Abstract
Rationale
Theories of drug tolerance differentiate between associative and behavioral (instrumental) drug tolerance. However, there is little research comparing these two forms of drug tolerance beyond alcohol and morphine.
Objective
We examined the time course development of associative and behavioral tolerance to the analgesic effects of nicotine.
Methods and results
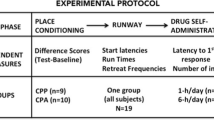
Associative tolerance was investigated by giving independent groups of rats one, five, 15, ten or 20 administrations of nicotine either explicitly paired or unpaired with a distinctive context. Associative tolerance, assessed in the tail flick, developed more rapidly and reached greater magnitude when nicotine and distinctive context were explicitly paired than when they were unpaired. This effect was evidenced after the fifth conditioning session and was maintained through the tenth, 15th, and 20th sessions. Contextual tolerance, assessed in the hot plate, was first evident after ten sessions. However, this effect disappeared safter 15 and 20 sessions. A second study examined the acquisition of behavioral tolerance to the disruptive effects of nicotine on the hot-plate response. Animals that practiced the test response while drugged developed greater tolerance than animals receiving as much nicotine and hot-plate practice but with these two conditions explicitly unpaired. This effect was evident in two different environments but did not generalize to the tail-flick test.
Conclusions
The findings suggest that contextual tolerance to drug effects is test specific, with tail-flick responses depending on cue-associative tolerance processes and hot-plate responses requiring procedures that allow the animal to practice the test response while drugged.



Similar content being viewed by others
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, DC, Author
Baker TB, Tiffany ST (1985) Morphine tolerance as habituation. Psychol Rev 92:78–108
Caggiula AR, Epstein LH, Antelman SM, Saylor S et al (1993) Acute stress or corticosterone administration reduces responsiveness to nicotine: implications for a mechanism of conditioned tolerance. Psychopharmacology 111:499–507
Caggiula AR, Epstein LH, Perkins KA, Saylor S et al (1995) Different methods of assessing nicotine-induced antinociception may engage different neural mechanisms. Psychopharmacology 122:301–306
Carter BL, Tiffany ST (1996) Cross-tolerance of associative and nonassociative morphine tolerance in the rat with mu- and kappa-specific opioids. Psychopharmacology 123:289–296
Cepeda-Benito A, Short P (1997) Morphine’s interoceptive stimuli as cues for the development of associative morphine tolerance in the rat. Psychobiology 25:236–240
Cepeda-Benito A, Tiffany ST (1992) Effect of number of conditioning trials on the development of associative tolerance to morphine. Psychopharmacology 109:172–176
Cepeda-Benito A, Tiffany ST (1995) Role of drug-administration cues in the associative control or morphine tolerance in the rat. Psychopharmacology 122:312–316
Cepeda-Benito A, Tiffany ST (1996) Test-specific manifestations of associative tolerance to the analgesic effects of morphine in the rat. Psychobiology 24:327–332
Cepeda-Benito A, Reynoso J, McDaniel E (1998) Associative tolerance to nicotine analgesia in the rat: tail-flick and hot-plate tests. Exp Clin Psychopharmacol 6:248–254
Cepeda-Benito A, Reynoso J, Erath ST (2000) Dose response analyses of associative tolerance to nicotine analgesia in the rat: tail-flick and hot-plate tests. Exp Clin Psychopharmacol 8:112–116
Dafters RI, Bach L (1985) Absence of environment-specificity in morphine tolerance acquired in nondistinctive environments: habituation or stimulus overshadowing? Psychopharmacology 87:101–106
D’Amour FE, Smith DL (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79
Demellweek C, Goudie AJ (1983) Behavioral tolerance to amphetamine and other psychostimulants: the case for considering behavioral mechanisms. Psychopharmacology 80:287–307
Eddy NB, Leimbach D (1953) Synthetic analgesics II. Dithenylbutanol and dithenylbutylamines. J Pharmacol Exp Ther 107:385–393
Fernandes M, Kluwe S, Coper H (1982) The development of tolerance to morphine in the rat. Psychopharmacology 54:197–201
Grisel JE, Wiertelak EP, Watkins LR, Maier SF (1994) Route of morphine administration modulates conditioned analgesic tolerance and hyperalgesia. Pharmacol Biochem Behav 49:1029–1035
Iwamoto T, Marion L (1993) Adrenergic, serotonergic and cholinergic components of nicotinic antinociception in rats. J Pharmacol Exp Ther 265:777–789
Johansen JP, Fields HL, Manning BH (2001) The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A 98:8077–8082
Littleton J (2001) Receptor regulation as a unitary mechanism for drug tolerance and physical dependence—not quite as simple as it seemed! Addiction 96:87–101
Pauly JR, Grun EU, Collins AC (1990) Chronic corticosterone administrations modulate nicotine sensitivity and brain nicotine receptor binding in C3H mice. Psychopharmacology 101:310–316
Pauly JR, Grun EU, Collins AC (1992) Tolerance to nicotine following chronic treatment by injections: a potential role for corticosterone. Psychopharmacology 108:33–39
Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS (1993) Why some people smoke and others do not: new perspectives. J Consult Clin Psychol 61:723–773
Poulos CX, Cappell H (1991) Homeostatic theory of drug tolerance: a general model of physiological adaptation. Psychol Rev 98:390–408
Ramsay DS, Woods SC (1997) Biological consequences of drug administration: implications for acute and chronic tolerance. Psychol Rev 104:170–193
Siegel S (1975) Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol 89:498–506
Siegel S (1977) Morphine tolerance acquisition as an associative process. J Exp Psychol Anim Behav Process 3:1–13
Siegel S, Baptista MAS, Kim JA, McDonald RV, Weise-Kelly L (2000) Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol 8:276–293
Tiffany ST (1994) The function of classical conditioning in drug taking. In: Drummond DC, Tiffany ST, Glautier S, Remington B (eds) Addiction: cue exposure, theory and practice. Wiley, London, pp 47–71
Tiffany ST, Drobes DJ, Cepeda-Benito A (1992) Contribution of associative and nonassociative processes to the development of morphine tolerance. Psychopharmacology 109:185–190
Trujillo KA (2000) Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology 151:121–141
Trujillo KA, Akil H (1995) Excitatory amino acids and drugs of abuse: a role for N-methyl-d-aspartate receptors in drug tolerance, sensitization and physical dependence. Drug Alcohol Depend 38:139–154
Wolgin DL (1989) The role of instrumental learning in behavioral tolerance to drugs. In: Goudie AJ, Emmit-Ogiesby MW (eds) Psychoactive drugs: tolerance and sensitization. Humana Press, Clifton, NJ, pp 17–114
Yoburn BC, Morales R, Kelly DD, Inturrisi EC (1984) Constraints on the tail-flick assay: morphine analgesia and tolerance are dependent upon locus of tail stimulation. Life Sci 34:1755–1762
Young AM, Goudie AJ (1994) Adaptive processes regulating tolerance to behavioral effects of drugs. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the fourth generation of progress. Raven Press, New York, pp 657–811
Acknowledgements
Funds for this research were provided by Grant # R01 DA121159 from the National Institute on Drug Abuse awarded to Antonio Cepeda-Benito.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cepeda-Benito, A., Davis, K.W., Reynoso, J.T. et al. Associative and behavioral tolerance to the analgesic effects of nicotine in rats: tail-flick and paw-lick assays. Psychopharmacology 180, 224–233 (2005). https://doi.org/10.1007/s00213-005-2151-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2151-4