely caused by the larger size of potassium cation as the tight packing of atoms in the case of Li12F13 −K+ structure may result in destabilizing valence repulsion effects which reduce the BE value; see Fig. 4. In contrast, analogous difference in binding energy between Li12F13 −Mg2+ and Li12F13 −Zn2+ compounds (ca. 20 kcal/mol) may be caused by the larger positive partial atomic charge located on the Zn atom (+1.3 a.u.) in comparison with the Mg atom (+0.7 a.u.).
Due to the fact that each of the Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, and Li12F13 −Zn2+ represents a strongly bound ionic complex whose equilibrium structure consists of a cation hidden inside the Li12F13 − trap, none of these metal ions can directly react with molecular species in the surrounding area. Therefore, the Li12F13 − anion itself can be treated as a promising steric shielding agent with respect to selected metal ions (whose ionic radius does not exceed that of K+). This novel property of the polynuclear high-symmetry superhalogen anions (which the Li12F13 − anion is a representative example of) might be utilized in the future and, analogously to crown ethers, could enable the entering of metal ions into various solutions.
4 Conclusions
The electronic and thermodynamic stability of the polynuclear Li12F13 − anion and its possible application as an effective steric shielding system embedding selected metal cations (Na+, K+, Mg2+, and Zn2+) were investigated on the basis of the ab initio calculations carried out at the MP2/6-311+G(d) level (for equilibrium geometries and force constants) and the QCISD/6-311+G(d) and MP4(SDQ)/6-311+G(d) levels (for electronic energies). The analysis of the results led us to the following conclusions:
-
1.
The Li12F13 − system is a strongly bound and thermodynamically stable polynuclear superhalogen anion whose vertical excess electron detachment energy was evaluated as equal to 9.85 eV.
-
2.
The equilibrium geometry of the Li12F13 − anion corresponds to the high-symmetry (C4v) compact structure and resembles a molecular basket with the negatively charged cavity inside. The size of the cavity (about 3 Å) allows for trapping selected metal ions (Na+, K+, Mg2+, and Zn2+).
-
3.
The ionic Li12F13 −Na+, Li12F13 −K+, Li12F13 −Mg2+, and Li12F13 −Zn2+ are characterized by the large binding energies (83.5–426.5 kcal/mol) between the Li12F13 − and M+/2+ components.
-
4.
In each equilibrium structure of the complexes investigated the cation is hidden inside the Li12F13 − molecular trap and thus cannot react with any species in the surrounding area. Therefore, the Li12F13 − anion can be considered as a steric shielding agent with respect to selected metal ions.
References
Gutsev GL, Boldyrev AI (1981) Chem Phys 56:277–283
Hotop H, Lineberger WC (1985) J Phys Chem Ref Data 14:731–750
Gutsev GL, Boldyrev AI (1987) Russ Chem Rev 56:519–531
Sobczyk M, Sawicka A, Skurski P (2003) Eur J Inorg Chem 2003:3790–3797
Anusiewicz I (2008) Aust J Chem 61:712–717
Freza S, Skurski P (2010) Chem Phys Lett 487:19–23
Czapla M, Skurski P (2015) J Phys Chem A 119:12868–12875
Wang XB, Ding CF, Wang LS, Boldyrev AI, Simons J (1999) J Chem Phys 110:4763–4771
Elliott BM, Koyle E, Boldyrev AI, Wang XB, Wang LS (2005) J Phys Chem A 109:11560–11567
Aleksandrowa AN, Boldyrev AI, Fu YJ, Yang X, Wang XB, Wang LS (2004) J Chem Phys 121:5709–5719
Sikorska C, Skurski P (2012) Chem Phys Lett 536:34–38
Ko YJ, Wang H, Pradhan K, Koirala P, Kandalam AK, Bowen KH, Jena P (2011) J Chem Phys 135:244312
Li Y, Zhang S, Wang Q, Jena P (2013) J Chem Phys 138:054309
Wu MM et al (2011) Angew Chem Int Ed 50:2568–2572
Yin B, Li J, Bai H, Wen Z, Jiang Z, Huang Y (2012) Phys Chem Chem Phys 14:1121–1130
Ding LP, Kuang XY, Shao P, Zhong MM, Zhao YR (2013) J Chem Phys 139:104304
Paduani C, Jena P (2012) J Phys Chem A 116:1469–1474
Czapla M, Freza S, Skurski P (2015) Chem Phys Lett 619:32–35
Sikorska C, Skurski P (2011) Inorg Chem 50:6384–6391
Marchaj M, Freza S, Skurski P (2014) Chem Phys Lett 612:172–176
Marchaj M, Freza S, Rybacka O, Skurski P (2013) Chem Phys Lett 574:13–17
Czapla M, Skurski P (2015) Chem Phys Lett 630:1–5
Czapla M, Anusiewicz I, Skurski P (2016) Chem Phys 465–466:46–51
Giri S, Behera S, Jena P (2014) Angew Chem Int Ed 53:13916–13919
Jena P (2015) J Phys Chem Lett 6:1119–1125
Fang H, Jena P (2016) J Mater Chem A 4:4728–4737
Fang H, Jena P (2016) J Phys Chem Lett 7:1596–1603
Wileńska D, Skurski P, Anusiewicz I (2014) J Fluor Chem 168:99–104
Kölmel C, Ewig CS (2001) J Phys Chem B 105:8538–8543
Fernandez-Lima FA, Neto OPV, Pimentel AS, Pacheco MAC, Ponciano CR, Nascimento MAC, da Silveira EF (2009) J Phys Chem A 113:15031–15040
Fernandez-Lima FA, Neto OPV, Pimentel AS, Pacheco MAC, Ponciano CR, Nascimento MAC, da Silveira EF (2009) J Phys Chem A 113:1813–1821
Wu SX, Kan YH, Li HB, Zhao L, Wu Y, Su ZM (2015) J Phys Chem Lett 6:2950–2958
Sangthong W, Bromley ST, Illas F, Limtrakul J (2009) Nanotechnology 3:304–307
Hermann K, Ruan Y, Hardin AM, Hadad CM, Badjić JD (2015) Chem Soc Rev 44:500–514
West R, Fink MJ, Michl J (1981) Science 214:1343–1344
Vonnemann J, Liese S, Kuehne C, Ludwig K, Dernedde J, Böttcher C, Netz RR, Haag R (2015) J Am Chem Soc 137:2572–2579
Takeda Y, Kohno R, Kudo Y, Fukada N (1989) Bull Chem Soc Jpn 62:999–1003
Blair SM, Brodbelt JS, Marchand AP, Chong HS, Alihodžić S (2000) J Am Soc Mass Spectrom 11:884–891
Kumondai K, Toyoda M, Ishihara M, Katakuse I, Takeuchi T, Ikeda M, Iwamoto K (2005) J Chem Phys 123:024314
Inokuchi Y, Ebata T, Ikeda T, Haino T, Kimura T, Guo H, Furutani Y (2015) New J Chem 39:8673–8680
Head-Gordon M, Pople JA, Frisch MJ (1988) Chem Phys Lett 153:503–506
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166:275–280
McLean AD, Chandler GS (1980) J Chem Phys 72:5639–5648
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
Raghavachari K, Pople JA (1978) Int J Quantum Chem 14:91–100
Trucks GW, Watts JD, Salter EA, Bartlett RJ (1988) Chem Phys Lett 153:490–495
Trucks GW, Salter EA, Sosa C, Bartlett RJ (1988) Chem Phys Lett 147:359–366
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968–5975
Gauss J, Cremer D (1988) Chem Phys Lett 150:280–286
Salter EA, Trucks GW, Bartlett RJ (1989) J Chem Phys 90:1752–1766
Purvis GD III, Bartlett RJ (1982) J Chem Phys 76:1910–1918
Zakrzewski VG, Ortiz JV, Nichols JA, Heryadi D, Yeager DL, Golab JT (1996) Int J Quantum Chem 60:29–36
Simons J (1971) J Chem Phys 55:1218–1230
Ortiz JV (1988) J Chem Phys 89:6348–6352
Rowe DJ (1968) Rev Mod Phys 40:153–166
Cederbaum LS (1975) J Phys B At Mol Phys 8:290–303
Simons J (1972) J Chem Phys 57:3787–3792
Simons J, Smith WD (1973) J Chem Phys 58:4899–4907
Zakrzewski VG, Ortiz JV (1995) Int J Quantum Chem 53:583–590
Zakrzewski VG, Ortiz JV (1994) Int J Quantum Chem 52:23–27
Sikorska C, Ignatowska D, Freza S, Skurski P (2011) J Theor Comput Chem 10:93–109
Zakrzewski VG, Dolgounitcheva O, Ortiz JV (1996) J Chem Phys 105:8748–8753
Besler BH, Merz KM Jr, Kollman PA (1990) J Comput Chem 11:431–439
Frisch MJ et al (2009) Gaussian09 (Rev.A.02). Gaussian, Inc, Wallingford
Sergeeva AP, Averkiev BB, Zhai HJ, Boldyrev AI, Wang LS (2011) J Chem Phys 134:224304
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education (Grant Numbers: DS 530-8375-D499-16, BMN/538-8375-B025-15, and BMN/538-8375-B025-16). The calculations have been carried out using resources provided by Wroclaw Centre for Networking and Supercomputing (http://wcss.pl). Grant No. 350.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Czapla, M. Polynuclear Li12F13 − anion as a steric shielding agent with respect to selected metal ions. Theor Chem Acc 135, 231 (2016). https://doi.org/10.1007/s00214-016-1992-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1992-8