Abstract
Rationale and objectives
Ethanol ataxia experiments with rats investigated cue effects on conditioned tolerance. Spontaneous recovery (SR) was assessed 1 day and 18 days after extinction with conditioned stimuli (CSs) paired or unpaired with an ethanol unconditioned stimulus (US). Behavioral tolerance was assessed by not tilting the apparatus during conditioning. Non-associative processes were assessed post-conditioning with or without a buzzer cue. Bouton’s (1993, Psychol Bull 114:80–99) memory theory was tested using an extinction cue and an associatively neutral cue presented during SR testing.
Methods
Tolerance was conditioned to a room + strobelight CS by ethanol injections experienced on a tilting floor (standard conditioning). Controls received no ethanol or ethanol, either during the CS without the floor tilting or 11 h post-CS. SR testing occurred 1 day or 18 days after extinction (experiment 1). Conditioning was followed by tolerance and CR tests either with or without a 15-s buzzer cue (experiment 2). In extinction, the CS and cue occurred without ethanol; the cue occurred before 7% or none of the extinction trials. Testing occurred 18 days after extinction with or without that cue (experiment 3), or with an equally familiar (“neutral‘’) cue presented before conditioning (experiment 4).
Results
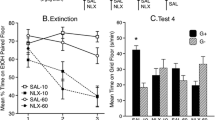
Tolerance developed without floor tilting. CS–US unpairings prevented tolerance. Tolerance SR occurred 18 days but not 1 day after extinction only after CS–US pairings (experiment 1). Post-conditioning tests showed no unconditioned effects of the cue (experiment 2). Testing with no cue 1 day after extinction with the cue resulted in no tolerance increase. The extinction cue reduced SR (experiments 3 and 4); the neutral cue did not (experiment 4).
Conclusions
Cues correlated with extinction reduce SR. Non-associative and practice processes, Bouton’s (1993, Psychol Bull 114:80–99) memory theory, alternative interpretations, and clinical implications are discussed.


Similar content being viewed by others
References
Aguado L, de Brugada I, Hall G (2001) Tests for inhibition after extinction of a conditioned stimulus in the flavour aversion procedure. Q J Exp Psychol B 54B:201–217
Bouton ME (1991) Context and retrieval in extinction and in other examples of interference in simple associative learning. In: Dachowski L, Flaherty CF (eds) Current topics in animal learning: brain, emotion, and cognition. Erlbaum, Hillsdale, pp 25–53
Bouton ME (1993) Context time and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull 114:80–99
Bouton ME, Bolles RC (1979) Contextual control of the extinction of conditioned fear. Learn Mot 10:445–466
Bouton ME, Nelson JB (1998) The role of context in classical conditioning: some implications for cognitive behavior therapy. In: O’Donohue W (ed) Learning and behavior therapy. Allyn and Bacon, Boston, pp 59–84
Brooks DC (2000) Recent and remote extinction cues reduce spontaneous recovery. Q J Exp Psychol B 53B:25–58
Brooks DC, Bouton ME (1993) A retrieval cue for extinction attenuates spontaneous recovery. J Exp Psychol Anim Behav Process 19:77–89
Brooks DC, Bouton ME (1994) A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. J Exp Psychol Anim Behav Process 20:366–379
Brooks DC, Bowker JL (2001) Further evidence that conditioned inhibition is not the mechanism of an extinction cue’s effect: a reinforced cue prevents spontaneous recovery. Anim Learn Behav 29:381–388
Brooks DC, Palmatier MI, Garcia EO, Johnson JL (1999) A retrieval cue for extinction reduces spontaneous recovery of a conditioned taste aversion. Anim Learn Behav 27:77–88
Brooks DC, Karamanlian BR, Foster VL (2001) Extinction and spontaneous recovery of ataxic tolerance to ethanol in rats. Psychopharmacology 153:491–196
Brooks DC, Bowker JL, Anderson JE, Palmatier MI (2003) Impact of brief or extended extinction of a taste aversion on inhibitory associations: evidence from summation, retardation, and preference tests. Anim Learn Behav 31:69–84
Burdick CK, James JP (1970) Spontaneous recovery of conditioned suppression of licking by rats. J Comp Physiol Psychol 72:467–470
Calton JL, Mitchell KG, Schachtman TR (1996) Conditioned inhibition produced by extinction of a conditioned stimulus. Learn Mot 27:335–361
Collins BN, Brandon TH (2002) Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol 70:390–397
Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatment. Addiction 97:155–167
Cunningham CL (1981) Association between the elements of a bivalent compound stimulus. J Exp Psychol Anim Behav Process 7:425–436
Denniston JC, Miller RR (2003) The role of temporal variables in inhibition produced through extinction. Learn Behav 31:35–48
Ellson DG (1939) Spontaneous recovery of the galvanic skin response as a function of the recovery interval. J Exp Psychol 25:586–600
Gleitman H (1971) Forgetting of long-term memories in animals. In: Honig WK, James PHR (eds) Animal memory. Academic, New York, pp 1–44
Howell DC (1987) Statistical methods for psychology, 2nd edn. PWS-Kent, Boston
Institute of Laboratory Animal Resources (1996) Guide for the care and use of laboratory animals. National Research Council, National Academy Press, Washington, D.C.
Konorski J (1948) Conditioned reflexes and neuron organization. Cambridge University Press, Cambridge
Larson SJ, Siegel S (1998) Learning and tolerance to the ataxic effect of ethanol. Pharmacol Biochem Behav 61:131–142
Lewis DJ (1956) Acquisition, extinction, and spontaneous recovery as a function of percentage of reinforcement and intertrial intervals. J Exp Psychol 51:45–53
Meil WM, See RE (1996) Conditioned cue recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol 7:754–763
O’Brien CP, Childress AR, McLellan AT, Ehrman R (1992) A learning model of addiction. In: O’Brien CP, Jaffe JH (eds) Addictive states (Association for Research in Nervous and Mental Disease, vol 70). Raven, New York, pp 157–177
Pavlov IP (1927) Conditioned reflexes. Oxford University Press, London
Poulos CX, Hinson RE, Siegel S (1981) The role of Pavlovian processes in drug tolerance and dependence: implications for treatment. Addict Behav 6:205–211
Rescorla RA (1969) Pavlovian conditioning inhibition. Psychol Bull 72:77–94
Rescorla R (1996) Spontaneous recovery after training with multiple outcomes. Anim Learn Behav 24:11–18
Rescorla R (1997) Spontaneous recovery after Pavlovian conditioning with multiple outcomes. Anim Learn Behav 25:99–107
Rescorla RA, Cunningham C (1978) Recovery of the US representation over time during extinction. Learn Mot 9:373–391
Robbins SJ (1990) Mechanisms underlying spontaneous recovery in autoshaping. J Exp Psychol Anim Behav Process 16:235–249
Savastano HI, Cole RP, Barnet RC, Miller RR (1999) Reconsidering conditioned inhibition. Learn Mot 30:101–127
Siegel S (1975) Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol 89:498–506
Siegel S (1978) Tolerance to the hyperthermic effect of morphine in the rat is a learned response. J Comp Physio Psychol 92:1137–1149
Siegel S (1983) Classical conditioning, drug tolerance, and drug dependence. In: Israel Y, Glaser FB, Kalant H, Popham RE et al (eds) Research advances in alcohol and drug problems, vol 7. Plenum, New York, pp 207–246
Siegel S (1991) Classical conditioning and opiate tolerance and withdrawal. In: Balfour DJK (ed) Psychotropic drugs of abuse. Pergamon Press, New York, pp 59–85
Siegel S, Larson SJ (1996) Disruption of tolerance to the ataxic effect of ethanol by an extraneous stimulus. Pharmacol Biochem Behav 55:125–130
Siegel S, Ramos BMC (2002) Applying laboratory research: drug anticipation and the treatment of drug addiction. Exp Clin Psychopharm 10:162–183
Siegel S, Hinson RE, Krank MD, McCully (1982) Heroin “overdose” death: the contribution of drug-associated environmental cues. Science 216:436–437
Siegel S, Baptista M, Kim JA, McDonald R, Weise-Kelly L (2000) Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol 8:276–293
Skinner BF (1950) Are theories of learning necessary? Psychol Rev 57:193–216
Sokolowska M, Siegel S, Kim JA (2002) Intraadministration associations: conditional hyperalgesia elicited by morphine onset cues. J Exp Psychol Anim Behav Process 28:309–320
Spear NE (1978) The processing of memories: forgetting and retention. Erlbaum, Hillsdale
Thomas DR, Sherman L (1986) An assessment of the role of handling cues in spontaneous recovery after extinction. J Exp Anal Behav 46:305–314
Vogel-Sprott M (1992) Alcohol tolerance and social drinking: learning the consequences. Guilford, New York
White AM, Roberts DC, Best PJ (2002) Context-specific tolerance to the ataxic effects of alcohol. Pharmacol Biochem Behav 72:107–110
Williams DA, Overmier JB, LoLordo VM (1992) A reevaluation of Rescorla’s early dictums about Pavlovian conditioned inhibition. Psychol Bull 111:275–290
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by funding from Denison University.
Rights and permissions
About this article
Cite this article
Brooks, D.C., Vaughn, J.M., Freeman, A.J. et al. An extinction cue reduces spontaneous recovery of ataxic ethanol tolerance in rats. Psychopharmacology 176, 256–265 (2004). https://doi.org/10.1007/s00213-004-1882-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-1882-y