Abstract
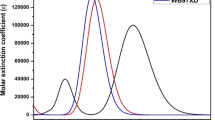
Quantum chemical calculations were carried out by using density functional theory and time-dependant density functional theory at B3LYP/6-31G(d) and TD-B3LYP/6-31G(d) level of theories. The absorption spectra have been computed with and without solvent. The calculated absorption spectra in ethanol, acetonitrile, and methanol are in good agreement with experimental evidences. The absorption spectra are red shifted compared to System1. On the basis of electron injection and electronic coupling constant, we have shed light on the nature of different sensitizers. The coplanarity between the benzene near anchoring group having LUMO and the bridge (N–N) is broken in System6 and System7 that would hamper the recombination process. The electron injection of System2–System10 is superior to System1. The highest electronic coupling constant has been observed for System6 that followed the System7 and System8. The light-harvesting efficiency of all the sensitizers enlarged in acetonitrile and ethanol. The long-range-corrected functional (LC-BLYP), Coulomb-attenuating method (CAM-B3LYP), and BH and HLYP functional underestimate the excitation energies while B3LYP is good to reproduce the experimental data. Moreover, we have investigated the effect of cyanoacetic acid as anchoring group on the electron injection.



Similar content being viewed by others
References
Fabregat-Santiago F, Garcia-Canadas J, Palomares E, Clifford JN, Haque SA, Durrant JR, Garcia-Belmonte G, Bisquert J (2004) The origin of slow electron recombination processes in dye-sensitized solar cells with alumina barrier coatings. J Appl Phys 96:6903–6907
Figgemeier E, Hagfeldt A (2004) Are dye-sensitized nano-structured solar cells stable? An overview of device testing and component analyses. Int J Photoenergy 6:127–140
Furube A, Katoh R, Yoshihara T, Hara K, Murata S, Arakawa H, Tachiya M (2004) Ultrafast direct and indirect electron-injection processes in a photoexcited dye-sensitized nanocrystalline zinc oxide film: the importance of exciplex intermediates at the surface. J Phys Chem B 108:12583–12592
Hongwei H, Xingzhong Z, Jian L (2005) Enhancement in photoelectric conversion properties of the dye-sensitized nanocrystalline solar cells based on the hybrid TiO2 electrode. J Electrochem Soc 152:A164–A166
Kim JH, Kang M-S, Kim YJ, Won J, Park N-G, Kang YS (2004) Dye-sensitized nanocrystalline solar cells based on composite polymer electrolytes containing fumed silica nanoparticles. Chem Commun 1662–1663
Miyasaka T, Kijitori Y (2004) Low-temperature fabrication of dye-sensitized plastic electrodes by electrophoretic preparation of mesoporous TiO2 layers. J Electrochem Soc 151:A1767–A1773
O’Reagan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dyesensitized colloidal TiO2 films. Nature 353:737–740
Grätzel M (2001) Photoelectrochemical cells. Nature 414:338–344
Grätzel M (2004) Corrigendum to “Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells”. J Photochem Photobiol, A 168:235
Nazeeruddin MK, Kay A, Rodicio L, Humphry-Baker R, Muller E, Liska P, Vlachopoulos N, Grätzel M (1993) Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc 115:6382–6390
Nazeeruddin MK, Pe′chy P, Renouard T, Zakeeruddin SM, Humphry-Baker R, Comte P, Liska P, Cevey L, Costa E, Shklover V, Spiccia L, Deacon GB, Bignozzi CA, Grätzel M (2001) Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J Am Chem Soc 123:1613–1624
Chen C-Y, Wu S-J, Li J-Y, Wu C-G, Chen J-G, Ho K-C (2007) A new route to enhance the light-harvesting capability of ruthenium complexes for dye-sensitized solar cells. Adv Mater 19:3888–3891
Chen C-Y, Chen J-G, Wu S-J, Li J-Y, Wu C-G, Ho K-C (2008) Multifunctionalized ruthenium-based super sensitizers for highly efficient dye-sensitized solar cells. Angew Chem Int Ed 47:7342–7345
Ito S, Zakeeruddin M, Hummphrey-Baker R, Liska P, Charvet R, Comte P, Nazeeruddin MK, Pe′chy P, Takata M, Miura H, Uchida S, Gratzel M (2006) High-efficiency organic-dye-sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv Mater 18:1202–1205
Choi H, Baik C, Kang SO, Ko J, Kang M-S, Nazeeruddin MK, Grätzel M (2008) Highly efficient and thermally stable organic sensitizers for solvent-free dye-sensitized solar cells. Angew Chem Int Ed 47:327–330
Zhang G, Bala H, Cheng Y, Shi D, Lv X, Yu Q, Wang P (2009) High efficiency and stable dye-sensitized solar cells with an organic chromophore featuring a binary π-conjugated spacer. Chem Commun 2198–2200
Wang Z-S, Li F-Y, Huang C-H (2001) Photocurrent enhancement of hemicyanine dyes containing RSO3-group through treating TiO2 films with hydrochloric acid. J Phys Chem B 105:9210–9217
Thomas KRJ, Lin JT, Hsu Y-C, Ho K-C (2005) Organic dyes containing thienylfluorene conjugation for solar cells. Chem Commun 4098–4100
Tian H, Yang X, Chen R, Pan Y, Li L, Hagfeldt A, Sun L (2007) Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem Commun 3741–3743
Li S-L, Jiang K-J, Shao K-F, Yang L-M (2006) Novel organic dyes for efficient dye-sensitized solar cells. Chem Commun 2792–2794
Wang Z-S, Cui Y, Dan-oh Y, Kasada C, Shinpo A, Hara K (2007) Thiophene-functionalized coumarin dye for efficient dye-sensitized solar cells: electron lifetime improved by coadsorption of deoxycholic acid. J Phys Chem C 111:7224–7230
Ito S, Miura H, Uchida S, Takata M, Sumioka K, Liska P, Comte P, Pechy P, Gratzel M (2008) High-conversion-efficiency organic dye-sensitized solar cells with a novel indoline dye. Chem Commun 5194–5196
Hara K, Kurashige M, Dan-oh Y, Kasada C, Shinpo A, Suga S, Sayama K, Arakawa H (2003) Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells. New J Chem 27:783–785
Mishra A, Fischer MKR, Bauerle P (2009) Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew Chem Int Ed 48:2474–2499
De Angelis F, Fantacci S, Selloni A (2004) Time-dependent density functional theory study of the absorption spectrum of [Ru(4,4′-COOH-2,2′-bpy)2(NCS)2] in water solution: influence of the pH. Chem Phys Lett 389:204–208
De Angelis F, Fantacci S, Selloni A, Nazeeruddin MK (2005) Time dependent density functional theory study of the absorption spectrum of the [Ru(4,4′-COO−-2,2′-bpy)2(X)2]4− (X = NCS, Cl) dyes in water solution. Chem Phys Lett 415:115–120
Xu Y, Chen WK, Cao MJ, Liu SH, Li JQ, Philippopoulos AI, Falaras P (2006) A TD-DFT study on the electronic spectrum of Ru(II)L2 [L = bis(5′-methyl-2,2′-bipyridine-6-carboxylato)] in the gas phase and DMF solution. Chem Phys 330:204–211
Ito S, Zakeeruddin SM, Humphry-Baker R, Liska P, Charvet R, Comte P, Nazeeruddin MK, Péchy P, Takata M, Miura H, Uchida S, Grätzel M (2006) High-efficiency organic-dye- sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv Mater 18:1202–1205
De Angelis F, Fantacci S, Selloni A, Grätzel M, Nazeeruddin MK (2007) Influence of the sensitizer adsorption mode on the open-circuit potential of dye-sensitized solar cells. Nano Lett 7:3189–3195
De Angelis F, Fantacci S, Selloni A, Nazeeruddin MK, Grätzel M (2007) Time-dependent density functional theory investigations on the excited states of Ru(II)-dye-sensitized TiO2 nanoparticles: the role of sensitizer protonation. J Am Chem Soc 129:14156–14157
De Angelis F, Fntacci S, Selloni A (2008) Alignment of the dye’s molecular levels with the TiO2 band edges in dye-sensitized solar cells: a DFT–TDDFT study. Nanotechnology 19:424002–424009
Di Censo D, Fantacci S, De Angelis F, Klein C, Evans N, Kalyanasundaram K, Bolink HJ, Grätzel M, Nazeeruddin MK (2008) Synthesis, characterization, and DFT/TD-DFT calculations of highly phosphorescent blue light-emitting anionic iridium complexes. Inorg Chem 47:980–989
Kurashige Y, Nakajima T, Kurashige S, Hirao K, Nishikitani Y (2007) Theoretical investigation of the excited states of coumarin dyes for dye-sensitized solar cells. J Phys Chem A 111:5544–5548
Balanay MP, Kim DH (2008) DFT/TD-DFT molecular design of porphyrin analogues for use in dye-sensitized solar cells. Phys Chem Chem Phys 10:5121–5127
Satoh N, Cho JS, Higuchi M, Yamamoto K (2003) Novel triarylamine dendrimers as a hole-transport material with a controlled metal-assembling function. J Am Chem Soc 125:8104–8105
Satoh N, Nakashima T, Yamamoto K (2005) Metal-assembling dendrimers with a triarylamine core and their application to a dye-sensitized solar cell. J Am Chem Soc 127:13030–13038
Wang Q, Zakeeruddin SM, Cremer J, Bäuerle P, Humphry-Baker R, Gratzel M (2005) Cross surface ambipolar charge percolation in molecular triads on mesoscopic oxide films. J Am Chem Soc 127:5706–5713
Al-Sehemi AG, Irfan A, Asiri AM, Ammar YA (2012) Synthesis, characterization and DFT study of methoxybenzylidene containing chromophores for DSSC materials. Spectrochimica Acta Part A. doi:10.1016/j.saa.2012.01.016
Al-Sehemi AG, Irfan A (2012) Dye-Sensitized nanocrystalline TiO2 solar cell: toward high sun light to electricity conversion (submitted)
Stein T, Kronik L, Baer R (2009) Prediction of charge-transfer excitations in coumarin-based dyes using a range-separated functional tuned from first principles. J Chem Phys 131:244119–244123
Wong BM, Piacenza M, Sala FD (2009) Absorption and fluorescence properties of oligothiophene biomarkers from long-range-corrected time-dependent density functional theory. Phys Chem Chem Phys 11:4498–4508
Wong BM, Cordaro JG (2008) Coumarin dyes for dye-sensitized solar cells: a long-range-corrected density functional study. J Chem Phys 129:214703–214710
Lange AW, Rohrdanz MA, Herbert JM (2008) Charge-transfer excited states in a π-stacked adenine dimer, as predicted using long-range-corrected time-dependent density functional theory. J Phys Chem B 112:6304–6308
Rohrdanz MA, Herbert JM (2008) Simultaneous benchmarking of ground- and excited-state properties with long-range-corrected density functional theory. J Chem Phys 129:034107–034115
Toulouse J, Colonna F, Savin A (2005) Short-range exchange and correlation energy density functionals: beyond the local-density approximation. J Chem Phys 122:014110–014119
Livshits E, Baer R (2007) A well-tempered density functional theory of electrons in molecules. Phys Chem Chem Phys 9:2932–2941
Preat J (2010) Photoinduced energy-transfer and electron-transfer processes in dye-sensitized solar cells: TDDFT insights for triphenylamine dyes. J Phys Chem C 114:16716–16725
Preat J, Michaux C, Jacquemin D, Perpète EA (2009) Enhanced efficiency of organic dye-sensitized solar cells: Triphenylamine derivatives. J Phys Chem C 113:16821–16833
Magyar RJ, Tretiak S (2007) Dependence of spurious charge-transfer excited states on orbital exchange in TDDFT: large molecules and clusters. J Chem Theory Comput 3:976–987
Irfan A, Al-Sehemi AG (2012) Donor-bridge-acceptor effect on the electron injection in triphenylamine based sensitizers: density functional theory investigations (submitted)
Irfan A, Al-Sehemi AG (2012) Quantum chemical investigations of the electron injection in triphenylamine based sensitizers (submitted)
Peach MJG, Benfield P, Helgaker T, Tozer DJ (2008) Excitation energies in density functional theory: an evaluation and a diagnostic test. J Chem Phys 128:044118
Bertolino CA, Ferrari AM, Barolo C, Viscardi G, Caputo S, Coluccia G (2006) Solvent effect on indocyanine dyes: a computational approach. Chem Phys 330:52–59
Jacquemin D, Perpète EA, Scalmani G, Frisch MJ, Kobayashi R, Adamo C (2007) Assessment of the efficiency of long-range corrected functionals for some properties of large compounds. J Chem Phys 126:144105
Guillaumont D, Nakamura S (2000) Calculation of the absorption wavelength of dyes using time-dependent density-functional theory (TD-DFT). Dyes Pigment 46:85–92
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Lynch BJ, Fast PL, Harris M, Truhlar DG (2000) Adiabatic connection for kinetics. J Phys Chem A 104:4811–4815
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687
Walsh PJ, Gordon KC, Officer DL, Campbell WM (2006) A DFT study of the optical properties of substituted Zn(II)TPP complexes. J Mol Struct THEOCHEM 759:17–24
Cleland DM, Gordon KC, Officer DL, Wagner P, Walsh PJ (2009) Tuning the optical properties of ZnTPP using carbonyl ring fusion. Spectrochimica Acta Part A 74:931–935
Zhang CR, Liang WZ, Chen HS, Chen YH, Wei ZQ, Wu YZ (2008) Theoretical studies on the geometrical and electronic structures of N-methyle-3,4-fulleropyrrolidine. J Mol Struct THEOCHEM 862:98–104
Sun J, Song J, Zhao Y, Liang WZ (2007) Real-time propagation of the reduced one-electron density matrix in atom-centered Gaussian orbitals: application to absorption spectra of silicon clusters. J Chem Phys 127:234107–234113
Matthews D, Infelta P, Grätzel M (1996) Calculation of the photocurrent-potential characteristic for regenerative, sensitized semiconductor electrodes. Sol Energy Mater Sol Cells 44:119–155
Frisch MJ, et al. (2009) Gaussian 09, revision A.1. Gaussian, Inc., Wallingford
Cossi M, Barone V (2001) Time-dependent density functional theory for molecules in liquid solutions. J Chem Phys 115:4708–4717
Amovilli C, Barone V, Cammi R, Cancès E, Cossi M, Mennucci B, Pomelli CS, Tomasi J (1998) Recent advances in the description of solvent effects with the polarizable continuum model. Adv Quantum Chem 32:227–261
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094
Preat J, Jacquemin D, Perpete E (2010) Design of new triphenylamine-sensitized solar cells: a theoretical approach. Environ Sci Technol 44:5666–5671
Preat J (2010) Photoinduced energy-transfer and electron-transfer processes in dye-sensitized solar cells: TDDFT insights for triphenylamine dyes. Sol Energy Mater Sol Cells 114:16716–16725
Pourtois G, Beljonne J, Ratner MA, Bredas JL (2002) Photoinduced electron-transfer processes along molecular wires based on phenylenevinylene oligomers: a quantum-chemical insight. J Am Chem Soc 124:4436–4447
Hsu C (2009) The electronic couplings in electron transfer and excitation energy transfer. Acc Chem Res 42:509–518
Marcus RA (1993) Electron transfer reactions in chemistry. Theory and experiment. Rev Mod Phys 65:599–610
Asbury JB, Wang YQ, Hao E, Ghosh H, Lian T (2001) Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res Chem Intermed 27:393–406
Katoh R, Furube A, Yoshihara T, Hara K, Fujihashi G, Takano S, Murata S, Arakawa H, Tachiya M (2004) Efficiencies of electron injection from excited N3 dye into nanocrystalline semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) films. J Phys Chem B 108:4818–4822
Hagfeldt A, Grätzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95:49–68
Barbara PF, Meyer TJ, Ratner MA (1996) Contemporary issues in electron transfer research. J Phys Chem 100:13148–13168
Nalwa HS (2001) Handbook of advanced electronic and photonic materials and devices. Academic, San Diego
Cassida M (1995) Recent advances in density functional methods: time dependent density functional response theory for molecules; DP Chong, Singapore
Harris DC, Bertolucci MD (1998) Symmetry and spectroscopy. Dover, New York
Liu D, Fessenden RW, Hug GL, Kamat PV (1997) Dye capped semiconductor nanoclusters. Role of back electron transfer in the photosensitization of SnO2 nanocrystallites with cresyl violet aggregates. J Phys Chem B 101:2583–2590
Ning Z, Zhang Q, Wu W, Pei H, Liu B, Tian H (2008) Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J Org Chem 73:3791–3797
Acknowledgments
The present work has been carried out under project No. 08-NAN155-7 funded by KAUST (King Abdulaziz City for Science and Technology) through the Long Term Comprehensive National Plan for Science, Technology and Innovation program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Sehemi, A.G., Irfan, A. & Asiri, A.M. The DFT investigations of the electron injection in hydrazone-based sensitizers. Theor Chem Acc 131, 1199 (2012). https://doi.org/10.1007/s00214-012-1199-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1199-6