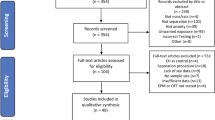
Abstract
Rationale
Unconditioned tasks in rodents have been the mainstay of behavioural assessment for decades, but their validity and sensitivity to detect the behavioural consequences of early life stress (ELS) remains contentious and highly variable.
Objectives
In the present study, we carried out a meta-analysis to investigate whether persistent behavioural effects, as assessed using unconditioned procedures in rats, are a reliable consequence of early repeated maternal separation, a commonly used procedure in rodents to study ELS.
Methods
A literature search identified 100 studies involving maternally separated rats and the following unconditioned procedures: the elevated plus maze (EPM); open field test (OFT); sucrose preference test (SPT) and forced swim task (FST). Studies were included for analysis if the separation of offspring from the dam was at least 60 min every day during the pre-weaning period prior to the start of adolescence.
Results
Our findings show that unconditioned tasks are generally poor at consistently demonstrating differences between control and separated groups with pooled effect sizes that were either small or non-existent (EPM: Hedge’s g = − 0.35, p = 0.01, OFT: Hedge’s g = − 0.32, p = 0.05, SPT: Hedge’s g = − 0.33, p = 0.21, FST: Hedge’s g = 0.99, p = 0.0001). Despite considerable procedural variability between studies, heterogeneity statistics were low; indicating the lack of standardization in the maternal separation protocol was the not the cause of these inconsistent effects.
Conclusions
Our findings indicate that in general, unconditioned tests of depression and anxiety are not sufficient to reveal the full behavioural repertoire of maternal separation stress should not be relied upon in isolation. We argue that more objective tasks that sensitively detect specific cognitive processes are better suited for translational research on stress-related disorders such as depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organisation (WHO) estimates that at least 5% of all adults presently suffer with depression, now the leading cause of morbidity worldwide (WHO 2022). However, despite this burden, advances in our understanding of the aetiology and treatment of depression have slowed since the introduction of selective serotonin reuptake inhibitors or SSRIs (Hyman 2013; Malhi and Mann 2018). This unmet need extends to the deleterious effects of early life stress (ELS), a widely recognised risk factor for depression and anxiety disorders (Chandan et al. 2019; Hughes et al. 2017; WHO 2020). Indeed, roughly one in five adults in the UK report having suffered from neglect, physical, sexual, or emotional abuse during childhood, with roughly half of these individuals reporting several forms of abuse (Elkin 2020). Child abuse, along with other forms of childhood trauma and uncontrollable stress, including the loss of a parent, a parent with a mental illness or witnessing domestic violence, collectively make up Adverse Childhood Experiences (ACEs) — a commonly used term to describe a variety of early life stresses (ELS) (CDC 2021; Hughes et al. 2017). ELS exposure notably increases the incidence of morbidities later in life, specifically mental health disorders such as depression and anxiety (Chandan et al. 2019; Hughes et al. 2017).
Translational experimental approaches are widely used to investigate the mechanisms underlying symptom-based diagnoses in humans, which extend to the effects of ELS on the neurobiology of depression and other mental health disorders. However, humans exposed to ELS do not always develop depression later in life (Hughes et al. 2017) and there are no empirical biomarkers of depression at present (de Aguiar Neto and Rosa 2019; Gururajan et al. 2016; Lopez et al. 2018). Experimental approaches in animals are therefore necessary for gaining a deeper mechanistic understanding of ELS that would inform novel therapeutic intervention. Repeated maternal separation (MS) is a form of ELS that involves the separation of young animals from their mother for at least 60 min each day for several days during the pre-adolescent period (Schmidt et al. 2011; Tractenberg et al. 2016). MS produces long lasting immunological (Dutcher et al. 2020), neurobiological (Nishi 2020) and behavioural (Nishi 2020) outcomes in animals that resemble the effects of early life adversity in humans (Heim et al. 2008). However, the specific behavioural changes pertaining to depression-relevant phenotypes in MS animals have been difficult to identify because behavioural validation is often assessed using "quick and dirty" tests derived from observations of spontaneous reactions to various stimuli or contexts (Reardon 2019). As examples, the elevated plus maze (EPM) and open field test (OFT) both exploit a rodent’s intrinsic fear of open spaces (Treit et al. 1993), such that when animals are more anxious, they increase their avoidance of open arms on the EPM and central areas of a field arena (Hlinák et al. 2009; Mechiel Korte and De Boer 2003). The sucrose preference test (SPT), relies on the preference of animals for sucrose over water when given the option to drink either freely. An attenuation of this preference is usually interpreted as anhedonia or a loss of interest in previously enjoyed aspects of life during depressive episodes. Finally, for the purposes of this article, the forced swim task (FST) exploits the innate reaction of an animal to escape when placed in a vessel of water (Yankelevitch-Yahav et al. 2015). Eventually, it is inferred that the rat shows helplessness (Porsolt et al. 1977) by no longer attempting to escape from the vessel — hypothetically a passive coping behaviour (Yankelevitch-Yahav et al. 2015). In stressed rats, the latency to the onset of passive coping decreases and the time spent immobile versus swimming increases (Bogdanova et al. 2013). Such behaviour is typically compared with symptoms of despair and fatigue during depressive episodes in humans (American Psychiatric Association 2013; Carvalho et al. 2021).
A widely cited issue with MS is the enormous variability in the separation protocol used by different research groups (Schmidt et al. 2011; Tractenberg et al. 2016). Such variation may underlie the variability in behavioural outcomes caused by early separation stress in both rats (Schmidt et al. 2011) and mice (Tractenberg et al. 2016). A previous meta-analysis of the effect of MS in rats on anxiety measures (Wang et al. 2020) listed sex, length of separation, temperature during isolation, single versus whole litter isolation, treatment of the control group, and age of testing as the main sources of variation between different studies. In humans, an increased burden of childhood stress correlates with more severe mental health outcomes (Hughes et al. 2017). The parallel for this burden may be an extended period of separation of rat offspring throughout the preadolescent period. Earlier onset post-natal stress (i.e. separation starting at an earlier postnatal day) may also have a differential impact on behavioural outcomes (Leichtweis et al. 2020). We thus assessed not only the duration of separation but also the starting age of this procedure in our analysis of the MS protocol, in addition to examining sex-dependent differences. We also investigated the effects of ambient temperature on the behavioural outcomes of MS, which shows considerable variability across different studies (Harshaw and Alberts 2012; Melo et al. 2018; Zimmerberg and Shartrand 1992).
The impact of MS ultimately depends on how this stressor interacts with the hypothalamic–pituitary–adrenal (HPA) axis in developing pups (Smith et al. 1997; Stanton et al. 1988). These changes result in differential stress reactivity in rats as they age, implying that changes in behaviour seen after MS may be exacerbated following a second stress in adulthood (van Bodegom et al. 2017). Separated pups also show long-lasting changes in the response of the immune system to later stress in life (Dutcher et al. 2020). We therefore included studies that identified behavioural differences before and after a second stress in both MS and control groups. Our primary objective here was to identify common effects in unconditioned tasks for depression and anxiety phenotypes in rodents following MS. Our analysis also considered procedural differences in the MS protocol to establish the robustness of reported behavioural outcomes of animals exposed to ELS.
Methods
Search strategy
Relevant studies were identified by searching the online databases of PubMed and Web of Science on February 22nd, 2022. The following search criteria were used: (maternal separation) AND (Rats OR Rat) AND (Elevated Plus Maze OR Open Field Test OR Sucrose Preference Test OR Forced Swim Test). A manual search was also adopted to ensure all relevant studies were included in the meta-analysis. The search was not limited by year of publication.
Study selection
Studies were included in the analysis if they conformed to the following criteria: (1) rats were used; (2) pups had been separated from their dams for at least 1 h daily for at least 7 days in the pre-weaning period. This differs from criteria in previous works that excluded studies that left their pups in the home cage during the separation period (Wang et al. 2020); (3) studies that measured depressive or anxiety-related behaviours on the EPM, OFT, SPT or FST; (4) studies that specified the sex of the animals, but unlike other approaches (Wang et al. 2020), if the data were pooled for both sexes the study was still included but separate analyses for pooled data were conducted; (5) studies were peer-reviewed and published in English; (6) studies reporting means ± SEM to allow the calculation of effect size.
Figure 1 shows a PRISMA flow diagram indicating the study selection strategy. Over 700 studies were identified from searches with 65 studies deemed eligible for inclusion in the present meta-analysis. The 65 studies yielded 100 cohorts with at least 60 min separation from the dam on multiple days in early life. Thirteen cohorts that had been separated for 15 min only were identified but were not included in the present analysis. Across 36 papers, there were 58 cohorts of rats studied using the elevated plus maze (EPM) (n = 1364). Nineteen papers contributed a total 32 cohorts of rats tested in the open field test (OFT) (n = 793). Across 10 papers, there were 17 cohorts of rats studied using the sucrose preference test (SPT) (n = 416). Across twenty papers, 28 cohorts on the forced swim test (FST) (n = 561). Fifteen cohorts were tested on one or more of the behavioural outcomes of interest following a further stress later in life. A summary of the included studies and pertinent procedural characteristics is provided in Supplementary Material, Table 1.
Data extraction
For each article, the following information was extracted: author; year of publication; rat strain; sex; presence of artificial heat source during separation; duration of each separation event in min; length of separation protocol in days; day of separation commencement; housing and treatment conditions of the control group; whole litter or individual pup separation; age at behavioural testing; whether a secondary adult stressor was applied, and the behavioural outcomes of each task (i.e. % of time in the open arm of the EPM, % time in the centre of the open field, % sucrose preference, time (s) immobile in the FST.
Behavioural outcomes were recorded as mean ± SEM for each subgroup. Where this was not explicitly available in the publication, data were extracted from graphs using the WebPlotDigitizer distance measurement function (Rohatgi 2021). Where one article contained multiple cohorts with different MS protocols, if available, the data were extracted and stored as a separate cohort. Where these different test cohorts had a common control group, the control group was split proportionally to avoid undue between-cohort correlations or the “unit-of-analysis” problem (Harrer et al. 2021: Chapter 3.5.2).
Statistical analyses
Data analyses were carried out using R (R Core Team 2021) and meta (Balduzzi et al. 2019), metafor (Viechtbauer 2010) and dmetar ((Harrer et al. 2021): Appendix D). Hedge’s g effect size was calculated for the behavioural measures of interest, chosen for its robustness to small sample sizes in comparison to Cohen’s d. Confidence intervals were calculated using t-table values for studies where total n < 30 or z-values of ± 1.96 for larger samples. Pooled effect size (Hedge’s g) was calculated for each behavioural outcome using a random effects model. Knapp-Hartung adjustments (Knapp and Hartung 2003) were used to calculate the confidence interval around the pooled effect size. Heterogeneity was assessed using the Q test (Cochran 1954) and I 2 statistics (Higgins and Thompson 2002), though in general tests for heterogeneity are weak, especially with low numbers of studies in a typical meta-analysis (Ioannidis 2007). Random effects modelling was used over fixed effects even where estimated heterogeneity was low based on our hypotheses of variability. Publication bias was assessed using funnel plots and Egger’s test (Egger et al. 1997). Moderators of interest were either categorical or continuous. Categorical moderators were assessed using χ2 test for subgroup differences. Continuous moderators, following collinearity analysis, were fitted by forced entry to a meta-regression using a mixed effects model using maximum likelihood for τ estimator. Then significant moderators, as analysed by subgroup analysis, were entered into the model and moderators removed by backwards stepwise regression, removing all moderators where p > 0.05 until only significant moderators remained. It is noted that stepwise models increase the likelihood off overfitting (Chatfield 1995; Whittingham et al. 2006) but our low number of moderators and precedent for use of this model (Wang et al. 2020) balances this concern. Studies that included exposure to an adult stress were treated as a subgroup; as such χ2 test for subgroup differences was applied.
Results
Variability in MS protocols
Of the included studies, the most common experimental design was the separation of pups for 3 h, each day, starting from PND 2 to PND 4. When separated from their dams, pups were kept with the rest of their litter in a temperature-controlled environment, in a different experimental room to the dam. The separated group was most often compared with a control group that had standard animal facility rearing — though this differed from study to study and was marginally more common than a control group that was entirely undisturbed. There was no significant collinearity between the three continuous predictors, thus all were used in each primary meta-regression. There were 7 identified secondary stressors, the most used was a variable chronic stress paradigm. All secondary stressors were applied following weaning, most often around PND 50.
Small or non-existent effects of MS on unconditioned behavioural measures
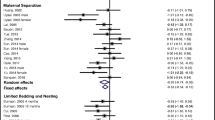
The categorisation of age groups was defined on the basis of previous work indicating major developmental age ranges in the rat (McCutcheon and Marinelli 2009). Figure 2 summarises the effect sizes reported for different age groups across the different behavioural tasks analysed in this study. Pooled Hedge’s g statistics indicate significant behavioural differences between MS and control animals for the EPM (Fig. 3) and FST (Fig. 6) only [EPM g = − 0.35 (95% CI = − 0.61, − 0.09), t = − 2.69, p = 0.0095, k = 58; FST g = 0.99 (95% CI = 0.63, 1.34), t = 5.64, p = 0.0001, k = 28]. Hedge’s g values for individual cohorts varied widely for each task. In the EPM, individual Hedge’s g clustered around 0 and had the largest prediction interval (see Fig. 3, prediction interval: − 1.76, 1.07). All effect sizes calculated were small (0.2 < g < 0.5) or moderate (0.5 < g < 0.8) except for the pooled g for FST where in peri-adolescents and older adults and OFT in older adult rats, a large effect size not including 0 was identified (see Fig. 4). In relation to the SPT, reported effect sizes across all age groups were not significant (see Fig. 5). In addition, there was no significant evidence of publication bias for any of the behavioural measures, as indicated by the symmetrical funnel plots and concordant non-significant Egger’s test values (see S2).
Effect size trends for different age group on each behavioural task. A down arrow indicates a decrease in performance of that measure in MS animals compared with controls. Uncoloured cells represent effect sizes that have confidence intervals including zero. Light blue cells indicate effect sizes that have confidence intervals that do not contain zero. Dark blue cells indicate effect sizes that have confidence intervals that do not contain zero and have a large effect size (greater than 1 or less than − 1). Left/right arrows indicate effect sizes within 0.2 of zero
Forest plot showing a meta-analysis of the effect of MS on % of time in open arms of the EPM. Blue boxes indicate studies in males, red are studies in females and grey boxes indicate studies that had pooled sex analysis. The Hedge’s g is depicted for each individual cohort with 95% CI, labelled on the left. The pooled Hedge’s g calculated by random effects model is displayed for each age group with a diamond and total for all cohorts at the bottom of the plot. The red bar indicates the overall prediction interval. The size of the box for individual cohorts correlates with the weighting attributed by the REM for the pooled g
Forest plot showing a meta-analysis of the effect of MS on % of time in open areas of the OFT. Blue boxes indicate studies in males, red are studies in females and grey boxes indicate studies that had pooled sex analysis. The Hedge’s g is depicted for each individual cohort with 95% CI, labelled on the left. The pooled Hedge’s g calculated by random effects model is displayed for each age group with a diamond and total for all cohorts at the bottom of the plot. The red bar indicates the overall prediction interval. The size of the box for individual cohorts correlates with the weighting attributed by the REM for the pooled g
Forest plot showing a meta-analysis of the effect of MS on % sucrose preference. Blue boxes indicate studies in males, red are studies in females and grey boxes indicate studies that had pooled sex analysis. The Hedge’s g is depicted for each individual cohort with 95% CI, labelled on the left. The pooled Hedge’s g calculated by random effects model is displayed for each age group with a diamond and total for all cohorts at the bottom of the plot. The red bar indicates the overall prediction interval. The size of the box for individual cohorts correlates with the weighting attributed by the REM for the pooled g
Lack of effect of methodological variables on behavioural outcomes of MS
I 2 values for all the behavioural tasks were not significant, thereby indicating that variability in reported effect sizes was not due to true differences in subgroup effect sizes. This conclusion was supported by a moderator analysis where no significant effect of subgroup was noted for the reported effect sizes for any of the behavioural tasks, both in subgroup χ2 analysis and meta-regression. Nevertheless, for the OFT and FST, the difference between MS and controls was greater in males than in females [OFT: male g = − 0.67 (95% CI = − 1.16, − 0.18), female g = − 0.05 (95% CI = − 0.39, 0.27), χ2 = 6.59, p = 0.04][FST: male g = 1.17 (95% CI = 0.82, 1.54), female g = − 0.15 (95% CI = − 1.68, 1.37), χ2 = 11.58, p = 0.003]. The date of first maternal separation was the only continuous moderator that had a significant impact on effect size in meta-regression. An earlier start day for MS predicted a greater effect size on the EPM (β = 0.44, se = 0.16, (95%CI = 0.12, 0.76), t = 2.73, p = 0.008), an effect that was robust to permutation tests (p* = 0.007) but was not demonstrated for any other task (Fig. 6).
Forest plot showing a meta-analysis of the effect of MS on immobility time in the FST. Blue boxes indicate studies in males, red are studies in females and grey boxes indicate studies that had pooled sex analysis. The Hedge’s g is depicted for each individual cohort with 95% CI, labelled on the left. The pooled Hedge’s g calculated by random effects model is displayed for each age group with a diamond and total for all cohorts at the bottom of the plot. The red bar indicates the overall prediction interval. The size of the box for individual cohorts correlates with the weighting attributed by the REM for the pooled g
Effect of a second uncontrollable stressor in MS animals
Seventeen cohorts were exposed to a second stress and these varied both in nature and timing. Cohorts were mostly exposed to multiple day variable chronic mild stress, or chronic restraint stress, though some cohorts used alternate stresses such as isolation and sleep deprivation. The day of application of second stress was predominantly in young adulthood and varied from PND 26 to PND 91. A detailed subgroup analysis failed to identify a significant difference in the reported effect sizes between cohorts tested with or without a secondary stress in adulthood. Thus, for the EPM, OFT and SPT effect sizes clustered around g = 0 with wide CIs (see Fig. 7). A larger effect size was observed for the FST but was also accompanied by a large CI that encompassed zero.
Discussion
As far as we are aware, this is the first meta-analysis of spontaneous behaviours claimed to assess depression- and anxiety-like phenotypes in adult rats exposed to repeated early maternal separation. Sixty-five studies were selected, encompassing one hundred cohorts across four widely used behavioural paradigms. Repeated maternal separation is a widely used experimental procedure to investigate the consequences of early life stress with long lasting behavioural (Nishi 2020), immune (Dutcher et al. 2020) and neurobiological (Nishi 2020) effects. Given the enormous burden of depression and anxiety-related disorders in humans (Bromet et al. 2011; Global Health Data Exchange, n.d.; WHO 2022), it has never been more important to develop robust behavioural procedures in animals to investigate underlying neurobiological mechanisms to help inform the development of new treatments.
The main findings of our analysis indicate that in general unconditioned tasks are not robustly sensitive to behavioural phenotypic changes in rats exposed to MS. Nevertheless, significant effect sizes were detected for the EPM and FST (g = − 0.35; g = 0.99 respectively), and therefore these two tasks should be included as part of a battery of tests to investigate elements of depressogenic and anxiogenic phenotypes associated with maternal separation stress. However, the FST in particular faces challenges of power and validity (Reardon 2019; RELACS Consortium et al. 2021) and we would not use the large effect size seen here to justify its continued use. Moreover, many of the moderators of interest did not explain the variability in effect sizes across the different cohorts. Although the literature regarding the impact of a second stress in MS animals is sparse, we found no evidence that the four tasks analysed in the present study are sufficiently sensitive to detect differential impacts of a secondary stressor in MS animals.
Unconditioned measures are not robust behavioural validations of maternal separation
As noted above, sample sizes in preclinical studies tend to be small (Vesterinen et al. 2014), with limited power to detect differences with small effect sizes, such as found here in the EPM. This was particularly the case for studies that have used the EPM where our analysis unequivocally demonstrated that this procedure is unsuitable as a behavioural screen for animals according to their previous history of stress. The utility of the EPM for mechanistic investigations into the neurobehavioural sequelae of maternal separation is therefore limited to a single observational time-point of unconditioned anxiety-like behaviour. Emphasis should be placed on baseline preference for safer environments rather than nuanced interpretations of the anxiety around open environments; especially given the lack of correspondence of this measure with the OFT. The EPM is underpinned by an ethological fear of heights and open spaces and therefore the task itself is likely to be stressful for the animal. Therefore, when using the EPM within a battery of tasks the effect of a stressful experience on other tasks should be duly noted.
The FST is also widely used to characterise stress-related disorders, notably depression-like behaviour in rodents (Carvalho et al. 2021; Yankelevitch-Yahav et al. 2015). Our analysis demonstrates the utility of this task in identifying meaningful differences between controls and maternally separated animals. However, growing concerns about the construct validity of this procedure have discouraged funding bodies and the pharmaceutical industry from continuing to invest in research that adopts this task (Reardon 2019). The main argument for the utility of this task in fact usually rests with predictive validity. Thus, drugs with clinical efficacy in depression such as SSRIs increase swim time and delay the onset to passive coping (Yankelevitch-Yahav et al. 2015). However, this beneficial effect results from acute systemic administration and does not resemble the much slower onset of clinical efficacy in humans (Thompson 2002). Although the FST exhibits sensitivity in identifying anti-depressant like compounds, especially compounds that modulate the monoaminergic systems (Cryan et al. 2005), not all compounds that reduce immobility times have anti-depressant activity and consequently fail in clinical trials (Bowman and Daws 2019; Cryan et al. 2005; Cryan and Mombereau 2004). Moreover, a more mixed response is observed with alternative anti-depressant therapies (Yankelevitch-Yahav et al. 2015). As questions of validity grow, so do ethical concerns of performing such an aversive task in animals (Reardon 2019).
Previous summary analysis in rodents has only addressed anxiolytic tests, they found a significant impact on EPM and OFT in rats (Wang et al. 2020). The effect sizes reported are comparable to those identified for the same behavioural tasks in this current work, yet we did not identify most of these effect sizes to be significant. This suggests that the literature added due to wider inclusion criteria and date of publication in this current study has a similar overall effect size as identified in Wang et al. (2020) but increased variation in reported effect size reduces the significance of the results, calling into question if these effect sizes represent meaningful behavioural differences following MS.
Rather surprisingly, the SPT returned the smallest effect size and highest variability of the measures analysed in this study. The SPT is claimed to assess anhedonia, but sucrose preference per se does not reflect ingestive behaviour associated with depressive episodes in humans with depression (Criteria 3 depression symptoms: American Psychiatric Association 2013; Simmons et al. 2020). Moreover, DSM-V criteria for ‘disinterest in previously liked activities’ is not limited to food preferences (American Psychiatric Association 2013). Nevertheless, the SPT has still been considered the gold standard procedure for the assessment of anhedonia (Bacharach and Calu 2019; Tõnissaar et al. 2006) and is routinely used in pre-clinical research (Cui et al. 2020a; Dallé et al. 2020; Jiang et al. 2021), despite its dependence on subjective ‘face validity’. Although our findings question the utility of this task in the behavioural assessment of ELS, we acknowledge that conditioned tests of affective disorder are often motivated by food reward (Bari et al. 2010; Enkel et al. 2010; Harding et al. 2004; Papciak et al. 2013; Phillips et al. 2018). Indeed, MS rats tested during adulthood show an attenuation of food conditioned anticipatory locomotor activity (Matthews et al. 1996). Taken together, MS appears to cause long lasting changes in incentive learning processes rather than the consumption of, or preference for, palatable food.
Lack of effect of maternal separation on unconditioned behaviours is not due to methodological variability
As there is no standard protocol for MS, it is not surprising that considerable variability exists in the precise way this procedure is carried out. Overall, most of the moderators of interest, based on prior findings, did not account for the variability in the calculated effect sizes. Thus, the variables of sex, age and MS start day were the only moderators across the four tasks to affect behavioural outcomes, but their impact was not consistent across tasks. Our analysis did identify that MS start day has a significant effect on the predicted Hedge’s g for EPM following MS, indicating that the time during development that a stressor is applied may create a more anxiogenic phenotype. Our analysis identified that the start date of MS has a significant effect on the predicted Hedge’s g for the EPM task following MS. However, this observation is tempered by the analysis of Wang (2020) where separation duration, rather than start date, was a significant moderator of MS outcomes. Although collinearity analysis did not indicate significant moderator co-dependence, it is reasonable to assume that both moderators represent the intensity of MS stress. We interpret these findings to suggest that EPM may be useful at identifying phenotypic differences following maternal separation stress but the sensitivity of this procedure may depend on the severity of the stress, including the start time (PND < 2) and duration of separation.
We calculated the I2 heterogeneity statistic or the proportion of the variability in observed effect sizes that was due to variability in the true effect size rather than random error (Borenstein et al. 2017). Since our reported effect sizes did not vary significantly, a low I2 value implies that any variability was due to random error rather than the precise parameters of the MS procedure.
Beyond unconditioned measures of depression and anxiety
In this section, we discuss alternative behavioural tasks to assess depression and anxiety-related phenotypes in rats previously exposed to ELS. These tasks are generally computer automated as well as objectively scored and based on analogous tasks in humans that assess comparable psychological processes, thereby facilitating translational research. Though these tasks have been used less often than traditional unconditioned tests, and therefore we cannot yet conduct a meta-analysis of this scale to assess their utility, Table 1 provides a summary of the main tasks in common use that fall within this category for discussion of their potential. A significant limitation of conditioned behavioural tasks is that they often require weeks of pre-training before the test phase and thus where unconditioned tasks may provide information about the acute phenotypic state, conditioned tasks are more useful to assess parameters such as learning rate, change in performance over time and acute changes in performance following life events.
Ambiguous cue task
Ambiguous cue tasks are increasingly used in humans and experimental animals (Enkel et al. 2010; Gethin et al. 2017; Harding et al. 2004; Papciak et al. 2013; Stuart et al. 2019; Surguladze et al. 2004) to assess negative affective bias and slowed processing speed in major depressive disorder (MDD), especially where individuals are exposed to ELS (Saleh et al. 2017). In humans, depressed and control individuals identify positive, negative, and neutral emotional faces with equivalent accuracies and response latencies (Gur et al. 1992; Hale 1998; Surguladze et al. 2004) but when presented with ambiguous face cues depressed individuals are more likely to label positive ambiguous faces as neutral and near-negative faces as negative (Surguladze et al. 2004). This shift in positive bias is pervasive through immediate processing to recall of episodic memory (Gethin et al. 2017). Furthermore, MDD patient populations with a history of ELS are more likely to lack a positive affective bias compared to both MDD without ELS and controls (Gethin et al. 2017) during and between depressive episodes. In humans, a lack of positive affective bias is thought to represent a risk marker for depression (Gotlib and McCann 1984; Hales et al. 2014).
Analogous ambiguous cue tasks have been developed in experiment animals. Positive and negative valence can be assigned to individual auditory cues that are linked to an appetitive task representing gain of reward or avoidance of punishment during training (Enkel et al. 2010; Harding et al. 2004; Papciak et al. 2013; Stuart et al. 2019). Rodents with a history of stress are slower to respond to ambiguous auditory cues (Harding et al. 2004) and show a negative affective bias (Enkel et al. 2010; Harding et al. 2004; Papciak et al. 2013). When investigating the effects of stress in adulthood on depression, the use of punishment in this task could result in the task itself acting as a secondary physiological stressor. In this situation, an altered judgement bias task may be more useful whereby the valence of the options are both positive, but one is more positive (Hales et al. 2016, 2021). This ‘pessimistic’ expectation of rodents when faced with an ambiguous cue is seen following a variety of models to induce stress phenotypes (Enkel et al. 2010; Harding et al. 2004; Papciak et al. 2013; Stuart et al. 2019). Notably, the MS procedure is sufficient to induce this pessimistic phenotype on an ambiguous cue task (Stuart et al. 2019). MS rats are less able to integrate rewarding information to adjust biases (Stuart et al. 2019) indicating a lack of positive bias as opposed to a negative affective bias. This more closely mirrors the findings in MDD and humans with an experience of ELS. Further support for the translatability of this task is limited by the investigation of pharmacological intervention focussing on acute dosing. Acute systemic administration of SSRIs such as citalopram and escitalopram produce dose-dependent shifts in bias as assessed using ambiguous cue tasks (Drozd et al. 2019; Rygula et al. 2014), with the tricyclic monoamine inhibitor desipramine also inducing a shift in negative bias, but d-amphetamine at higher doses producing a positive bias (Rygula et al. 2014). With respect to the PRL task, selective brain 5-HT depletion (Bari et al. 2010) and 5HT2C receptor antagonism (Phillips et al. 2018) leads to a decrease in the proportion of win-stay responses. Moreover, the atypical antidepressant agomelatine decreased loss-shift behaviour in the PRL and ambiguous cue tasks, suggesting that treated animals were more robust to negative feedback (Drozd et al. 2019). Such effects in the ambiguous cue task were only apparent in animals identified as trait “pessimistic” (Drozd et al. 2019) suggesting that drug effects may be importantly modulated by baseline differences in lose-shift / win-stay behaviour.
Probabilistic reversal learning
Integration of reward-based information is an integral component of probabilistic reversal learning (PRL) tasks. PRL measures cognitive flexibility in the context of decision-making and the ability to integrate fluctuations in reward likelihood in both humans and animals (Bari et al. 2010; Mukherjee et al. 2020; Murphy et al. 2009; Phillips et al. 2018; Taylor Tavares et al. 2008). PRL tasks are used in a clinical setting to assess the cognitive symptomology in depression states (Murphy et al. 2009; Taylor Tavares et al. 2008). Patients with MDD are slower to learn and less responsive to reversals on PRL tasks (Mukherjee et al. 2020; Wilkinson et al. 2021). In addition, individuals with a history of ELS show reduced responsiveness to positive feedback and reduced positive reinforcement learning for high magnitude rewards (Wilkinson et al. 2021).
Rodents can be trained on PRL tasks that are remarkably similar to those used in human participants. Two cues can be presented to the rats and correct responses corresponding with selecting the cue with the higher probability of reward. Following a criterion having been attained of correct responses, stimulus-reward contingencies are reversed, which allows for a number of behavioural measures to be assessed including the latency to respond following a contingency reversal and the proportion of correct win-stay or loss-shift responses when the outcome does not match the expected return. Computational modelling based on reinforcement learning theory can also be applied to both human and experimental animal performance on the PRL task (Robbins and Cardinal 2019). Pharmacological models of depression in rats have elucidated permutations in PRL behaviour in rats (Bari et al. 2010; Phillips et al. 2018). The proportion of win-stay responses is decreased following serotonin depletion (Bari et al. 2010) and 5HT2C receptor antagonism (Phillips et al. 2018) highlighting in rats the same decreased attention to positive reinforcement identified in humans (Wilkinson et al. 2021). Interesting work using both the PRL and the ambiguous cue task indicates that administration of the atypical antidepressant agomelatine reduces loss-shift behaviour, indicating they are more robust to negative feedback, but only in animals identified as trait “pessimistic” using the ambiguous cue (Drozd et al. 2019). This work supports our general suggestion that combinations of tasks can be used to describe both cognitive changes in depressogenic models and to explain individual differences in susceptibility to certain types of pharmacological intervention. Unpublished data using the PRL task in rodents who have been exposed to MS indicates ELS has a long-lasting impact on the response latency in PRL (Dutcher E, unpublished findings) and this is exacerbated by later life stress. MS is sufficient to induce PRL behavioural changes, but a “double-hit” with a later life stress exacerbates these effects. There are also sexually dimorphic sensitisations to negative outcomes to influence learning which indicate the potential of the PRL task in ELS rats to illustrate the reinforcement learning blunting seen in humans (Wilkinson et al. 2021).
Concluding remarks
The present meta-analysis has drawn on a large body of empirical evidence using raw means to calculate effect sizes across many cohorts. While most studies analysed contained all the procedural details we wished to extract, maximizing power for moderator analysis, including of a wide literature base in the pooled analysis allowed for robust conclusions to be drawn on the utility of measuring spontaneous behaviours for the assessment of anxiety and depression phenotypes. Where Wang (2020) identified evidence of potential publication bias (i.e. the tendency to not publish negative results) particularly for studies using EPM, we did not. This could explain why they previously noted a significant and moderate effect size for cohorts tested on EPM, and we did not, if our inclusion criteria increased the proportion of studies with null and negative findings. Further, our lack of evidence of publication bias indicates appropriate use of inclusion criteria for this meta-analysis such that studies that more likely had null and negative results were not disproportionately excluded. We included cohorts of both sexes and had a large sample size for both males and females. Nevertheless, a few limitations in our analysis should be noted. For example, a lack of observable pooled effect size across the tasks limited our ability to analyse moderator effects and the impact of a second stressor in adulthood after MS. In particular, the small number of cohorts that applied a second stressor in adulthood further limited this assessment. A further limitation of this analysis is that whilst wide inclusion criteria allow for a broad overview of the literature, we did not include qualitative assessments of the included studies. We also note that while we have suggested some alternate behavioural metrics to assess the depression phenotype, there are still too few studies using contemporary methods such as automated touchscreen tasks to make strong claims regarding their usefulness. Conditioned tasks such as the ambiguous cue and PRL are not being proposed here as like-for-like replacements of unconditioned tasks but have the advantage of revealing cognitive processes less obviously identifiable with unconditioned tasks.
Research on mental health is increasingly focused on mechanisms to guide treatment options, overturning the historical search for new drugs on the back of old drugs. To facilitate the pursuit for relevant biomarkers, including those relating to stress reactivity, robust experimental approaches and behavioural tasks are needed in experimental animals. Although this study was unable to verify any task as particularly suitable for behavioural screening, our analysis highlights variation in MS protocol as a moderator of effect size. In line with contemporary inclusion criteria, both males and females should be included in MS studies, especially as sex was the only moderator in our hands to impact effect size in more than one task.
In conclusion, the present meta-analysis of four unconditioned tasks indicates that the effect sizes for depression- and anxiety-like phenotypic outcomes of ELS are small and highly variable. Although the FST reveals significant difference between control and separated animals, the underlying neurobiology that mediates this effect is poorly understood. Given the additional poor translatability of unconditioned tasks to MDD in humans, we suggest that any further use of unconditioned tasks is within a battery of tasks that assess conditioned and unconditioned behaviours and are not relied upon in isolation. The MS procedure however remains a valuable experimental approach for revealing the underlying neurobehavioural mechanisms of ELS (Schmidt et al. 2011; Tractenberg et al. 2016). Looking forward, translational preclinical research incorporating automated operant or instrumental procedures, including touchscreen tasks measuring different aspects of goal-directed behaviour, promise exciting new ways of exploring persistent behavioural sequelae of MS and impacts of future uncontrollable stressors.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (Fifth Edition). American Psych Assoc. https://doi.org/10.1176/appi.books.9780890425596
Bacharach SZ, Calu DJ (2019) Stability of individual differences in sucralose taste preference. PLoS ONE 14(5):e0216431. https://doi.org/10.1371/journal.pone.0216431
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evidence Based Mental Health 22(4):153–160. https://doi.org/10.1136/ebmental-2019-300117
Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW (2010) Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology 35(6):1290–1301. https://doi.org/10.1038/npp.2009.233
Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF (2013) Factors influencing behavior in the forced swim test. Physiol Behav 118:227–239. https://doi.org/10.1016/j.physbeh.2013.05.012
Borenstein M, Higgins JPT, Hedges LV, Rothstein HR (2017) Basics of meta-analysis: I 2 is not an absolute measure of heterogeneity: I 2 is not an absolute measure of heterogeneity. Res Synth Methods 8(1):5–18. https://doi.org/10.1002/jrsm.1230
Bowman MA, Daws LC (2019) Targeting serotonin transporters in the treatment of juvenile and adolescent depression. Front Neurosci 13:156. https://doi.org/10.3389/fnins.2019.00156
Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lépine J-P, Levinson D, Matschinger H, Mora MEM, Browne MO, Posada-Villa J, … Kessler RC (2011) Cross-national epidemiology of DSM-IV major depressive episode. BMC Med 9(1) 90https://doi.org/10.1186/1741-7015-9-90
Carvalho C, Herrmann K, Marques TA, Knight A (2021) Time to abolish the forced swim test in rats for depression research? J App Animal Ethics Res (published online ahead of print 2021). https://doi.org/10.1163/25889567-bja10026
CDC (2021) Adverse Childhood Experiences. CDC Fast Facts. https://www.cdc.gov/violenceprevention/aces/index.html#
Chandan JS, Thomas T, Gokhale KM, Bandyopadhyay S, Taylor J, Nirantharakumar K (2019) The burden of mental ill health associated with childhood maltreatment in the UK, using The Health Improvement Network database: A population-based retrospective cohort study. Lancet Psychiatr 6(11):926–934. https://doi.org/10.1016/S2215-0366(19)30369-4
Chatfield C (1995) Model uncertainty, data mining and statistical inference. J Roy Stat Soc Ser A (Statistics in Society) 158(3):419. https://doi.org/10.2307/2983440
Cochran WG (1954) Some methods for strengthening the common χ 2 tests. Biometrics 10(4):417. https://doi.org/10.2307/3001616
Cryan JF, Mombereau C (2004) In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry 9(4):326–357. https://doi.org/10.1038/sj.mp.4001457
Cryan JF, Valentino RJ, Lucki I (2005) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29(4–5):547–569. https://doi.org/10.1016/j.neubiorev.2005.03.008
Cui Y, Cao K, Lin H, Cui S, Shen C, Wen W, Mo H, Dong Z, Bai S, Yang L, Shi Y, Zhang R (2020a) Early-life stress induces depression-like behavior and synaptic-plasticity changes in a maternal separation rat model: gender difference and metabolomics study. Front Pharmacol 11:102. https://doi.org/10.3389/fphar.2020.00102
Dallé E, Daniels WMU, Mabandla MV (2020) Long-term treatment with fluvoxamine decreases nonmotor symptoms and dopamine depletion in a postnatal stress rat model of Parkinson’s disease. Oxid Med Cell Longev 2020:1–15. https://doi.org/10.1155/2020/1941480
de Aguiar Neto FS, Rosa JLG (2019) Depression biomarkers using non-invasive EEG: a review. Neurosci Biobehav Rev 105:83–93. https://doi.org/10.1016/j.neubiorev.2019.07.021
Drozd R, Rychlik M, Fijalkowska A, Rygula R (2019) Effects of cognitive judgement bias and acute antidepressant treatment on sensitivity to feedback and cognitive flexibility in the rat version of the probabilistic reversal-learning test. Behav Brain Res 359:619–629. https://doi.org/10.1016/j.bbr.2018.10.003
Dutcher EG, Pama EAC, Lynall M-E, Khan S, Clatworthy MR, Robbins TW, Bullmore ET, Dalley JW (2020) Early-life stress and inflammation: a systematic review of a key experimental approach in rodents. Brain Neurosci Adv 4:239821282097804. https://doi.org/10.1177/2398212820978049
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (clinical Research Ed) 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Elkin M (2020) Child abuse extent and nature, England and Wales: Year ending March 2019. ONS UK. https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/childabuseextentandnatureenglandandwales/yearendingmarch2019#:~:text=1.,years%20(8.5%20million%20people).
Enkel T, Gholizadeh D, von Bohlen Und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, Gass P, Vollmayr B (2010) Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 35(4):1008–1015. https://doi.org/10.1038/npp.2009.204
Gethin JA, Lythe KE, Workman CI, Mayes A, Moll J, Zahn R (2017) Early life stress explains reduced positive memory biases in remitted depression. Eur Psychiatry: J Assoc Eur Psychiatr 45:59–64. https://doi.org/10.1016/j.eurpsy.2017.06.011
Global Health Data Exchange. (n.d.). Institute of Health Metrics and Evaluation. Retrieved April 13, 2022, from http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b
Gotlib IH, McCann CD (1984) Construct accessibility and depression: an examination of cognitive and affective factors. J Pers Soc Psychol 47(2):427–439. https://doi.org/10.1037/0022-3514.47.2.427
Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC (1992) Facial emotion discrimination: II Behavioral Findings in Depression. Psychiatry Res 42(3):241–251. https://doi.org/10.1016/0165-1781(92)90116-k
Gururajan A, Clarke G, Dinan TG, Cryan JF (2016) Molecular biomarkers of depression. Neurosci Biobehav Rev 64:101–133. https://doi.org/10.1016/j.neubiorev.2016.02.011
Hale WW (1998) Judgment of facial expressions and depression persistence. Psychiatry Res 80(3):265–274. https://doi.org/10.1016/S0165-1781(98)00070-5
Hales CA, Bartlett JM, Arban R, Hengerer B, Robinson ES (2021) Effects of pro-depressant and immunomodulatory drugs on biases in decision-making in the rat judgement bias task. Eur J Neurosci 55(9–10):2955–2970. https://doi.org/10.1111/ejn.15127
Hales CA, Robinson ESJ, Houghton CJ (2016) Diffusion modelling reveals the decision making processes underlying negative judgement bias in rats. PLoS ONE 11(3):e0152592. https://doi.org/10.1371/journal.pone.0152592
Hales CA, Stuart SA, Anderson MH, Robinson ESJ (2014) Modelling cognitive affective biases in major depressive disorder using rodents: rodent models of cognitive affective biases. Br J Pharmacol 171(20):4524–4538. https://doi.org/10.1111/bph.12603
Harding EJ, Paul ES, Mendl M (2004) Cognitive bias and affective state. Nature 427(6972):312–312. https://doi.org/10.1038/427312a
Harrer M, Cuijpers P, Furukawa T, Ebert D (2021) Doing meta-analysis with R: a hands-on guide (First edition). CRC Press/Taylor & Francis Group.
Harshaw C, Alberts JR (2012) Group and individual regulation of physiology and behavior: a behavioral, thermographic, and acoustic study of mouse development. Physiol Behav 106(5):670–682. https://doi.org/10.1016/j.physbeh.2012.05.002
Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008) The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 33(6):693–710. https://doi.org/10.1016/j.psyneuen.2008.03.008
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
Hlinák Z, Hynie S, Krejcí I, Klenerová V (2009) Novel and simple behavioral paradigm for assessing anxiety in rats: effect of diazepam. Neuro Endocrinol Lett 30(1):25–31
Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP (2017) The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health 2(8):e356–e366. https://doi.org/10.1016/S2468-2667(17)30118-4
Hyman SE (2013) Psychiatric drug development: diagnosing a crisis. Cerebrum: The Dana Forum on Brain Science, 2013;5.
Ioannidis JPA (2007) Why most published research findings are false: author’s reply to Goodman and Greenland. PLoS Med 4(6):e215. https://doi.org/10.1371/journal.pmed.0040215
Jiang Z, Zhu Z, Zhao M, Wang W, Li H, Liu D, Pan F (2021) H3K9me2 regulation of BDNF expression in the hippocampus and medial prefrontal cortex is involved in the depressive-like phenotype induced by maternal separation in male rats. Psychopharmacology 238(10):2801–2813. https://doi.org/10.1007/s00213-021-05896-7
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22(17):2693–2710. https://doi.org/10.1002/sim.1482
Leichtweis KS, Carvalho M, Morais-Silva G, Marin MT, Amaral VCS (2020) Short and prolonged maternal separation impacts on ethanol-related behaviors in rats: Sex and age differences. Stress 23(2):162–173. https://doi.org/10.1080/10253890.2019.1653847
Lopez JP, Kos A, Turecki G (2018) Major depression and its treatment: microRNAs as peripheral biomarkers of diagnosis and treatment response. Curr Opin Psychiatry 31(1):7–16. https://doi.org/10.1097/YCO.0000000000000379
Malhi GS, Mann JJ (2018) Depression. The Lancet 392(10161):2299–2312. https://doi.org/10.1016/S0140-6736(18)31948-2
Matthews K, Wilkinson LS, Robbins TW (1996) Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol Behav 59(1):99–107. https://doi.org/10.1016/0031-9384(95)02069-1
McCutcheon JE, Marinelli M (2009) Age matters. Eur J Neurosci 29(5):997–1014. https://doi.org/10.1111/j.1460-9568.2009.06648.x
Mechiel Korte S, De Boer SF (2003) A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol 463(1–3):163–175. https://doi.org/10.1016/S0014-2999(03)01279-2
Melo C, Vizin RCL, Silva NU, Ishikawa DT, Echeverry MB, Carrettiero DC, Almeida MC (2018) Early maternal separation promotes alterations in the thermoregulatory profile of adult Wistar rats. J Therm Biol 78:151–160. https://doi.org/10.1016/j.jtherbio.2018.09.013
Mukherjee D, Filipowicz ALS, Vo K, Satterthwaite TD, Kable JW (2020) Reward and punishment reversal-learning in major depressive disorder. J Abnorm Psychol 129(8):810–823. https://doi.org/10.1037/abn0000641
Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ (2009) Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194(6):535–540. https://doi.org/10.1192/bjp.bp.108.056093
Nishi M (2020) Effects of early-life stress on the brain and behaviors: implications of early maternal separation in rodents. Int J Mol Sci 21(19):7212. https://doi.org/10.3390/ijms21197212
Papciak J, Popik P, Fuchs E, Rygula R (2013) Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous-cue interpretation paradigm. Behav Brain Res 256:305–310. https://doi.org/10.1016/j.bbr.2013.08.036
Phillips BU, Dewan S, Nilsson SRO, Robbins TW, Heath CJ, Saksida LM, Bussey TJ, Alsiö J (2018) Selective effects of 5-HT2C receptor modulation on performance of a novel valence-probe visual discrimination task and probabilistic reversal learning in mice. Psychopharmacology 235(7):2101–2111. https://doi.org/10.1007/s00213-018-4907-7
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266(5604):730–732. https://doi.org/10.1038/266730a0
R Core Team (2021). R: A Language and Environment for Statistical Computing (4.1.2). R Foundation for Statistical computing. https://www.R-project.org/
Reardon S (2019) Depression researchers rethink popular mouse swim tests. Nature 571(7766):456–457. https://doi.org/10.1038/d41586-019-02133-2
RELACS Consortium, Bonapersona V, Hoijtink H, Sarabdjitsingh RA, Joëls M (2021) Increasing the statistical power of animal experiments with historical control data. Nat Neurosci 24(4):470–477. https://doi.org/10.1038/s41593-020-00792-3
Robbins TW, Cardinal RN (2019) Computational psychopharmacology: a translational and pragmatic approach. Psychopharmacology 236(8):2295–2305. https://doi.org/10.1007/s00213-019-05302-3
Rohatgi A (2021) WebPlotDigitizer (4.5). https://automeris.io/WebPlotDigitizer
Rygula R, Papciak J, Popik P (2014) The effects of acute pharmacological stimulation of the 5-HT, NA and DA systems on the cognitive judgement bias of rats in the ambiguous-cue interpretation paradigm. Eur Neuropsychopharmacol 24(7):1103–1111. https://doi.org/10.1016/j.euroneuro.2014.01.012
Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, Taylor WD (2017) Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med 47(1):171–181. https://doi.org/10.1017/S0033291716002403
Schmidt MV, Wang X-D, Meijer OC (2011) Early life stress paradigms in rodents: Potential animal models of depression? Psychopharmacology 214(1):131–140. https://doi.org/10.1007/s00213-010-2096-0
Simmons WK, Burrows K, Avery JA, Kerr KL, Taylor A, Bodurka J, Potter W, Teague TK, Drevets WC (2020) Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol Psychiatry 25(7):1457–1468. https://doi.org/10.1038/s41380-018-0093-6
Smith MA, Kim S-Y, van Oers HJJ, Levine S (1997) Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology 138(11):4622–4628. https://doi.org/10.1210/endo.138.11.5529
Stanton ME, Gutierrez YR, Levine S (1988) Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci 102(5):692–700. https://doi.org/10.1037/0735-7044.102.5.692
Stuart SA, Hinchcliffe JK, Robinson ESJ (2019) Evidence that neuropsychological deficits following early life adversity may underlie vulnerability to depression. Neuropsychopharmacology 44(9):1623–1630. https://doi.org/10.1038/s41386-019-0388-6
Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML (2004) Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology 18(2):212–218. https://doi.org/10.1037/0894-4105.18.2.212
Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC (2008) Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage 42(3):1118–1126. https://doi.org/10.1016/j.neuroimage.2008.05.049
Thompson C (2002) Onset of action of antidepressants: results of different analyses. Hum Psychopharmacol Clin Exp 17(S1):S27–S32. https://doi.org/10.1002/hup.386
Tõnissaar M, Herm L, Rinken A, Harro J (2006) Individual differences in sucrose intake and preference in the rat: circadian variation and association with dopamine D2 receptor function in striatum and nucleus accumbens. Neurosci Lett 403(1–2):119–124. https://doi.org/10.1016/j.neulet.2006.04.023
Tractenberg SG, Levandowski ML, de Azeredo LA, Orso R, Roithmann LG, Hoffmann ES, Brenhouse H, Grassi-Oliveira R (2016) An overview of maternal separation effects on behavioural outcomes in mice: evidence from a four-stage methodological systematic review. Neurosci Biobehav Rev 68:489–503. https://doi.org/10.1016/j.neubiorev.2016.06.021
Treit D, Menard J, Royan C (1993) Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav 44(2):463–469. https://doi.org/10.1016/0091-3057(93)90492-C
van Bodegom M, Homberg JR, Henckens MJAG (2017) Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 11:87. https://doi.org/10.3389/fncel.2017.00087
Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, Antonic A, Howells DW, Macleod MR (2014) Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221:92–102. https://doi.org/10.1016/j.jneumeth.2013.09.010
Viechtbauer W (2010) Conducting Meta-Analyses in R with the metafor Package. J Stat Softw 36(3) https://doi.org/10.18637/jss.v036.i03
Wang D, Levine JLS, Avila-Quintero V, Bloch M, Kaffman A (2020) Systematic review and meta-analysis: effects of maternal separation on anxiety-like behavior in rodents. Transl Psychiatry 10(1):174. https://doi.org/10.1038/s41398-020-0856-0
Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour?: stepwise modelling in ecology and behaviour. J Anim Ecol 75(5):1182–1189. https://doi.org/10.1111/j.1365-2656.2006.01141.x
WHO (2020) Child Maltreatment. WHO.
WHO (2022) Depression: Let’s talk. WHO GENEVA.
Wilkinson MP, Slaney CL, Mellor JR, Robinson ESJ (2021) Investigation of reward learning and feedback sensitivity in non-clinical participants with a history of early life stress. PLoS ONE 16(12):e0260444. https://doi.org/10.1371/journal.pone.0260444
Yankelevitch-Yahav R, Franko M, Huly A, Doron R (2015) The forced swim test as a model of depressive-like behavior. J vis Exp 97:52587. https://doi.org/10.3791/52587
Zimmerberg B, Shartrand AM (1992) Temperature-dependent effects of maternal separation on growth, activity, and amphetamine sensitivity in the rat. Dev Psychobiol 25(3):213–226. https://doi.org/10.1002/dev.420250306
References used for the meta-analysis
Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez M (2008) Effects of maternal separation onhypo-thalamic–pituitary–adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience 154(4):1218–1226. https://doi.org/10.1016/j.neuroscience.2008.05.011
Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ (2007) Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 32(3):256–266. https://doi.org/10.1016/j.psyneuen.2006.12.013
Alves RL, Oliveira P, Lopes IM, Portugal CC, Alves CJ, Barbosa F, Summavielle T, Magalhães A (2020) Early-life stress affects drug abuse susceptibility in adolescent rat model independently of depression vulnerability. Sci Rep 10(1):13326. https://doi.org/10.1038/s41598-020-70242-4
Babygirija R, Yoshimoto S, Gribovskaja-Rupp I, Bülbül M, Ludwig K, Takahashi T (2012) Social interaction attenuates stress responses following chronic stress in maternally separated rats. Brain Res 1469:54–62. https://doi.org/10.1016/j.brainres.2012.06.007
Bianco CD, Hübner IC, Bennemann B, de Carvalho CR, Brocardo PS (2021) Effects of postnatal ethanol exposure and maternal separation on mood, cognition and hippocampal arborization in adolescent rats. Behav Brain Res 411:113372. https://doi.org/10.1016/j.bbr.2021.113372
Borges-Aguiar AC, Schauffer LZ, de Kloet ER, Schenberg LC (2018) Daily maternal separations during stress hyporesponsive period decrease the thresholds of panic-like behaviors to electrical stimulation of the dorsal periaqueductal gray of the adult rat. Behav Brain Res 344:132–144. https://doi.org/10.1016/j.bbr.2018.02.020
Cao B, Wang J, Zhang X, Yang X, Poon DC-H, Jelfs B, Chan RH, Wu JC-Y, Li Y (2016) Im-pairment of decision making and disruption of synchrony between basolateral amygdala and anterior cingulate cortex in the maternally separated rat. Neurobiol Learn Mem 136:74–85. https://doi.org/10.1016/j.nlm.2016.09.015
de Melo SR, de David Antoniazzi CT, Hossain S, Kolb B (2018) Neonatal Stress Has a Long-Lasting Sex-Dependent Effect on Anxiety-Like Behavior and Neuronal Morphology in the Prefrontal Cortex and Hip-pocampus. Dev Neurosci 40(2):93–103. https://doi.org/10.1159/000486619
Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan J, Dinan T (2010) Effects of the probiotic Bifidobac-terium infantis in the maternal separation model of depression. Neuroscience 170(4):1179–1188. https://doi.org/10.1016/j.neuroscience.2010.08.005
de Souza JA, da Silva MC, de Souza Ferraz Junior JC, de Souza FL, de Souza SL (2022) Mater-nal separation in the light or dark phase of the circadian cycle has different effects on the corticosterone levels and anxiety-like behavior in male adult rats. Physiol Behav 247:113725. https://doi.org/10.1016/j.physbeh.2022.113725
Dimatelis JJ, Vermeulen IM, Bugarith K, Stein DJ, Russell VA (2016) Female rats are resistant to developing the depressive phenotype induced by maternal separation stress. Metab Brain Dis 31(1):109–119. https://doi.org/10.1007/s11011-015-9723-8
Dimatelis J, Stein D, Russell V (2012) Behavioral changes after maternal separation are reversed by chronic constant light treatment. Brain Res 1480:61–71. https://doi.org/10.1016/j.brainres.2012.07.013
Egerton S, Donoso F, Fitzgerald P, Gite S, Fouhy F, Whooley J, Dinan TG, Cryan JF, Culloty SC, Ross RP, Stanton C (2022) Investigating the potential of fish oil as a nutraceutical in an animal model of early life stress. Nutr Neurosci 25(2):356–378. https://doi.org/10.1080/1028415X.2020.1753322
Eiland L, McEwen BS (2012) Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22(1):82–91. https://doi.org/10.1002/hipo.208624
Englund J, Haikonen J, Shteinikov V, Amarilla SP, Atanasova T, Shintyapina A, Ryazantseva M, Par-tanen J, Voikar V, Lauri SE (2021) Downregulation of kainate recetors regulating GABAergic transmis-sion in amygdala after early life stress is associated with anxiety-like behavior in rodents. Transl Psy-Chiatry 11(1):538. https://doi.org/10.1038/s41398-021-01654-7
Faure J, Uys JDK, Marais L, Stein DJ, Daniels WMU (2007) Early maternal separation alters the response to traumatization: Resulting in increased levels of hippocampal neurotrophic factors. Metab Brain Dis 22(2):183–195. https://doi.org/10.1007/s11011-007-9048-3
Frank D, Zlotnik A, Kofman O, Grinshpun J, Severynovska O, Brotfain E, Kut R, Natanel D, Melamed I, Boyko M (2019) Early life stress induces submissive behavior in adult rats. Behav Brain Res 372:112025. https://doi.org/10.1016/j.bbr.2019.112025
Frankola KA, Flora AL, Torres AK, Grissom EM, Overstreet S, Dohanich GP (2010) Effects of early rearing conditions on cognitive performance in prepubescent male and female rats. Neurobiol Learn Mem 94(1):91–99. https://doi.org/10.1016/j.nlm.2010.04.005
Gazzo G, Melchior M, Caussaint A, Gieré C, Lelièvre V, Poisbeau P (2021) Overexpression of chlo-ride importer NKCC1 contributes to the sensory-affective and sociability phenotype of rats following neonatal maternal separation. Brain Behav Immun 92:193–202. https://doi.org/10.1016/j.bbi.2020.12.010
Gildawie KR, Ryll LM, Hexter JC, Peterzell S, Valentine AA, Brenhouse HC (2021) A two-hit adversity model in developing rats reveals sex-specific impacts on prefrontal cortex structure and behavior. Dev Cogn Neurosci 48:100924. https://doi.org/10.1016/j.dcn.2021.100924
González-Pardo H, Arias JL, Vallejo G, Conejo NM (2019) Environmental enrichment effects after early stress on behavior and functional brain networks in adult rats (A. Kavushansky, Ed.). Plos One 14(12):0226377. https://doi.org/10.1371/journal.pone.0226377
Huang J, Shen C, Ye R, Shi Y, Li W (2021) The Effect of Early Maternal Separation Combined With Adolescent Chronic Unpredictable Mild Stress on Behavior and Synaptic Plasticity in Adult Female Rats. Front Psychiatry 12:539299. https://doi.org/10.3389/fpsyt.2021.539299
Hui J-J, Zhang Z-J, Liu S-S, Xi G-J, Zhang X-R, Teng G-J, Chan KC, Wu EX, Nie B-B, Shan B-C, Li L-J, Reynolds GP (2011) Hippocampal neurochemistry is involved in the behavioural effects of neo-natal maternal separation and their reversal by post-weaning environmental enrichment: A magnetic resonance study. Behav Brain Res 217(1):122–127. https://doi.org/10.1016/j.bbr.2010.10.014
Jaimes-Hoy L, Gutiérrez-Mariscal M, Vargas Y, Pérez-Maldonado A, Romero F, Sánchez-Jaramillo E, Charli J-L, Joseph-Bravo P (2016) Neonatal Maternal Separation Alters, in a Sex-Specific Manner, the Expression of TRH, of TRH-Degrading Ectoenzyme in the Rat Hypothalamus, and the Response of the Thy-roid Axis to Starvation. Endocrinology 157(8):3253–3265. https://doi.org/10.1210/en.2016-1239
Jin S, Zhao Y, Jiang Y, Wang Y, Li C, Zhang D, Lian B, Du Z, Sun H, Sun L (2018) Anxiety-like behaviour assessments of adolescent rats after repeated maternal separation during early life. NeuroReport 29(8):643–649. https://doi.org/10.1097/WNR.0000000000001010
Jones NC, Kumar G, O’Brien TJ, Morris MJ, Rees SM, Salzberg MR (2009) Anxiolytic effects of rapid amygdala kindling, and the influence of early life experience in rats. Behav Brain Res 203(1):81–87. https://doi.org/10.1016/j.bbr.2009.04.023
Kim H-B, Yoo J-Y, Yoo S-Y, Suh SW, Lee S, Park JH, Lee J-H, Baik T-K, Kim H-S, Woo R-S (2020) Early-life stress induces EAAC1 expression reduction and attention-deficit and depressive behaviors in adolescent rats. Cell Death Discovery 6:73. https://doi.org/10.1038/s41420-020-00308-9
Lajud N, Roque A, Cajero M, Gutiérrez-Ospina G, Torner L (2012) Periodic maternal separation de-creases hippocampal neurogenesis without affecting basal corticosterone during the stress hyporesponsive peri-od, but alters HPA axis and coping behavior in adulthood. Psychoneuroendocrinology 37(3):410–420. https://doi.org/10.1016/j.psyneuen.2011.07.011
Lee J-H, Kim JY, Jahng JW (2014) Highly Palatable Food during Adolescence Improves Anxiety-Like Behaviors and Hypothalamic-Pituitary-Adrenal Axis Dysfunction in Rats that Experienced 5 Neonatal Mater-nal Separation. Endocrinol Metab 29(2):169. https://doi.org/10.3803/EnM.2014.29.2.169
Lee YJ, Koe AS, Ashokan A, Mitra R (2020) Female rats are resilient to the behavioral effects of ma-ternal separation stress and exhibit stress-induced neurogenesis. Heliyon 6(8):e04753. https://doi.org/10.1016/j.heliyon.2020.e04753
León Rodríguez DA, Dueῆas Z (2013) Maternal Separation during Breastfeeding Induces GenderDependent Changes in Anxiety and the GABAA Receptor AlphaSubunit in Adult Wistar Rats (J Hombergr Ed). PLoS ONE 8(6):68010
Li M, Xue X, Shao S, Shao F, Wang W (2013) Cognitive, emotional and neurochemical effects of re-peated maternal separation in adolescent rats. Brain Res 1518:82–90. https://doi.org/10.1016/j.brainres.2013.04.026
Majcher-Maślanka I, Solarz A, Chocyk A (2019) Maternal separation disturbs postnatal development of the medial prefrontal cortex and affects the number of neurons and glial cells in adolescent rats. Neuroscience 423:131–147. https://doi.org/10.1016/j.neuroscience.2019.10.033
Maniam J, Morris MJ (2010a) Long-term postpartum anxiety and depression-like behavior in mother rats subjected to maternal separation are ameliorated by palatable high fat diet. Behav Brain Res 208(1):72–79. https://doi.org/10.1016/j.bbr.2009.11.005
Maniam J, Morris MJ (2010) Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology 35(5):717–728. https://doi.org/10.1016/j.psyneuen.2009.10.013
Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WM (2008) Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corti-costerone and neurotrophin levels in the hippocampus. Neurosci Res 61(1):106–112. https://doi.org/10.1016/j.neures.2008.01.011
Melo C, Vizin R, Silva N, Ishikawa D, Echeverry M, Carrettiero D, Almeida M (2018) Early maternal separation promotes alterations in the thermoregulatory profile of adult Wistar rats. J Therm Biolo-Gy 78:151–160. https://doi.org/10.1016/j.jtherbio.2018.09.013
Oines E, Murison R, Mrdalj J, Grønli J, Milde AM (2012) Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol Behav 105(4):1058–1066. https://doi.org/10.1016/j.physbeh.2011.11.024
Papadakakis A, Sidiropoulou K, Panagis G (2019) Music exposure attenuates anxiety- and depression-like behaviors and increases hippocampal spine density in male rats. Behav Brain Res 372:112023. https://doi.org/10.1016/j.bbr.2019.112023
Peng H-H, Tsai T-C, Huang W-Y, Wu H-M, Hsu K-S (2019) Probiotic treatment restores normal de-velopmental trajectories of fear memory retention in maternally separated infant rats. Neuropharmacology 153:53–62. https://doi.org/10.1016/j.neuropharm.2019.04.026
Ploj K, Roman E, Nylander I (2002) Effects of maternal separation on brain nociceptin/orphanin FQ peptide levels in male Wistar rats. Pharmacol Biochem Behav 73(1):123–129. https://doi.org/10.1016/S0091-3057(02)00778-5
Roque A, Ruiz-González R, Pineda-López E, Torner L, Lajud N (2020) Prenatal immobilization stress and postnatal maternal separation cause differential neuroendocrine responses to fasting stress in adult male rats. Dev Psychobiol 62(6):737–748. https://doi.org/10.1002/dev.21947
Roque A, Valles Méndez KM, Ruiz R, Pineda E, Lajud N (2021) Early life stress induces a transient increase in hippocampal corticotropin-releasing hormone in rat neonates that precedes the effects on hypotha-lamic neuropeptides. Eur J Neurosci. https://doi.org/10.1111/ejn.15193
Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M (2014) The behavioral and immunolog-ical impact of maternal separation: A matter of timing. Front Behav Neurosci 8:192. https://doi.org/10.3389/fnbeh.2014.00192
Ruiz R, Roque A, Pineda E, Licona-Limón P, José Valdéz-Alarcón J, Lajud N (2018) Early life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk. Psychoneuroendocrinology 96:203–211. https://doi.org/10.1016/j.psyneuen.2018.07.0126
Sadeghi M, Peeri M, Hosseini M-J (2016) Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol Behav 163:177–183. https://doi.org/10.1016/j.physbeh.2016.05.017
Salberg S, Noel M, Burke NN, Vinall J, Mychasiuk R (2020) Utilization of a rodent model to examine the neurological effects of early life adversity on adolescent pain sensitivity. Dev Psychobiol 62(3):386–399. https://doi.org/10.1002/dev.21922
Salzberg M, Kumar G, Supit L, Jones NC, Morris MJ, Rees S, O’Brien TJ (2007) Early Postnatal Stress Confers Enduring Vulnerability to Limbic Epileptogenesis. Epilepsia 48(11):2079–2085. https://doi.org/10.1111/j.1528-1167.2007.01246.x
Seo MK, Ly NN, Lee CH, Cho HY, Choi CM, Nhu LH, Lee JG, Lee BJ, Kim G-M, Yoon BJ, Park SW, Kim YH (2016) Early life stress increases stress vulnerability through BDNF gene epigenetic changes in the rat hippocampus. Neuropharmacology 105:388–397. https://doi.org/10.1016/j.neuropharm.2016.02.009
Shi D-D, Zhang Y-D, Ren Y-Y, Peng S-Y, Yuan T-F, Wang Z (2021) Predictable maternal separa-tion confers adult stress resilience via the medial prefrontal cortex oxytocin signaling pathway in rats. Mol Psychiatry. https://doi.org/10.1038/s41380-021-01293-w
Shu C, Xiao L, Tang J, Wang G, Zhang X, Wang X (2015) Blunted behavioral and molecular respons-es to chronic mild stress in adult rats with experience of infancy maternal separation. Tohoku J Exp Med 235(2):81–87. https://doi.org/10.1620/tjem.235.81
Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KCF (2006) Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: Gender-dependent effects. Brain Res 1097(1):123–132. https://doi.org/10.1016/j.brainres.2006.04.066
Solarz A, Majcher-Maślanka I, Kryst J, Chocyk A (2021) A Search for Biomarkers of Early-life Stress-related Psychopathology: Focus on 70-kDa Heat Shock Proteins. Neuroscience 463:238–253. https://doi.org/10.1016/j.neuroscience.2021.02.026
Stevenson CW, Meredith JP, Spicer CH, Mason R, Marsden CA (2009) Early life programming of innate fear and fear learning in adult female rats. Behav Brain Res 198(1):51–57. https://doi.org/10.1016/j.bbr.2008.10.021
Trujillo V, Durando PE, Suárez MM (2016) Maternal separation in early life modifies anxious behav-ior and Fos and glucocorticoid receptor expression in limbic neurons after chronic stress in rats: Effects of tian-eptine. Stress (Amsterdam, Netherlands) 19(1):91–103. https://doi.org/10.3109/10253890.2015.1105958
Tsotsokou G, Nikolakopoulou M, Kouvelas ED, Mitsacos A (2021) Neonatal maternal separation affects metabotropic glutamate receptor 5 expression and anxiety-related behavior of adult rats. Eur J Neurosci 54(2):4550–4564. https://doi.org/10.1111/ejn.15358
van Zyl PJ, Dimatelis JJ, Russell VA (2013) Changes in behavior and ultrasonic vocalizations during antidepressant treatment in the maternally separated Wistar-Kyoto rat model of depression. Metab Brain Dis. https://doi.org/10.1007/s11011-013-9463-6
Vargas J, Junco M, Gomez C, Lajud N (2016) Early Life Stress Increases Metabolic Risk, HPA Axis Re-activity, and Depressive-Like Behavior When Combined with Postweaning Social Isolation in Rats (C. M. McCormick, Ed.). PLOS ONE 11(9):e0162665. https://doi.org/10.1371/journal.pone.0162665
Wang Q, Li M, Du W, Shao F, Wang W (2015) The different effects of maternal separation on spatial learning and reversal learning in rats. Behav Brain Res 280:16–23. https://doi.org/10.1016/j.bbr.2014.11.040
Wei Y, Wang G, Wang H, He J, Zhang N, Wu Z, Xiao L, Yang C (2018) Sex-dependent impact of different degrees of maternal separation experience on OFT behavioral performances after adult chronic un-predictable mild stress exposure in rats. Physiol Behav 194:153–161. https://doi.org/10.1016/j.physbeh.2018.04.0347
Xiong G-J, Yang Y, Wang L-P, Xu L, Mao R-R (2014) Maternal separation exaggerates spontaneous recovery of extinguished contextual fear in adult female rats. Behav Brain Res 269:75–80. https://doi.org/10.1016/j.bbr.2014.04.015
Acknowledgements
JWD acknowledges funding from a GlaxoSmithKline (GSK)-Varsity award. OS acknowledges funding from the MB/PhD programme at Cambridge University. The Behavioural and Clinical Neuroscience Institute at Cambridge University received core funding from the Medical Research Council (G1000183) and Wellcome Trust (093875/Z/10/Z).
Disclosures
JWD has received funding from GSK and Boehringer Ingelheim Pharma GmbH & Co. TWR consults for Cambridge Cognition, and is in receipt of a research grant from Shionogi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on Innovating translational models of affective disorders
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stupart, O., Robbins, T.W. & Dalley, J.W. “The wrong tools for the right job”: a critical meta-analysis of traditional tests to assess behavioural impacts of maternal separation. Psychopharmacology 240, 2239–2256 (2023). https://doi.org/10.1007/s00213-022-06275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06275-6