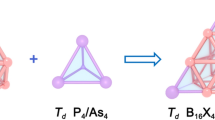
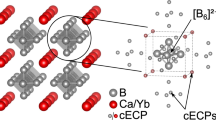
Abstract
Tight-binding calculations with an extended Hückel Hamiltonian were performed on Ba2/3Pt3B2 and LuOs3B2. Hypothetical linear metal boride chains present in these materials are analyzed with a three-dimensional model that contains a trigonal bipyramidal T3B2 (T = transition metal) building unit for the compounds. The geometrical structure for the T3B2 trigonal bipyramids depends on the number of electrons. For systems that have greater than 36 electrons in its trigonal bipyramidal building unit, a structural distortion is expected. Electron back donation from the electron-rich M3 fragment to the empty e′ set on B2 creates boron–boron interaction along the z-axis. Boron–boron pairing then participates as an electron sink and causes a trigonal distortion of the platinum Kagome net. On the other hand, a system with <35 electrons should have an undistorted, CeCo3B2 type structure. The electronic factors that create the breathing motion are discussed and analyzed with the aid of molecular and solid-state models. The metal–metal bonding associated with the structural properties also has been examined.









Similar content being viewed by others
References
Nesper R (1991) Angew Chem Int Ed 30:788
Cotton FA (1966) Q Rev Chem Soc 20:389
Cotton FA, Murillo CA, Walton RA (2005) Multiple bonds between metal atoms, 3rd edn. Springer, New York
Mingos DMP, Wales DJ (1990) Introduction to cluster chemistry. Prentice Hall, Englewood Cliffs
Matthias BT, Corenzwit E, Vandenberg JM, Barz H (1977) Proc Natl Acad Sci USA 74:1334
Vandenberg JM, Matthias BT (1977) Proc Natl Acad Sci USA 74:1336
Shenoy GK, Dunlap BD, Fradin FY (1981) Ternary superconductors. Elsevier, New York
Kuz’ma YuB, Krip’yakevich PI, Biloninhko NS (1969) Dopovidi Akad Nauk USSR A10:939
Niihara K, Yashima S (1973) Bull Chem Soc Jpn 46:770
Rogl P (1973) Monatsh Chem 104:1623
Rogl P (1975) Monatsh Chem 106:1624
Valovka IP, Kuz’ma YuB (1978) Inorg Mater 14:356
Johnston DC (1977) Solid State Commun 24:699
Ku HC, Meisner GP, Acker F, Johnston DC (1980) Solid State Commun 35:91
Ku HC, Meisner GP (1981) J Less-Common Met 78:99
Vandenberg JM, Barz H (1980) Mater Res Bull 15:1493
Barz H (1980) Mater Res Bull 15:1489
Voroshilov YuV, Kripyakevich PI, Kuz’ma YuB (1971) Sov Phys Crystallogr 15:813
Rogl P (1980) J Nucl Mater 92:292
Shelton RN (1978) J Less-Common Met 62:191
Jung W, Quentmeier D (1984) Z Kristallogr 151:172
Wells AF (1977) Three-dimensional nets and polyhedra. Wiley, New York
Hahn T (ed) (1983) International tables for crystallography, vol. A: space-group symmetry. Reidel Dordrecht, Netherlands
Laves F (1967) In: Westbrook JH (ed) Intermetallic compounds. Wiley, New York, p 129
Pearson WB (1972) The crystal chemistry and physics of metals and alloys. Wiley, New York
Johnston RL, Hoffmann R (1990) Polyhedron 9:1901
Johnston RL, Hoffmann R (1992) Z Anorgan Allgem Chem 616:105
Cenzual K, Chabot B, Parthe E (1988) Acta Cryst C44:221
Voroshilov YuV, Krypyakevich PI, Kuz’ma YuB (1971) Sov Phys Cryst 15:813
Jung W, Quentemier D (1980) Z Krist 151:172
Hiebl K, Rogl P, Uhl E, Sienko MJ (1980) Inorg Chem 19:3316
Chevalier B, Cole A, Lejay P, Etourneau J (1981) Mater Res Bull 16:1067
Vandenberg JM, Barz H (1980) ibid 15:1493
Umarji AM, Dhar SK, Malik SK, Vijayaraghavan R (1987) Phys Rev B 36:8929
Bailey MS, Lobkovsky EB, Hicks DG, Claus H, Hor YS, Schlueter JA, Mitchell JF (2007) J Solid State Chem 180:1333
Mirgel R, Jung WJ (1988) Less-Common Met 144:87
Takegahara K, Harima H, Kasuya T (1985) J Phys Soc Jpn 54:4743
Wade K (1976) Adv Inorg Chem Radiochem 9:446
Mingos DMP (1984) Acc Chem Res 17:311
Lauher JW (1978) J Am Chem Soc 100:5305
Jemmis ED, Jayasree EG (2003) Acc Chem Res 36:816
Jemmis ED, Balakrishnarajan MM, Pancharatna PD (2002) Chem Rev 102:93
King RB (1990) Inorg Chem 29:2164 has used a different electron counting scheme but also obtains the same number of valence electrons for the trigonal bipyramidal clusters. With 18 electrons, each edge then could be considered to be a localized two center—two electron bond
Dahr SK, Malik SK, Vijayaragavan R (1981) J Phys C: Solid State Phys 14:L321
Oesterreicher H, Parker FT, Misroch M (1977) Appl Phys 12:287
Whangbo M-H, Hoffmann R, Woodward RB (1979) Proc R Soc London Ser A 366:23
Hoffmann R (1963) J Chem Phys 39:1397
Hoffmann R, Lipscomb WN (1962) J Chem Phys 36:2179
Ammeter JH, Bürgi H-B, Thiebeault JC, Hoffmann R (1978) J Am Chem Soc 100:3686
Taylor NJ, Chieh PC, Carty AJ (1975) J Chem Soc, Chem Commun 448
Longoni G, Chini P (1976) J Am Chem Soc 98:7225
Cotton FA, Haas TE (1964) Inorg Chem 3:10
Wei CH, Dahl LF (1968) J Am Chem Soc 90:3960
Ruff JK (1971) ibid 93:2159
Lauher JW (1978) ibid 100:5305
Dedieu A, Hoffmann R (1978) ibid 100:2074
Schilling BER, Hoffmann R (1979) ibid 101:3456
Evans DG, Hughes GR, Mingos DMP, Bassett J-M, Welch AJ (1980) J Chem Soc Chem Commun 1255
Delley B, Manning MC, Ellis DE, Berkowitz J, Trogler WC (1982) Inorg Chem 21:2247
Rives AB, Xiao-Zeng Y, Fenske RF (1982) ibid 21:2286
Pacchioni G, Fantucci D, Valenti V (1982) Inorg Chem Acta 224:89
Fantucci P, Pacchioni G, Valenti V (1984) Inorg Chem 23:247
Chang KW, Wooley RG (1979) J Phys C: Solid State Phys 12:2745
Bullet DW (1985) Chem Phys Lett 115:450
Underwood DJ, Hoffmann R, Tatsumi K, Nakamura A, Yamamoto Y (1985) J Am Chem Soc 107:5968
Burdett JK, Canadell E, Miller GJ (1986) J Am Chem Soc 108:6561
Whangbo M-H, Lee C, Köhler J (2006) Angew Chem Int Ed 45:7545
Acknowledgments
We wish to thank the Robert A. Welch Foundation and the Texas Center for Superconducivity at the University of Houston for support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Eluvathingal Jemmis and published as part of the special collection of articles celebrating his 60th birthday; dedicated also in memoriam of Dr. Seeyearl Seong.
Seeyearl Seong: Deceased.
Rights and permissions
About this article
Cite this article
Seong, S., Choi, S.Y. & Albright, T.A. The bonding in hexagonal Ba2/3Pt3B2 and CeCo3B2 type ternary metal borides. Theor Chem Acc 131, 1091 (2012). https://doi.org/10.1007/s00214-012-1091-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1091-4