Abstract
Rationale
Chronic stress and corticosterone have been shown to affect serotonin (5-HT) neurotransmission; however, the influence of stress on the activity of the dorsal raphe nucleus (DRN), the main source of 5-HT in the forebrain, is not well understood. In particular, it is unknown if and how stress modifies DRN 5-HT7 receptors, which are involved in the modulation of the firing of local inhibitory interneurons responsible for regulating the activity of DRN projection cells.
Objectives
Our study aimed to investigate the effect of repeated corticosterone injections on the modulation of the inhibitory transmission within the DRN by 5-HT7 receptors and whether it could be reversed by treatment with a 5-HT7 receptor antagonist.
Methods
Male Wistar rats received corticosterone injections repeated twice daily for 14 days. Spontaneous inhibitory postsynaptic currents (sIPSCs) were then recorded from DRN projection cells in ex vivo slice preparations obtained 24 h after the last injection.
Results
Repeated corticosterone administration resulted in decreased frequency, but not amplitude, of sIPSCs in DRN projection cells. There were no changes in the excitability of these cells; however, corticosterone treatment suppressed the 5-HT7 receptor-mediated increase in sIPSC frequency. Administration of the 5-HT7 receptor antagonist SB 269970 for 7 days beginning on the eighth day of corticosterone treatment reversed the detrimental effects of corticosterone on 5-HT7 receptor reactivity and GABAergic transmission in the DRN.
Conclusions
Elevated corticosterone level reduces DRN 5HT7 receptor reactivity and decreases GABAergic transmission within the DRN, which can be reversed by the 5-HT7 receptor antagonist SB 269970.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic stress and elevated blood cortisol levels are risk factors causally related to the development of a number of human pathologies including depressive disorders (reviewed in: Claes 2004; Parker et al. 2003; Reagan et al. 2008). It has been established that the pathomechanism of depressive disorders involves dysfunctions of the brain serotonin (5-hydroxytryptamine, 5-HT) system (reviewed in: Stahl 1998; Köhler et al. 2016). Animal studies have shown that both acute and chronic stressors affect 5-HT neurotransmission, evoking alterations in 5-HT release and reuptake as well as changes in extracellular 5-HT and 5-HT receptors’ levels (Adell et al. 1988; reviewed in: Chaouloff 2000).
The dorsal raphe nucleus (DRN) is the main source of widespread 5-HT innervation, which modulates the activity of neuronal networks in target forebrain structures via several 5-HT receptor subtypes (reviewed in: Jacobs and Azmitia 1992; Celada et al. 2013). 5-HT may also influence the activity of DRN 5-HT neurons themselves as these cells express 5-HT1A, 5-HT1B, 5-HT1D, and possibly 5-HT2 autoreceptors (McDevitt and Neumaier 2011). 5-HT neurons of the DRN are known to express glucocorticoid receptors (GRs; Harfstrand et al. 1986). Chronic exposure to elevated corticosterone levels and uncontrollable stress have been demonstrated to induce a functional desensitization of 5-HT1A somatodendritic autoreceptors (Fairchild et al. 2003; Rainer et al. 2012; Rozeske et al. 2011; reviewed in: McDevitt and Neumaier 2011), which normally effectively attenuate spiking activity of DRN 5-HT projection neurons (Aghajanian et al. 1990). Apart from 5-HT projections cells, the DRN contains a population of GABAergic interneurons which provide inhibitory input onto DRN projection cells. These inhibitory interneurons express 5-HT2A/C and possibly 5-HT1A and 5-HT6 receptors (Asaoka et al. 2015; Gocho et al. 2013; Liu et al. 2000). Another serotonin receptor abundant in the DRN is the 5-HT7 receptor (Roberts et al. 2004). Its role in the regulation of the activity of DRN neuronal network in normal and pathological conditions is not completely understood. Importantly, 5-HT7 receptors in the DRN are present on GABAergic interneurons, but not on 5-HT cells (Monti et al. 2008; Kusek et al. 2015). 5-HT7 receptor activation increases the mean frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) recorded from DRN projection cells, as well as induces their hyperpolarization and decreases their firing frequency (Kusek et al. 2015). Systemic blockade of 5-HT7 receptors enhances 5-HT transmission and metabolism in target structures (Mnie-Filali et al. 2011; Kusek et al. 2015). While modifications of the GABAergic input onto DRN 5-HT neurons have been shown to play an important role in facilitating certain stress-induced behaviors (Challis et al. 2013; Crawford et al. 2013), the influence of prolonged elevation of corticosterone level on GABAergic transmission within the DRN and its modulation by the 5-HT7 receptor has not yet been investigated. Repeated corticosterone administration has been proposed as a preclinical rat model to study links between stress, glucocorticoids, and depressive behavior (reviewed in: Sterner and Kalynchuk 2010). In the present study, we aimed at determining the effects of corticosterone treatment lasting 14 days on the ability of the 5-HT7 receptor to modulate inhibitory transmission in the DRN.
5-HT7 receptor blockade has been shown to induce antidepressant-like effects in animal models (reviewed in: Ciranna and Catania 2014; Nikiforuk 2015). These effects occur faster than those induced by conventional antidepressant drugs (Mnie-Filali et al. 2011; reviewed in: Tokarski et al. 2012a). We have previously demonstrated that treatment with the selective 5-HT7 receptor antagonist SB 269970 counteracted repeated restraint stress-induced modifications of glutamatergic transmission and synaptic plasticity in the rat frontal cortex (Tokarski et al. 2011). However, the plausible influence of 5-HT7 receptor antagonism on the effects of prolonged corticosterone treatment on functional properties of DRN neurons has not yet been investigated. Therefore, the second aim of the present study was to determine whether blocking 5-HT7 receptors with SB 269970 could reverse corticosterone-induced alterations in 5-HT7 receptor-dependent modulation of GABAergic transmission in the DRN.
Experimental procedures
Animals
The experimental procedures were approved by the Local Ethics Committee for Animal Experiments at the Institute of Pharmacology, Polish Academy of Sciences, and were carried out in accordance with the European Community guidelines for the use of experimental animals and the national law. Male Wistar rats (Charles River, Germany) weighing approx. 150 g at the beginning of the experiment were housed in standard laboratory cages and maintained on a 12-h light/dark schedule (lights on at 07:00 and off at 19:00) with free access to standard food and tap water.
Treatment
The animals were assigned to four groups. In the first experimental group (termed: Cort + SB), animals received corticosterone injections subcutaneously (dose 10 mg/kg, volume 1 ml/kg; in 1% solution of Tween 80 in water; Zahorodna and Hess 2006) twice daily for 14 days and, beginning on the eighth day of corticosterone treatment, additionally the 5-HT7 receptor antagonist, SB 269970 (Tocris) for 7 days. SB 269970 was dissolved in 0.9% NaCl and injected intraperitoneally (dose 2.5 mg/kg, volume 1 ml/kg) once daily. The second group (termed: Cort + NaCl) received corticosterone for 14 days and, beginning on the eighth day of corticosterone treatment, injections of 0.9% NaCl for 7 days. Control rats for these treatments received 1% Tween 80 for 14 days and SB 269970 for 7 days (termed: Tween + SB) or 1% Tween 80 for 14 days and 0.9% NaCl for 7 days (termed: Tween + NaCl). The number of animals in each group was 10.
Slice preparation and incubation
Brain slices were prepared 24 h after the last substance administration to avoid acute effects of corticosterone and SB 269970 (Hagan et al. 2000; Droste et al. 2008). Rats were anesthetized with isoflurane (Aerrane, Baxter, UK) and decapitated. Their brains were quickly removed and immersed in an ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) NaCl (130), KCl (5), CaCl2 (2.5), MgSO4 (1.3), KH2PO4 (1.25), NaHCO3 (26), and d-glucose (10), bubbled with a mixture of 95% O2–5% CO2. Coronal midbrain slices containing the DRN (thickness 300 μm) were cut using a vibrating microtome (Leica VT1000) and subsequently stored submerged in ACSF at 30 ± 0.5 °C. Two or three slices were obtained from each animal.
Whole-cell recording
After at least 3 h of preincubation, an individual slice was placed in the recording chamber and superfused without recycling at 2.5 ml/min with warm (32 ± 0.5 °C; Bacon and Beck 2000, Liu et al. 2000), modified ACSF of the following composition (in mM): NaCl (132), KCl (2), CaCl2 (2.5), MgSO4 (1.3), KH2PO4 (1.25), NaHCO3 (26), and d-glucose (10), bubbled with 95% O2–5% CO2. Modified ACSF also contained N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY 100635, Tocris, 2 μM) to block 5-HT1A receptors, as during the recordings, 5-carboxyamidotryptamine (5-CT, Tocris; 250 nM), a nonselective 5-HT1A and 5-HT7 receptor agonist, was used to activate 5-HT7 receptors.
Whole-cell recordings were obtained from the dorsal part of the midline region of the DRN. Neurons were visualized using the Zeiss Axioscope 2 upright microscope (Nomarski optics), a × 40 water immersion objective and an infrared camera. Recording pipettes were pulled from borosilicate glass capillaries (Harvard Apparatus), using the Sutter Instrument P97 puller. The pipette solution contained (in mM) K-gluconate (130), NaCl (5), CaCl2 (0.3), MgCl2 (2), HEPES (10), Na2-ATP (5), Na-GTP (0.4), and EGTA (1). Osmolarity and pH were adjusted to 290 mOsm and 7.2, respectively. Pipettes had an open tip resistance of approx. 6 MΩ. Signals were recorded using the SEC 05LX amplifier (NPI, Germany), filtered at 2 kHz and digitized at 20 kHz using a Digidata 1440A interface and Clampex 10 software (Molecular Devices, USA).
Putative 5-HT DRN projection neurons were identified by their response to hyper- and depolarizing current pulses (duration 400 ms; Galindo-Charles et al. 2008; Kusek et al. 2015). For each cell, the relationship between the injected current intensity and the number of spikes was plotted and gain was determined as the slope of a straight line fitted to experimental data.
Cells were voltage-clamped at 0 mV, and after 15 min of stabilization sIPSCs were recorded for 4 min as outwards currents. We have previously shown that these currents could be blocked by bicuculline (Kusek et al. 2015). Next, 5-carboxyamidotryptamine (5-CT, Tocris; 250 nM) was added to the ACSF (Kusek et al. 2015). Following 15 min of stabilization, sIPSCs were again recorded for 4 min. Data were accepted for analysis when the access resistance ranged between 15 and 18 MΩ and remained stable (< 25% change) during the recording.
Spontaneous IPSCs were detected offline using Mini Analysis software (Synaptosoft), and individual synaptic events were manually selected for further analysis. The threshold amplitude for the detection of an IPSC was set to 10 pA. IPSC kinetics were determined from the averaged IPSC for each cell. Rise time was measured as the time needed for the current to rise from 10 to 90% of the peak. The decay time constant (tau) was determined from fitting an exponential function to the decay phase of the IPSC.
Statistical analysis
Rats were weighed on a daily basis. Average growth curves for each experimental treatment group were constructed by fitting linear regression lines to raw data. Slopes for the individual fits were compared across groups using the two-way analysis of variance (ANOVA), followed by Sidak’s multiple comparisons test. Statistical analyses of the electrophysiological data were carried out using two-way ANOVA followed by Sidak’s multiple comparison test. For estimating the effects of 5-CT on synaptic activity in brain slices from the different treatment groups, the percentage change relative to baseline was used as the dependent variable. The analysis was performed in GraphPad Prism 7 software. The results are expressed as the mean ± SEM. The significance level was set at p = 0.05 for all comparisons.
Results
SB 269970 does not change the effect of corticosterone on animal body weight
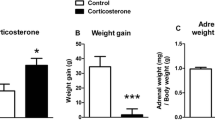
Rats from the Cort + NaCl group gained significantly less weight compared to the animals receiving the vehicle (Tween + NaCl group; Fig. 1). Concurrent administration of SB 269970 and corticosterone (Cort + SB group) did not modify the effect of corticosterone on body weight gain (measured as the slope of the linear regression line). No differences were evident between rats receiving the vehicle and SB 269970 injections (Tween + SB group) and the Tween + NaCl group. A significant main effect of corticosterone on body weight gain (F(1, 36) = 147.1, p < 0.0001) was observed. Animals from the Cort + NaCl and Cort + SB groups gained weight significantly slower than Tween + NaCl receiving rats (p < 0.0001, Sidak’s multiple comparisons test).
Effects of repeated corticosterone and 5-HT7 receptor antagonist SB 269970 injections on animal body weight. Rats from the Cort + NaCl group, as well as from the Cort + SB group, gained significantly less weight compared to control (Tween + NaCl) animals. Concurrent administration of SB 269970 and corticosterone (Cort + SB) did not modify the effect of corticosterone on body weight gain (measured as the slope of the linear regression line). No differences were evident between the Tween + SB and the Tween + NaCl group. The number of animals in each group was 10. Data are mean ± SEM. ***p < 0.001. The arrow indicates the first day of SB 269970 injections
Lack of effect of corticosterone and SB 269970, alone and in combination, on DRN neuronal excitability
All cells subjected to analysis, when stimulated by depolarizing current pulses fired broad action potentials with a “notch” on their descending phase (Fig. 2), characteristic of putative 5-HT projection neurons (see also: Galindo-Charles et al. 2008; Kusek et al. 2015). The resting membrane potential and input resistance of recorded DRN neurons did not differ significantly between groups (Table 1). In addition, there were no differences in neuronal excitability between any groups.
Repeated corticosterone and SB 269970 injections do not influence basic electrophysiological properties and excitability of DRN projection neurons. a Examples of single action potentials from all examined groups, with the “notch” on their descending phase marked with an asterisk. b Responses of representative projection neuron to different current injections (step: 20 pA) recorded in DRN slice prepared from control (Tween + NaCl) animal. c Relationship between spiking rate and injected current for the cell shown in panel b. The slope of the straight line fitted to experimental data represents gain. d Summary graph showing the mean gain (± SEM) of all neurons from the Tween + NaCl, Tween + SB, Cort + NaCl, and Cort + SB-treated rats. The differences between groups are not significant
SB 269970 reverses effects of corticosterone treatment on the inhibitory input to DRN neurons
There was a significant effect of corticosterone treatment (F(1, 60) = 14.66, p = 0.0003), SB 269970 treatment (F(1, 60) = 15.31, p = 0.0002), and their interaction (F(1, 60) = 14.99, p = 0.0003) on the frequency of sIPSCs. The sIPSC frequency was lower in the group receiving corticosterone and 0.9% NaCl injections (Cort + NaCl) compared to the Tween + NaCl group (0.27 ± 0.02 Hz vs. 0.82 ± 0.08 Hz, respectively; n = 17 and 14, t = 5.361, df = 60, p < 0.0001; Sidak’s multiple comparison test), as well as when compared to the Cort + SB group (0.27 ± 0.02 Hz vs. 0.83 ± 0.06 Hz, respectively; n = 17 and 18, t = 5.783, df = 60, p < 0.0001; Sidak’s multiple comparison test; Fig. 3b1, c1). No differences between the Cort + SB and Tween + NaCl groups were observed (0.83 ± 0.06 vs. 0.82 ± 0.08, respectively; n = 18 and 14, t = 0.059, df = 60, p = 0.9999; Sidak’s multiple comparison test). There were no significant effects of SB 269970 injections (Tween + SB) on sIPSC frequency compared to control (Tween + NaCl) (0.82 ± 0.10 vs. 0.82 ± 0.08, respectively; n = 15 and 14, t = 0.028, df = 60, p = 0.9999; Sidak’s multiple comparison test).
SB 269970 reverses the effect of repeated corticosterone administration on DRN GABAergic transmission. a Sample recordings from representative neurons in slices prepared from animals treated with Tween + NaCl (first trace), Tween + SB (second trace), Cort + NaCl (third trace), and Cort + SB (fourth trace). Dots mark spontaneous synaptic events. (b1) Cumulative probability plots of inter-event intervals of sIPSCs recorded from individual representative neurons from all four groups of rats. b2 Cumulative probability plots of amplitudes of sIPSCs recorded from individual representative neurons. c1 Summary graph showing the mean frequency (± SEM) of sIPSCs recorded from all neurons from the Tween + NaCl, Tween + SB, Cort + NaCl, and Cort + SB-treated rats. ***p < 0.001. c2 A comparison of the mean amplitude (± SEM) of sIPSCs recorded from all neurons of the four investigated groups of animals. Labels as in panel c1
The analysis did not reveal any effect of treatments (corticosterone treatment: F(1, 60) = 0.8172, p = 0.3696; SB 269970 treatment: F(1, 60) = 1.851, p = 0.1787) or their interaction (F(1, 60) = 0.2929, p = 0.5903) on sIPSC amplitude (Fig. 3b2, c2).
No significant effect of SB 269970 treatment (F(1, 60) = 0.4056, p = 0.5266) and interaction (F(1, 60) = 0.1992, p = 0.6569), but significant effect of corticosterone treatment (F(1, 60) = 6.739, p = 0.0118) on the rise time of sIPSCs was observed. However, multiple comparisons with Sidak’s test did not reveal any significant differences between groups. A significant effect of treatment with SB 269970 (F(1, 60) = 5.292, p = 0.0249) on the decay time constant of sIPSCs and interaction (F(1, 60) = 3.877, p = 0.0536) with no effect of corticosterone treatment (F(1, 60) = 1.929, p = 0.1700) was observed (Table 2). The decay time constant was found to be shorter in the group of animals receiving Tween and SB 269970 injections compared to control (6.58 ± 0.19 vs. 7.46 ± 0.17, respectively; n = 15 and 14, t = 2.886, df = 60, p = 0.032; Sidak’s multiple comparison test).
Changes in 5-HT7 receptor function induced by corticosterone treatment are reversed by SB 269970
A two-way ANOVA revealed a significant interaction between corticosterone and SB 269970 treatments on the 5-CT-induced percentage change in sIPSC frequency (F(1,60) = 8.767, p = 0.0044). This was not accompanied by significant main effects of either corticosterone treatment (F(1,60) = 2.966, p = 0.0902) or SB 269970 treatment (F(1,60) = 3.329, p = 0.0731). The 5-CT-induced increase in sIPSC frequency was similar in the Tween + NaCl and Tween + SB groups (114.14 ± 3.14%, n = 14, vs. 116.65 ± 6.66%, n = 15 respectively, adjusted p = 0.9709, post hoc Sidak’s multiple comparisons test). Corticosterone abolished the 5-HT7 effect as there was no 5-CT-mediated increase in sIPSC frequency in the Cort + NaCl group when compared to the Tween + NaCl group (97.49 ± 4.03%, n = 17, vs. 114.14 ± 3.14%, n = 14 respectively, adjusted p = 0.0110, post hoc Sidak’s multiple comparisons test). Co-administration of corticosterone and SB 269970 fully rescued the effect of 5-CT on sIPSC frequency, back to the level seen in the Tween + NaCl group (116.10 ± 3.91%, n = 18, vs. 114.14 ± 3.14%, n = 14, respectively, adjusted p > 0.9999, post hoc Sidak’s multiple comparisons test) (Fig. 4).
SB 269970 reverses the effect of repeated corticosterone administration on 5-HT7 receptor reactivity in the DRN. a1 Sample recordings from a representative neuron in slice prepared from the rat receiving Tween + NaCl before (upper trace) and after addition of 250 nM 5-CT to the ACSF (lower trace). a2 Sample recordings from a representative neuron in slice prepared from the animal receiving Cort + NaCl before (upper trace) and after addition of 250 nM 5-CT to the ACSF (lower trace). Gray dots mark spontaneous synaptic events accepted for analysis. b The effect of 5-HT7 receptor activation on the sIPSC frequency (mean ± SEM) shown as a percentage of basal sIPSC frequency (before 5-CT addition); *p < 0.05; **p < 0.01; ns non-significant effect
There were no significant effects of corticosterone treatment, SB 269970 treatment, or their interaction on 5-CT-induced percentage changes in sIPSC amplitude (main effect of corticosterone treatment: F(1, 60) = 0.3492, p = 0.8524; main effect of SB 269970 treatment: F(1, 60) = 0.5211, p = 0.4732; interaction: F(1, 60) = 0.1256, p = 0.7243), sIPSC rise time (main effect of corticosterone treatment: F(1, 60) = 0.1341, p = 0.7155; main effect of SB 269970 treatment: F(1, 60) = 0.5632, p = 0.4559; interaction: F(1, 60) = 3.2700, p = 0.0756), and sIPSC decay time constant (main effect of corticosterone treatment: F(1, 60) = 0.3060, p = 0.5822, main effect of SB 269970 treatment: F(1, 60) = 0.5458, p = 0.4629; interaction: F(1, 60) = 1.9230, p = 0.1706).
Discussion
The results of the present study demonstrate that corticosterone treatment lasting 14 days induced a decrease in the frequency of sIPSCs recorded from DRN projection neurons without significant changes in the mean amplitude and kinetic properties, apart from a small decrease in the decay time constant in the case of sIPSCs in cells from the Tween + SB group of animals. These observations are suggestive of a presynaptic location of corticosterone-induced effects on GABAergic transmission within the DRN. In line with this finding, a moderate, chronic increase in circulating corticosterone level has been shown to exert no significant effect on the response of rat DRN 5-HTergic cells to either GABAA or GABAB receptor activation (Judge et al. 2004). In the ventromedial DRN of stressed mice subjected to 5-day social defeat, a decrease in the frequency and a smaller decrease in the amplitude of sIPSCs have been observed (Crawford et al. 2013). It is thus likely that repeated administration of corticosterone brought about a decrease in neurotransmitter release from GABAergic terminals due to a decrease in spiking activity of DRN GABAergic interneurons. It should also be noted that since the DRN receives GABAergic inputs from extrinsic sources including the hypothalamus, substantia nigra, ventral tegmental area, and rostromedial tegmental nucleus (reviewed in: Soiza-Reilly and Commons 2014), it is conceivable that systemic administration of corticosterone reduced the activity of these inhibitory afferent projections as well.
Obtained results demonstrate that repeated corticosterone administration suppressed the reactivity of 5-HT7 receptors in the DRN. These receptors appear to be located on DRN GABA interneurons, but not projection neurons, and their activation has earlier been shown to increase the frequency of sIPSCs recorded from DRN 5-HT projection neurons (Mnie-Filali et al. 2011; Kusek et al. 2015), consistent with the fact that 5-HT7 receptors’ agonists raise the excitability of the neuron that expresses these receptors (Bacon and Beck 2000; Bickmeyer et al. 2002; Tokarski et al. 2003). Thus, another consequence of prolonged corticosterone exposure is a lack of 5-HT7 receptor-mediated indirect inhibitory effect on DRN projection cells. It is tempting to speculate that observed suppression of the reactivity of DRN 5-HT7 receptors resulted from enhanced 5-HT release within the DRN. It has been reported that treatment with corticosterone does not result in changes in the basal firing rate of DRN 5-HT neurons or the basal extracellular level of 5-HT in the DRN (Leitch et al. 2003; Rainer et al. 2012). However, GR-mediated reduction of the autoinhibitory function of 5-HT1A somatodendritic receptors in DRN 5-HT neurons has been found to occur after short- and long-term (4–7 weeks) treatment with corticosterone (Fairchild et al. 2003; Laaris et al. 1995; Rainer et al. 2012). Thus, the enhanced 5-HT release is likely to occur in the DRN of corticosterone-treated animals when excitatory inputs to the DRN (reviewed in: Lee et al. 2003) are active. Larger surges of 5-HT within the DRN would result in a stronger, than normal, activation of 5-HT receptors expressed by local neurons, which might lead to their desensitization and/or downregulation. Experiments in vitro have demonstrated that agonist treatment results in a downregulation of 5-HT7 receptors in hippocampal neuronal cultures (Vasefi et al. 2013) and desensitization of these receptors in astrocytes of the rat frontal cortex (Shimizu et al. 1998). It should also be noted that 5-HT7 receptor functional desensitization is likely to contribute to a decrease in spiking activity of DRN GABAergic interneurons and observed reduction in sIPSCs frequency in DRN projection neurons of corticosterone-treated rats.
It is also conceivable that GR activation might influence the expression level of 5-HT7 receptors, as is the case with 5-HT1A receptor in the DRN (Laaris et al. 1995); however, to the best of our knowledge, no data are available on the effects of elevated blood plasma corticosterone on the expression of 5-HT7 receptors in the DRN. While adrenalectomy results in an increase of 5-HT7 receptor mRNA expression in the hippocampus (Le Corre et al. 1997), we have previously found that repeated administration of corticosterone for 21 days changed neither the affinity (Kd) of hippocampal 5-HT7 receptors to [3H]-SB 269970 nor their maximum density (Bmax, Tokarski et al. 2009).
The present data demonstrate that administration of SB 269970, repeated for 7 days, normalized the frequency of sIPSCs in DRN projection cells of corticosterone-treated rats and restored the reactivity of 5-HT7 receptors in the DRN. It has been shown that SB 269970 rapidly enters the brain after intraperitoneal administration and is eliminated within less than 2 h (Hagan et al. 2000). Thus, in our experiments, the blockade of 5-HT7 receptors largely coincided with a rapid rise in corticosterone level in the brain which normalizes within 2 h after its subcutaneous administration (Droste et al. 2008). If observed suppression of the reactivity of 5-HT7 receptors in DRN GABA interneurons indeed resulted from corticosterone-induced enhancement of 5-HT release within DRN and excessive activation of 5-HT7 receptors, their temporal blockade might prevent the occurrence of this effect and lead to the restoration of their reactivity. Interestingly, chronic restraint stress-related hyperactivity of the hypothalamic-pituitary-adrenal axis of rats has recently been linked to an increased expression of 5-HT7 receptors in the adrenal cortex (reviewed in: Terrón 2014). It has been reported that SB-656104 (another 5-HT7 receptor antagonist) significantly inhibited the sensitized corticosterone response to acute restraint in chronically stressed animals (García-Iglesias et al. 2013). It is conceivable that similar mechanisms might be operable in our experimental model involving repeated corticosterone administration.
Administration of SB 269970 alone, repeated for 7 days, affected neither sIPSCs frequency nor the reactivity of 5-HT7 receptors in the DRN. It has been reported that 1-week long treatment of rats with the 5-HT7 receptor antagonist SB 269970, in a dose comparable to the one used in the present study, did not alter the firing of DRN 5-HT cells but resulted in desensitization of somatodendritic 5-HT1A autoreceptors, thus providing a prospective fast-acting antidepressant strategy (Mnie-Filali et al. 2011). It should be noted that SB 269970 may differently influence the expression of 5-HT7 receptors, as in vitro experiments demonstrated downregulation, upregulation, or lack of effect of SB 269970 on these receptors, depending on a particular splice variant (Andressen et al. 2015). We have demonstrated previously that treatment with SB 269970 induced functional desensitization of 5-HT7 receptors in the rat hippocampus (Tokarski et al. 2012b). An earlier study has shown that corticosterone administration lasting 7–21 days enhanced the excitatory effect of 5-HT7 receptor activation on the activity of hippocampal pyramidal neurons (Tokarski et al. 2009). Thus, the effects of prolonged corticosterone exposure on hippocampal (Tokarski et al. 2009, 2012b) and DRN (this study) 5-HT7 receptors are opposite. However, the results of the present study complement previous work which demonstrated that treatment with SB 269970 counteracts repeated restraint stress-induced detrimental modifications of the glutamatergic transmission and synaptic plasticity in the rat frontal cortex (Tokarski et al. 2011).
Repeated corticosterone administration, as a model of uncontrollable stress, has been suggested to represent a rodent model of depression and anxiety (Zhao et al. 2008; Rainer et al. 2012). The amount of corticosterone administered and the duration of treatment, which produce a depressive-like phenotype, differ between laboratories (reviewed in: Sterner and Kalynchuk 2010). However, repeated corticosterone administration generally produces behavioral and neurobiological alterations that resemble the symptoms and neurobiological changes associated with human depression, including increases in passive behavior and decreases in active behaviors in the forced swim test, inhibited sexual behavior, decreased sucrose intake, decreased responding for food, and decreased grooming (reviewed in: Sterner and Kalynchuk 2010). Importantly, a decrease in 5-HT level by a half in rat frontal cortex has been reported after corticosterone administration in drinking water lasting for 8 weeks (Luine et al. 1993). It should be noted that no change in the basal firing rate of DRN projection cells or extracellular level of 5-HT in the DRN has been found to occur in mice after 7 weeks of corticosterone treatment (Rainer et al. 2012), but this discrepancy might be attributed to species differences. A decreased activity of the serotonergic system has also been found to occur after chronic unpredictable stress, which induces depressive-like phenotype as well (Bambico et al. 2009, reviewed in: Mahar et al. 2014). On the other hand, after administration of corticosterone for 14 days, no change in basal extracellular 5-HT level has been observed in rat hippocampus (Leitch et al. 2003). After 12 days of corticosterone treatment, no change in 5-HT level but a significantly increased amount of 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) in rat frontal cortex has been reported, indicative of an increased metabolism of 5-HT in the DRN target structure (Inoue and Koyama 1996). Thus, corticosterone-induced decrease in the activity of the rat serotonergic system appears to develop only after prolonged exposure to corticosterone, while the early effects may include an increased excitability of DRN projection neurons primarily due to a reduction in the autoinhibitory function of 5-HT1A somatodendritic receptors (Fairchild et al. 2003; Laaris et al. 1995; Rainer et al. 2012) and a reduced GABAergic input from local DRN interneurons (this study). These effects may be accompanied by an elevation of the expression of gene encoding tryptophan hydroxylase 2 (tph2), the rate-limiting enzyme for brain 5-HT synthesis in the DRN, reported to occur after 3 weeks of treatment of rats with corticosterone (Donner et al. 2012).
Conclusion
In conclusion, the results of the present study indicate that repeated administration of exogenous corticosterone induces a reduction of 5-HT7 receptor reactivity and weakening of inhibitory transmission within the DRN neuronal network. Administration of a 5-HT7 receptor antagonist restores 5-HT7 receptor reactivity and reverses the effects of elevated corticosterone levels on inhibitory transmission within the DRN. These findings may be of importance for the treatment of stress-related disorders.
References
Adell A, Garcia-Marquez C, Armario A, Gelpi E (1988) Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem 50:1678–1681
Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K (1990) Electrophysiology of the central serotonin system: receptor subtypes and transducer mechanisms. Ann N Y Acad Sci 600:93–103
Andressen KW, Manfra O, Brevik CH, Ulsund AH, Vanhoenacker P, Levy FO, Krobert KA (2015) The atypical antipsychotics clozapine and olanzapine promote down-regulation and display functional selectivity at human 5-HT7 receptors. Br J Pharmacol 172:3846–3860. https://doi.org/10.1111/bph.13169
Asaoka N, Nagayasu K, Nishitani N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S (2015) Olanzapine augments the effect of selective serotonin reuptake inhibitors by suppressing GABAergic inhibition via antagonism of 5-HT6 receptors in the dorsal raphe nucleus. Neuropharmacology 95:261–268. https://doi.org/10.1016/j.neuropharm.2015.03.032
Bacon WL, Beck SG (2000) 5-Hydroxytryptamine7 receptor activation decreases slow after hyperpolarization amplitude in CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther 294:672–679
Bambico FR, Nguyen NT, Gobbi G (2009) Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur Neuropsychopharmacol 19:215–228. https://doi.org/10.1016/j.euroneuro.2008.11.005
Bickmeyer U, Heine M, Manzke T, Richter DW (2002) Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur J Neurosci 16:209–218. https://doi.org/10.1046/j.1460-9568.2002.02072.x
Celada P, Puig MV, Artigas F (2013) Serotonin modulation of cortical neurons and networks. Front Integr Neurosci 7:25. https://doi.org/10.3389/fnint.2013.00025
Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O (2013) Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci 33:13978–13988. https://doi.org/10.1523/jneurosci.2383-13.2013
Chaouloff F (2000) Serotonin, stress and corticoids. J Psychopharmacol 14:139–151
Ciranna L, Catania MV (2014) 5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: physiological role and possible implications in autism spectrum disorders. Front Cell Neurosci 8:250. https://doi.org/10.3389/fncel.2014.00250
Claes SJ (2004) CRH, stress, and major depression: a psychobiological interplay. Vitam Horm 69:117–150
Crawford LK, Rahman SF, Beck SG (2013) Social stress alters inhibitory synaptic input to distinct subpopulations of raphe serotonin neurons. ACS Chem Neurosci 4:200–209
Donner NC, Montoya CD, Lukkes JL, Lowry CA (2012) Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37(5):645–661. https://doi.org/10.1016/j.psyneuen.2011.08.008
Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC (2008) Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149:3244–3253. https://doi.org/10.1210/en.2008-0103
Fairchild G, Leitch MM, Ingram CD (2003) Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology 45:925–934
Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduño J, Frías-Dominguez C, Drucker-Colin R, Mihailescu S (2008) Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse 62:601–615. https://doi.org/10.1002/syn.20526
García-Iglesias BB, Mendoza-Garrido ME, Gutiérrez-Ospina G, Rangel-Barajas C, Noyola-Díaz M, Terrón JA (2013) Sensitization of restraint-induced corticosterone secretion after chronic restraint in rats: involvement of 5-HT7 receptors. Neuropharmacology 71:216–227. https://doi.org/10.1016/j.neuropharm.2013.03.013
Gocho Y, Sakai A, Yanagawa Y, Suzuki H, Saitow F (2013) Electrophysiological and pharmacological properties of GABAergic cells in the dorsal raphe nucleus. J Physiol Sci 63:147–154. https://doi.org/10.1007/s12576-012-0250-7
Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR (2000) Characterization of SB-269970-a, a selective 5-HT(7) receptor antagonist. Br J Pharmacol 130:539–548
Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson JA (1986) Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A 83:9779–9783
Inoue T, Koyama T (1996) Effects of acute and chronic administration of high-dose corticosterone and dexamethasone on regional brain dopamine and serotonin metabolism in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 20:147–156
Jacobs BL, Azmitia EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72:165–229
Judge SJ, Ingram CD, Gartside SE (2004) GABA receptor modulation of 5-HT neuronal firing: characterization and effect of moderate in vivo variations in glucocorticoid levels. Neurochem Int 45:1057–1065
Köhler S, Cierpinsky K, Kronenberg G, Adli M (2016) The serotonergic system in the neurobiology of depression: relevance for novel antidepressants. J Psychopharmacol 30:13–22. https://doi.org/10.1177/0269881115609072
Kusek M, Sowa J, Kamińska K, Gołembiowska K, Tokarski K, Hess G (2015) 5-HT7 receptor modulates GABAergic transmission in the rat dorsal raphe nucleus and controls cortical release of serotonin. Front Cell Neurosci 9:324. https://doi.org/10.3389/fncel.2015.00324
Laaris N, Haj-Dahmane S, Hamon M, Lanfumey L (1995) Glucocorticoid receptor-mediated inhibition by corticosterone of 5-HT1A autoreceptor functioning in the rat dorsal raphe nucleus. Neuropharmacology 34:1201–1210
Le Corre S, Sharp T, Young AH, Harrison PJ (1997) Increase of 5-HT7 (serotonin-7) and 5-HT1A (serotonin-1A) receptor mRNA expression in rat hippocampus after adrenalectomy. Psychopharmacology 130:368–374
Lee HS, Kim MA, Valentino RJ, Waterhouse BD (2003) Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res 963:57–71
Leitch MM, Ingram CD, Young AH, McQuade R, Gartside SE (2003) Flattening the corticosterone rhythm attenuates 5-HT1A autoreceptor function in the rat: relevance for depression. Neuropsychopharmacology 28:119–125
Liu R, Jolas T, Aghajanian G (2000) Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res 873:34–45
Luine VN, Spencer RL, McEwen BS (1993) Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Res 616:65–70
Mahar I, Bambico FR, Mechawar N, Nobrega JN (2014) Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38:173–192. https://doi.org/10.1016/j.neubiorev.2013.11.009
McDevitt RA, Neumaier JF (2011) Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J Chem Neuroanat 41:234–246. https://doi.org/10.1016/j.jchemneu.2011.05.001
Mnie-Filali O, Faure C, Lambás-Señas L, El Mansari M, Belblidia H, Gondard E, Etiévant A, Scarna H, Didier A, Berod A, Blier P, Haddjeri N (2011) Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 36:1275–1288
Monti JM, Leopoldo M, Jantos H (2008) The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphe nucleus suppresses REM sleep in the rat. Behav Brain Res 191:184–189. https://doi.org/10.1016/j.bbr.2008.03.025
Nikiforuk A (2015) Targeting the serotonin 5-HT7 receptor in the search for treatments for CNS disorders: rationale and Progress to date. CNS Drugs 29:265–275. https://doi.org/10.1007/s40263-015-0236-0
Parker KJ, Schatzberg AF, Lyons DM (2003) Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 43:60–66
Rainer Q, Nguyen HT, Quesseveur G, Gardier AM, David DJ, Guiard BP (2012) Functional status of somatodendritic serotonin 1A autoreceptor after long-term treatment with fluoxetine in a mouse model of anxiety/depression based on repeated corticosterone administration. Mol Pharmacol 81:106–112. https://doi.org/10.1124/mol.111.075796
Reagan LP, Grillo CA, Piroli GG (2008) The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol 585:64–75. https://doi.org/10.1016/j.ejphar.2008.02.050
Roberts C, Thomas DR, Bate ST, Kew JN (2004) GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacology 46:935–941
Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF (2011) Uncontrollable, but not controllable, stress desensitizes 5-HT1 receptors in the dorsal raphe nucleus. J Neurosci 31:14107–14115. https://doi.org/10.1523/jneurosci.3095-11.2011
Shimizu M, Nishida A, Zensho H, Miyata M, Yamawaki S (1998) Agonist-induced desensitization of adenylyl cyclase activity mediated by 5-hydroxytryptamine7 receptors in rat frontocortical astrocytes. Brain Res 784:57–62
Soiza-Reilly M, Commons KG (2014) Unraveling the architecture of the dorsal raphe synaptic neuropil using high-resolution neuroanatomy. Front Neural Circuits 8:105. https://doi.org/10.3389/fncir.2014.00105
Stahl SM (1998) Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord 51:215–235
Sterner EY, Kalynchuk LE (2010) Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuro-Psychopharmacol Biol Psychiatry 34:777–790. https://doi.org/10.1016/j.pnpbp.2010.03.005
Terrón JA (2014) Novel insights into the potential involvement of 5-HT7 receptors in endocrine dysregulation in stress-related disorders. Rev Neurosci 25:439–449. https://doi.org/10.1515/revneuro-2014-0017
Tokarski K, Zahorodna A, Bobula B, Hess G (2003) 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res 993:230–234. https://doi.org/10.1016/j.brainres.2003.09.015
Tokarski K, Pitra P, Duszynska B, Hess G (2009) Imipramine counteracts corticosterone-induced alterations in the effects of the activation of 5-HT(7) receptors in rat hippocampus. J Physiol Pharmacol 60:83–88
Tokarski K, Bobula B, Kusek M, Hess G (2011) The 5-HT(7) receptor antagonist SB 269970 counteracts restraint stress-induced attenuation of long-term potentiation in rat frontal cortex. J Physiol Pharmacol 62:663–667
Tokarski K, Bobula B, Grzegorzewska-Hiczwa M, Kusek M, Hess G (2012a) Stress- and antidepressant treatment-induced modifications of 5-HT7 receptor functions in the rat brain. Pharmacol Rep 64:1305–1315
Tokarski K, Zelek-Molik A, Duszyńska B, Satała G, Bobula B, Kusek M, Chmielarz P, Nalepa I, Hess G (2012b) Acute and repeated treatment with the 5-HT7 receptor antagonist SB 269970 induces functional desensitization of 5-HT7 receptors in rat hippocampus. Pharmacol Rep 64:256–265
Vasefi MS, Kruk JS, Heikkila JJ, Beazely MA (2013) 5-Hydroxytryptamine type 7 receptor neuroprotection against NMDA-induced excitotoxicity is PDGFβ receptor dependent. J Neurochem 125:26–36. https://doi.org/10.1111/jnc.12157
Zahorodna A, Hess G (2006) Imipramine and citalopram reverse corticosterone-induced alterations in the effects of the activation of 5-HT(1A) and 5-HT(2) receptors in rat frontal cortex. J Physiol Pharmacol 57:389–399
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581:113–120. https://doi.org/10.1016/j.ejphar.2007.12.005
Funding
This work is supported by the grant DEC-2013/11/B/NZ4/04743 financed by the National Science Center, Poland, and by statutory funds from the Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental procedures were approved by the Local Ethics Committee for Animal Experiments at the Institute of Pharmacology, Polish Academy of Sciences, and were carried out in accordance with the European Community guidelines for the use of experimental animals and the national law.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sowa, J., Kusek, M., Siwiec, M. et al. The 5-HT7 receptor antagonist SB 269970 ameliorates corticosterone-induced alterations in 5-HT7 receptor-mediated modulation of GABAergic transmission in the rat dorsal raphe nucleus. Psychopharmacology 235, 3381–3390 (2018). https://doi.org/10.1007/s00213-018-5045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5045-y