Abstract
Rationale
The serotonin (5-HT) system has been reported to be involved in decision-making. A key component of this neurotransmitter system is the 5-HT1A receptor, and research is beginning to show how this receptor can influence decision-making. However, this relationship has rarely been studied in humans.
Objectives
This study assessed whether individual variability in 5-HT1A availability correlates with decision-making in healthy volunteers.
Methods
We measured regional availability of the 5-HT1A receptor in the hippocampal complex and striatum using positron emission tomography and correlated this with performance on two decision-making tasks measuring sensitivity to probability, rewards and punishments and temporal discounting, respectively.
Results
No relationship between decision-making behaviour and 5-HT1A availability in the striatum was found. However, a positive correlation was detected between participants’ 5-HT1A availability in the hippocampal complex and their sensitivity to the probability of winning. Furthermore, there was a negative correlation between the degree to which participants discounted future rewards and 5-HT1A availability in the hippocampal complex.
Conclusions
These data support a role for the 5-HT1A receptor in the aberrant decision-making that can occur in neuropsychiatric disorders such as depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The serotonin (5-HT) system has been implicated in decision-making, with manipulations resulting in altered processing of rewards (Murphy et al 2002) and punishments (Cools et al 2008; Crockett et al 2009). Specifically, reduced 5-HT transmission has been implicated in impulsivity (Winstanley et al. 2004), a component of which is temporal discounting, the tendency to devalue temporally distant rewards. Experimentally increasing levels of 5-HT in rats via administration of an SSRI decreases discounting behaviours (Bizot et al 1988), whereas reducing 5-HT in humans via administration of acute tryptophan depletion (ATD) increases discounting (Schweighofer et al 2008). Interestingly, Takahashi et al (2008) reported that depressed patients, in whom 5-HT transmission is hypothesized to be disrupted, also exhibited elevated temporal discounting.
The striatum is known to play a central role in decision-making (Cools et al 2007). Schultz et al. (2000) demonstrated that neurons within the primate striatum fired in response to reward-predicting stimuli, while in humans Delgado et al. (2000) reported that the dorsal striatum was active during monetary reward processing. With respect to impulsivity, Pine et al. (2009) showed that delay discounting correlated with responses in the human striatum. 5-HT transmission in the striatum may also influence decision-making: Seymour et al. (2012) found that ATD altered the exchange rate by which rewards and punishments were compared, which was linked to an increase in striatal and prefrontal responses. Moreover, Tanaka et al. (2007) observed that the ventral striatum responded to short-term rewards after ATD, while the dorsal striatum responded to long-term rewards after acute tryptophan loading.
The hippocampus may also play a role in decision-making: Lisman and Grace (2005) argue that the hippocampus encodes novel information, with such novelty signals being conveyed to the VTA via the ventral striatum, which means that these signals may regulate striatal-dependent reward processing. Indeed Camara et al. (2008) showed increased striatal–hippocampal coupling during the processing of gains and losses in humans, and Guitart-Masip et al. (2010) reported that novel images (which activated the hippocampus) enhanced reward signals in the striatum elicited due to subsequently presented rewards. Regarding impulsivity, Mariano et al. (2009) reported that rats with hippocampal damage exhibited increased discounting of delayed rewards, which Schacter and Addis (2009) argue is due to the hippocampal complex being involved in the formation of past and future episodic representations. Similarly, Peters and Büchel (2010) suggest that temporal discounting is related to the subjective capacity for future episodic thought, with such ‘mental time travel’ involving the hippocampus. The hippocampus’ role in decision-making may also depend on 5-HT transmission: ATD results in poorer performance on episodic memory tasks (Riedel et al 1999), argued to be due to a decreased stimulation of excitatory 5-HT receptors (Meeter et al 2006). Mobini et al. (2000) reported that rats whose 5-HT systems had been destroyed with 5,7-dihydroxytryptamine became more impulsive, choosing smaller, sooner rewards, which correlated with the magnitude of 5-HT depletion in the hippocampus. Initial evidence implicates the 5-HT1A receptor in decision-making: Schmitz et al. (2009) showed that human subjects homozygous for the 5-HT1A C(-1019)G polymorphism, linked to increased 5-HT1A receptor expression, exhibited longer reaction times to potential rewards and shorter reaction times to potential punishments. With respect to temporal discounting, Miyazaki et al. (2012) demonstrated that injection of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)-tetralin into the rat dorsal raphe nucleus, which decreases 5-HT neuron firing rates, increased waiting errors for delayed but not immediate rewards.
Whilst not specifically relating to the 5-HT1A receptor, recent work by Macoveanu and colleagues has implicated another 5-HT receptor sub-type in decision-making: Macoveanu et al. (2013b) report that acute blockade of the 5-HT2A receptors with ketanserin made healthy volunteers more risk-averse and selectively reduced ventral striatal responses to negative outcomes. Further, ketanserin abolished the negative correlation between striatal responses to low-risk negative outcomes and risk-averse choice behaviour that was observed in the placebo condition. Using multi-modal neuroimaging, the same group also demonstrated that lateral prefrontal activation during response inhibition was attenuated following tryptophan depletion and that the magnitude of this reduction was greatest in those with high cortical 5-HT2A binding (Macoveanu et al. 2013a). Together these two studies indicate the ability of neuroimaging methods in helping researchers to better understand the relationship between decision-making and activity at a specific 5-HT receptor sub-type.
While 5-HT transmission in the striatum and hippocampus, particularly at the 5-HT1A receptor, may play an important role in decision-making, this hypothesis is difficult to test in humans as regionally specific pharmacological manipulations cannot be conducted non-invasively. To address this question, we utilized an individual differences approach, examining whether a relationship between decision-making and 5-HT transmission could be observed at the level of the receptor. Whilst this approach was used to demonstrate that dopamine transmission influences decision-making (Takahashi 2012), it has never been used to better understand 5-HT’s influence on decision-making (Takahashi et al 2010). We measured levels of the 5-HT1A receptor using positron emission tomography (PET) and correlated these with performance on two decision-making tasks, measuring sensitivity to reward and punishment information, and temporal discounting, respectively. We predicted that greater 5-HT1A availability in the striatum and hippocampus would predict increased sensitivity to rewards and punishments and decreased temporal discounting.
Materials and methods
Participants
Fifteen participants (13 males; mean age 50.9 years, range 35–63 years) underwent a PET scan at the Cyclotron Unit at the Hammersmith Hospital campus of Imperial College London and completed a separate behavioural session on a different day at the Institute of Cognitive Neuroscience, University College London. Subjects were free from past or present psychiatric disorders, as determined by assessment on the Mini International Neuropsychiatric Inventory (MINI; Sheehan et al 1998). None of the subjects had taken any psychotropic medication within 12 months of participating nor had they any previous history of alcohol/substance dependence. Written informed consent was obtained from all subjects prior to both scan and behavioural sessions, and the study was approved by the local research ethics committee. At the behavioural session, participants received £20 for compensation for their time and travel, and up to an additional £10 on each of the two cognitive tasks, depending on performance, meaning that each volunteer was compensated between £20 and £40 for this session.
PET procedure
To measure regional 5-HT1A availability, we used 11C-CUMI-101, a highly selective 5-HT1A PET ligand (Milak et al 2011). A full description of PET data acquisition and analysis methods is presented in Selvaraj et al. (2012). Briefly, participants had an intravenous canula sited in the ante-cubital vein flushed with a saline infusion followed by an injection of the 11C-CUMI ligand as a smooth bolus over a 30-s period at the beginning of the scan. The radiochemical purity of the ligand was high (between 95 % and 100 %, mean 97 %, SD 1.5 %).
PET scans were acquired from a GE Discovery RX PET/CT scanner with an axial field view of 15.7 cm. Twenty-two frames were acquired in total, each with 47 slices at 3 mm in thickness. The dynamic PET scans were acquired over 90 min. Time frames were of increasing duration: 30-s pre-injection background, 1 × 15 s, 3 × 5 s, 1 × 30 s, 4 × 60 s, 7 × 300 s, and 5 × 600 s. The dynamic scans were de-noised using a level 2, order 64 Battle Lemarie wavelet filter (Turkheimer et al 1999). Head movement in the dynamic PET acquisition was corrected for using frame-by-frame realignment using a mutual information algorithm (Studholme et al 1997).
For our analyses, we calculated whole-brain parametric images in order to assess relationships between 5-HT1A availability and behavioural performance on a voxel-wise basis. These were acquired from the dynamic images using Piwave software (Turkheimer et al. 2007) with the simplified reference tissue model method (Lammertsma and Hume 1996). Each subject’s parametric image was spatially normalised to the PET template within SPM8 software (statistical parametric mapping 8; www.fil.ion.ac.uk/spm/software/spm8/) and smoothed with an 8-mm full-width at half-maximum Gaussian kernel. The ROIs of the hippocampal complex (hippocampus and parahippocampal gyrus combined) and the striatum (nucleus accumbens, putamen and pallidum, due to very low binding values within the caudate) were defined using the HamNet probabilistic atlas (shown to have a very high reliability for these regions; Hammers et al 2007).
Behavioural session
Gambling task (Rogers et al. 2003)
Participants completed 80 trials, each of which required them to make a choice between two gambles. They were paid according to their winnings, with each point won being converted to one penny. Each gamble was represented as a bar, the height of which conveyed the probability (25, 50 or 75 %) of winning or losing a number of points. The sizes of potential gains and losses were displayed in green at the top and in red at the bottom of the bars, respectively (Fig. 1).
On each trial, participants had to choose between the ‘control’ gamble, which consisted of a 50 % chance of winning or losing ten points, and an ‘experimental’ gamble, which varied in terms of probability, high (75 %) or low (25 %), potential gains, high (80 points) or low (20 points) and potential losses high (80 points) or low (20 points), resulting in eight trial types. The gambling task also included two further trial types (gains only and losses only), which were not analysed further.
This task yields three main outcome measures: the proportion of choices of the experimental gamble over the control gamble as a function of (1) probability of winning, (2) the size of potential gains and (3) the size of potential losses. These measures were calculated by taking the difference between the proportion of experimental gamble chosen when each of these factors was high compared with when they were low (for clarity, the negative of this value is presented for loss sensitivity).
Temporal discounting task (Pine et al. 2009)
Participants completed 220 trials. In 200 of these trials, the subjects chose between two scenarios, one in which they would receive a smaller amount of money in the more immediate future and one in which they would receive a larger amount of money in the more distant future (Fig. 2). The remaining 20 trials were ‘catch’ trials, where participants could choose between receiving a smaller amount of money after a longer time delay, or a larger, sooner amount. This trial type was administered to ensure that participants were engaged with the task. The choices varied in magnitude (from £1 to £100) and delay (from 1 week to 1 year).
Subjects had to choose between a smaller, more immediate reward (left side of blue bar) and a larger but more delayed reward (right side). The amounts of money differed in magnitude (£1 to £100) and in delay (1 week to 1 year). Subjects completed 220 trials, giving them different scenarios each time
Values were assigned to each option within each trial, implemented in the context of a hyperbolic discount valuation model taken from Mazur (1987) and developed further by Pine et al. (2009). Critical to subjects’ choices in this task is the steepness of the discounting of expected reward values according to their delay and magnitude, denoted by:
where V is the subjective value (or ‘subjective discounted utility’) placed upon an expected reward of a magnitude M with a delay of d. D is the discount factor (ranging from 0 to 1) by which the utility (U) is discounted, being a hyperbolic function of the delay of the reward multiplied by the discount rate parameter K. A higher K value indicates a higher rate of discounting, while a ‘flat’ discounting rate (K = 0) implies no discounting at all, choosing the more delayed reward, no matter how long the wait. Finally, U is a negative exponential function of the magnitude of the reward, including a parameter, r, which describes the concavity/convexity of the participant’s utility function and hence their sensitivity to increasing magnitudes of reward. In addition to the number of smaller, sooner vs. larger, later options chosen, participants’ K and r values were the main outcome measures, determined using maximum likelihood estimation (see Pine et al. (2009) for details).
Analysis
Participants’ scores from each of the behavioural tasks were entered as group-level covariates in second-level SPM8 analyses including their whole-brain parametric PET images to identify correlations between behaviour and regional 5-HT1A availability. Each variable from each task was entered separately, resulting in five second-level models (win sensitivity, loss sensitivity and probability sensitivity from the gambling task, and discount rate (K) and utility concavity (r) from the temporal discounting task). We corrected for multiple comparisons, controlling the family-wise error rate at the voxel level. We were particularly interested in relationships between 5-HT1A availability and behaviour in striatal and hippocampal regions. Therefore, we used the hippocampal and striatal ROIs (see prior text) in order to constrain the search volume, resulting in small volume corrected (SVC) P-values. However, due to the exploratory nature of this study, we did not correct over the volume of merged ROIs but instead examined each (bilateral) ROI separately in order to ensure that any significant correlations with 5-HT1A availability were observed.
Results
Gambling task
Subjects chose the ‘experimental’ gamble significantly more often when the probability of winning was higher (t[14] = 15.0, P < 0.001), when the amount they could win was higher (t[14] = 2.9, P = 0.011) and when the amount they could lose was lower (t[14] = 3.0, P = 0.009). The mean sensitivity to win was 0.110 (SD = 0.147), the mean sensitivity to losses was 0.115 (SD = 0.146) and the mean sensitivity to probability was 0.694 (SD = 0.18). Sensitivities to wins and losses were strongly positively correlated (r = 0.722, P = 0.002).
In the voxel-wise analyses, positive correlations were identified between sensitivity to probability and 5-HT1A availability in the hippocampal complex bilaterally, though only the correlation in the right survived SVC correction for multiple comparisons (right peak voxel: [x = 20, y = 0, z = −38], Z = 3.63, P SVC = 0.028 (Fig. 3); left peak voxel: [x = −16, y = −2, z = −34], Z = 3.21, P SVC = 0.089). This correlation between 5-HT1A availability in the right hippocampal complex and sensitivity to probability remained significant, surviving SVC correction for multiple comparisons, even when the two female participants were excluded from the analysis ([x = 20, y = 0, z = −36], Z = 3.51, P SVC = 0.045). There was no significant relationship between 5-HT1A availability in the striatum and any of the parameters from the gambling task. For completeness, we provide a list of all regions surviving a threshold of P < 0.001 (uncorrected), minimum cluster size of 10 voxels, in Table 1 of the “Electronic supplementary material”.
Left statistical parametric map (SPM) depicting 11C-CUMI binding at baseline within the right hippocampal/parahippocampal ROI that positively correlates with participants’ sensitivity to probability (20, 0, −38, small volume correction analysis presented at P SVC = 0.028). Right scatterplot of correlation between participants’ sensitivity to probability and baseline 11C-CUMI binding within this cluster. This correlation shows that those participants who showed greater sensitivity to information pertaining to probability had greater baseline 5-HT1A binding within the right hippocampal complex
Temporal discounting task
All subjects chose the larger–sooner reward on above 90 % of the catch trials (mean 19.4/20, SD = 0.83). On average, participants chose the sooner, smaller option over the larger, greater option (mean 119.2/200, SD = 48.05).
Using the model of best fit from Pine et al. (2009), we found that participants discounted the value of future rewards hyperbolically (mean K = 0.099, SD = 0.057) and also exhibited a concave utility function (mean r = 0.0086, SD = 0.017), comparable to results reported previously on this task. Participants’ K values and r values were significantly correlated (r = 0.565, P = 0.028).
The voxel-wise analyses revealed significant negative correlations between K and 5-HT1A availability in the left hippocampal complex (Fig. 4) which survived correction for multiple comparisons (peak voxel: [x = −26, y = −14, z = −36], Z = 3.54, P SVC = 0.037). This correlation however did not remain significant when removing the two female participants from the analysis. Again there was no significant relationship between 5-HT1A availability in the striatum and either K or r values. A full list of all regions that survive a threshold of P < 0.001 (uncorrected), minimum cluster size of 10 voxels, is presented in Table 2 of the “Electronic supplementary material”.
Left SPM image depicting baseline 11C-CUMI binding within the left hippocampal/parahippocampal ROI that negatively correlates with participants’ discount factor (−26, −14, −36, small volume correction analysis presented at P SVC = 0.037). Right scatterplot of correlation between discount factor and baseline 5-HT1A receptor availability within this cluster. This correlation indicates that those participants who displayed increased discounting of rewards based upon their temporal delay had decreased baseline 5-HT1A receptor binding within the left hippocampal complex
Discussion
Several studies have suggested that 5-HT transmission in the striatum and hippocampus may play an important role in decision-making and impulsivity. Using an individual differences approach, we identified a positive correlation between participants’ sensitivity to probability on a gambling task and the 5-HT1A availability in the hippocampal complex, and a negative correlation between participants’ discount rate on the temporal discounting paradigm and 5-HT1A availability in the left hippocampal complex. However, no such relationships were found between performance on these tasks and 5-HT1A availability in the striatum.
The involvement of the hippocampal complex in participants’ sensitivity to probability supports previous research that has highlighted a role for this structure in reward and punishment processing (e.g. Camara et al 2008). Furthermore, it supports the notion that 5-HT may have a role to play in this relationship since those participants with greater 5-HT1A availability were more likely to use information about the probability of winning when making their decisions on the gambling task. This finding is consistent with that of Rogers et al. (1999), who showed that ATD resulted in a reduced selection of more probable gain outcomes. No such relationship was seen with regards to the amount that participants use information about the magnitude of potential wins or losses. This may be due in part to actual win, loss and probability sensitivity scores of 0.110, 0.115 and 0.694, respectively. These scores differ from those reported in a previous study using the same task where ATD reduced win sensitivity (Rogers et al 2003), which were 0.30, 0.25 and 0.52, respectively. In other words, participants in the present study tended to rely more strongly on information regarding the probability of an outcome rather than the outcome itself, which may have affected our sensitivity to identify correlations with gain and loss sensitivity measures.
The involvement of hippocampal complex 5-HT1A availability in participants’ intertemporal choices is consistent with previous research that has highlighted its role in temporal discounting (e.g. Mobini et al 2000; Schacter and Addis 2009) and previous work using ATD that has shown that 5-HT alters delay discounting (e.g. Schweighofer et al 2008). In the present study, participants with greater 5-HT1A availability discounted the value of delayed rewards to a lesser extent, leading to less impulsive choice. Although the hippocampus is typically associated with episodic memory processing and contextual learning, Peters and Büchel (2010) describe a manner in which this region may contribute to temporal discounting. These authors administered a standard discounting paradigm, but with the addition of a novel episodic condition which involved the presentation of relevant future episodes (i.e. vacation in Paris) that coincided with the later time point. They were able to show that these ‘episodic tags’ decreased participants’ discount rates and, through connectivity analyses, that this tag effect was associated with increased coupling between the ACC and the hippocampus bilaterally. Therefore, we speculate that ATD may increase delay discounting by impairing prospective memory ability through attenuated hippocampal complex activation and ACC coupling (van der Veen et al 2006); however, this hypothesis requires testing in future studies.
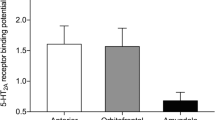
Whilst a relationship between hippocampal complex 5-HT1A availability and performance on both tasks was observed, and despite previous research indicating that striatal 5-HT may influence decision-making (e.g. Tanaka et al 2007), we identified no significant correlations between temporal discounting and/or reward and punishment processing. This may be due in part to the fact that this region has relatively few 5-HT1A receptors (Palacios et al 1990), meaning that sensitivity to observe correlations with 5-HT1A availability was limited. By contrast, the hippocampal complex ROI has a high 5-HT1A density (Pazos et al 1987), providing us with better sensitivity to identify correlations with the behaviour. This is mirrored by mean (SD) regional binding values within the hippocampal complex of 2.48 (0.32) compared with 0.19 (0.08) within the striatum. Therefore, our results cannot be interpreted as indicating that serotonergic transmission within the striatum is not involved in the cited decision-making tasks. However, in order to assess its involvement, it may be necessary to measure other 5-HT receptors (e.g. 5-HT2 receptor subtype) which are present in far greater numbers in the striatum (Joyce et al 1993).
It should also be noted that this study contained a limited sample size (N = 15), reducing our sensitivity to detect small effects, and an unbalanced gender distribution (two females). Whilst the correlation between 5-HT1A availability and sensitivity to probability on the gambling task remained significant when the female participants were excluded, the correlation between 5-HT1A availability and discount factors on the temporal discounting paradigm did not (though we note that statistical power would have been reduced due to the reduction in sample size, making this null result difficult to interpret). In order to maintain our already limited statistical power, we did not correct for the fact that we examined two ROIs and five task variables, which increases the likelihood of type I errors. Therefore, these results should be treated with caution until replicated. It will be important in future studies to include both a larger sample size and a balanced gender distribution in order to assess the impact of gender on these results.
In conclusion, we found that the influence of 5-HT on decision-making can be observed at the level of the receptor. This has implications for psychiatric disorders in which 5-HT transmission is hypothesised to be compromised, such as depression, in which abnormalities in reward/punishment processing and impulsivity have been reported (Eshel and Roiser 2010) and 5-HT1A receptors may be reduced (Drevets et al 1999). It would be of great interest to use the individual differences approach we adopted in the present study to investigate whether the 5-HT1A receptor is linked to poor decision-making in depression and other neuropsychiatric disorders.
References
Bizot JC, Thiébot MH, Le Bihan C, Soubrié P, Simon P (1988) Effects of imipramine-like drugs and serotonin uptake blockers on delay of reward in rats. Possible implication in the behavioral mechanism of action of antidepressants. J Pharmacol Exp Ther 246:1144–1151
Camara E, Rodriguez-Fornells A, Münte TF (2008) Functional connectivity of reward processing in the brain. Front Hum Neurosci 2:19
Cools R, Sheridan M, Jacobs E, D’Esposito M (2007) Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci 27:5506–5514
Cools R, Robinson OJ, Sahakian B (2008) Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology 33:2291–2299
Crockett MJ, Clark L, Robbins TW (2009) Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci 29:11993–11999
Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA (2000) Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84:3072–3077
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C (1999) PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 46:1375–1387
Eshel N, Roiser JP (2010) Reward and punishment processing in depression. Biol Psychiatry 68:118–124
Guitart-Masip M, Bunzeck N, Stephan KE, Dolan RJ, Düzel E (2010) Contextual novelty changes reward representations in the striatum. J Neurosci 30:1721–1726
Hammers A, Chen C-H, Lemieux L, Allom R, Vossos S, Free SL, Myers R, Brooks DJ, Duncan JS, Koepp MJ (2007) Statistical neuroanatomy of the human inferior frontal gyrus and probabilistic atlas in a standard stereotaxic space. Hum Brain Mapp 28:34–48
Joyce JN, Shane A, Lexow N, Winokur A, Casanova MF, Kleinman JE (1993) Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology 8:315–336
Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158
Lisman JE, Grace AA (2005) The hippocampal–VTA loop: controlling the entry of information into long-term memory. Neuron 46:703–713
Macoveanu J, Rowe JB, Hornboll B, Elliot R, Paulson OB, Kundsen GM, Siebner HR (2013a) Serotonin 2A receptors contribute to the regulation of risk-averse decisions. Neuroimage 83:35–44
Macoveanu J, Hornboll B, Elliot R, Erritzoe D, Paulson OB, Siebner HR, Knudsen GM, Rowe JB (2013b) Serotonin 2A receptors, citalopram and tryptophan depletion: a multi-modal imaging study of their interactions during response inhibition. Neuropsychopharmacology 38(6):996–1005
Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Rawlins JNP, Walton ME, Rushworth MFS, Baxter MG, Campbell TG (2009) Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci 30:472–484
Mazur JE (1987) An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H (eds) Quantitative analyses of behavior: vol. 5. The effect of delay and of intervening events on reinforcement value. Erlbaum, Hillsdale, pp 55–73
Meeter M, Talamini L, Schmitt JAJ, Riedel WJ (2006) Effects of 5-HT on memory and the hippocampus: model and data. Neuropsychopharmacology 31:712–720
Milak MS, Severance AJ, Prabhakaran J, Kumar JSD, Majo VJ, Ogden RT, Mann JJ, Parsey RV (2011) In vivo serotonin-sensitive binding of [11C]CUMI-101: a serotonin 1A receptor agonist positron emission tomography radiotracer. J Cereb Blood Flow Metab 31:243–249
Miyazaki KW, Miyazaki K, Doya K (2012) Activation of dorsal raphe serotonin neurons is necessary for waiting for delayed rewards. J Neurosci 32:10451–10457
Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E (2000) Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 152:390–397
Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ (2002) The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 163:42–53
Palacios JM, Waeber C, Hoyer D, Mengod G (1990) Distribution of serotonin receptors. Ann N Y Acad Sci 600:36–52
Pazos A, Probst A, Palacios JM (1987) Serotonin receptors in the human brain—III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21:97–122
Peters J, Büchel C (2010) Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal–mediotemporal interactions. Neuron 66:138–148
Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, Dolan RJ (2009) Encoding of marginal utility across time in the human brain. J Neurosci 29:9575–9581
Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG (1999) Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci 2:898–905
Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW (1999) Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19:9029–9038
Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS (2003) Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology 28:153–162
Schacter DL, Addis DR (2009) On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos Trans R Soc Lond B Biol Sci 364:1245–1253
Schmitz A, Kirsch P, Reuter M, Alexander N, Kozyra E, Kuepper Y, Osinsky R, Hennig J (2009) The 5-HT1A C(-1019)G polymorphism, personality and electrodermal reactivity in a reward/punishment paradigm. Int J Neuropsychopharmacol 12:383–392
Schultz W, Tremblay L, Hollerman JR (2000) Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex 10:272–284
Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, Doya K (2008) Low-serotonin levels increase delayed reward discounting in humans. J Neurosci 28:4528–4532
Selvaraj S, Turkheimer F, Rosso L, Faulkner P, Mouchlianitis E, Roiser JP, McGuire P, Cowen PJ, Howes O (2012) Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry 17:1254–1260
Seymour B, Daw ND, Roiser JP, Dayan P, Dolan R (2012) Serotonin selectively modulates reward value in human decision-making. J Neurosci 32:5833–5842
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33, quiz 34–57
Studholme C, Hill DL, Hawkes DJ (1997) Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 24:25–35
Takahashi H (2012) Monoamines and assessment of risks. Curr Opin Neurobiol 22:1062–1067
Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, Kusumi I, Masui T, Nakagawa S, Suzuki K, Tanaka T, Koyama T, Radford MHB (2008) Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects—an analysis based on Tsallis’ statistics. Neuro Endocrinol Lett 29:351–358
Takahashi H, Matsui H, Camerer C, Takano H, Kodaka F, Ideno T, Okubo S, Takemura K, Arakawa R, Eguchi Y, Murai T, Okubo Y, Kato M, Ito H, Suhara T (2010) Dopamine D1 receptors and nonlinear probability weighting in risky choice. J Neurosci 30:16567–16572
Tanaka SC, Schweighofer N, Asahi S, Shishida K, Okamoto Y, Yamawaki S, Doya K (2007) Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal striatum. PLoS ONE 2:e1333
Turkheimer FE, Brett M, Visvikis D, Cunningham VJ (1999) Multiresolution analysis of emission tomography images in the wavelet domain. J Cereb Blood Flow Metab 19:1189–1208
Turkheimer FE, Edison P, Pavese N, Roncaroli F, Anderson AN, Hammers A, Gerhard A, Hinz R, Tai YF, Brooks DJ (2007) Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nucl Med 48:158–167
Van der Veen FM, Evers EAT, van Deursen JA, Deutz NEP, Backes WH, Schmitt JAJ (2006) Acute tryptophan depletion reduces activation in the right hippocampus during encoding in an episodic memory task. Neuroimage 31:1188–1196
Winstanley CA, Dalley JW, Theobald DEH, Robbins TW (2004) Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29:1331–1343
Acknowledgments
This study was funded by a Medical Research Council (UK) grant to Dr. Howes (grant number MC-A656-5QD30) and a National Institute of Health Research Biomedical Research Council grant to King’s College London. Paul Faulkner was funded by a Medical Research Council studentship.
Conflict of interest
The authors have no conflicts of interest, with no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Oliver D. Howes and Jonathan P. Roiser contributed equally to this study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Faulkner, P., Selvaraj, S., Pine, A. et al. The relationship between reward and punishment processing and the 5-HT1A receptor as shown by PET. Psychopharmacology 231, 2579–2586 (2014). https://doi.org/10.1007/s00213-013-3426-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3426-9