Abstract
The physiological function and cellular role of some members of the TRPM family are poorly understood and still mysterious. Melastatin, the founding member of the TRPM group, is the most prominent example of the mysteries involved in understanding TRP channel function. Melastatin or TRPM1 was first cloned in 1998 and since then it has been suggested that it functions as a tumor suppressor protein in melanocytes. On the other hand, TRPM8 and TRPA1 have been described as cold receptors, TRPM4 and TRPM5 as calcium-activated nonselective cation channels, TRPM6 and TRPM7 as magnesium-permeable and magnesium-modulated cation channels, TRPM2 as an ADP-ribose-activated channel of macrophages, and TRPM3 as a hypo-osmolarity- and sphingosine-activated channel. There are many unsolved questions and many studies have to be performed to understand the overall function of the TRPM family. In addition to electrophysiological recordings and biochemical characterization, the use of compounds modulating TRPM channel function has often been helpful to study TRPM channels in a cellular context. Therefore, the review will summarize the known functions, activation mechanisms, and pharmacological modulations of the TRPM channels.
Similar content being viewed by others
Introduction
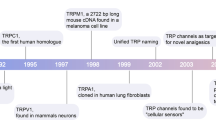
Organization of cells in a multicellular organism depends on rapid and accurate transmission of information. Electrical signals and chemical compounds induce changes of intracellular second messenger concentrations, e.g., calcium ions, cAMP and cGMP. In excitable cells, electrical signals induce increases in the intracellular calcium concentration necessary for cellular responses like hormone secretion, contraction of muscle cells and sensation, processing and responding to environmental stimuli. These functions are mediated by voltage-gated calcium channels and hyperpolarization-activated cyclic nucleotide-modulated channels. The molecular basis for the hormone-induced calcium transient in nonexcitable cells was unclear and began to be unraveled with the molecular characterization of the trp locus of the Drosophila genome. Montell and Rubin (1989) identified and cloned Drosophila TRP as a cation channel involved in Drosophila phototransduction. The sequencing of the different genomes accelerated the access to homologous proteins in worm and men, and soon the TRP channels grew up to a superfamily with more than 20 different genes coding for TRP-homologous channel proteins in men (Harteneck et al. 2000; Montell et al. 2002). The classification of the 20 TRP channels into at least three subfamilies (TRPC, TRPV, TRPM) was initially based on sequence comparison, later on functional data helped to structure the variety of proteins.
Proteins closely related to the Drosophila TRP channel protein on the basis of phylogeny and function form the family of classic TRP channels (TRPC; Clapham et al. 2003). These proteins are activated by intracellular signals generated upon hormonal stimulation (Zufall 2005; Dietrich et al. 2005; Plant and Schaefer 2005). The TRPV family is formed by proteins related to the vanilloid receptor 1 or capsaicin receptor 1 (Clapham et al. 2003). Two members of the TRPV family function as calcium transport proteins of vitamin D3-responsive epithelium whereas the other members are activated by physical and chemical stimuli. The TRPM group was named according to the first known member, melastatin, a protein identified in a comparative screen between benign and malignant transformed melanocytes (Duncan et al. 1998; Hunter et al. 1998; Deeds et al. 2000). Within the superfamily of TRP channels, the proteins of the TRPC and TRPV classes are best characterized, whereas there are deficits in understanding the function of the many TRPM members. Comparisons across the species resulted in one, three and eight TRPM members in Drosophila, C. elegans and mammals, respectively. Phylogenetic analysis of Fig. 1 allows reducing the variability of the mammalian proteins to four groups (TRPM1 and TRPM3, TRPM6 and TRPM7, TRPM4 and TRPM5, TRPM2 and TRPM8). Furthermore, Fig. 1 shows that the Drosophila and C. elegans members are closely related to the sequences of TRPM1/TRPM3 and TRPM6/TRPM7 and probably share similar cellular functions. Deciphering the activation mechanisms of TRP channels, the first compounds have been described allowing pharmacological modulation of TRPM channels (Table 1). Except the herbal products, the known compounds are currently more useful as experimental pharmacological tools than first model compounds for developing therapeutic drugs. The diversity of interesting functions and the close sequence relationship are the opposing conditions for the challenging aim to develop TRP-modulating drugs for the therapy. This review will summarize the activation mechanisms and the known compounds allowing pharmacological modulation of TRPM channels (Table 1).
TRPM1 and TRPM3
In a differential display screen analyzing mRNAs of different melanoma cell lines, the mRNA of TRPM1 was identified and named melastatin because of the absence of TRPM1 mRNA in malignant transformed melanoma cell lines, suggesting a tumor suppressor function of the channel protein (Duncan et al. 1998; Hunter et al. 1998; Deeds et al. 2000). The putative role of TRPM1 in cellular differentiation and proliferation processes is further confirmed by studies of hexamethylene bisacetamide (HMBA)-treated human pigmented melanoma cell lines (Fang and Setaluri 2000). HMBA-treated cells differentiate and change pattern of expressed proteins. Pigmented metastatic melanocytes selectively transcribe TRPM1 mRNA. The level of TRPM1 mRNA could be enhanced by incubation in the presence of 5 mM HMBA (Fang and Setaluri 2000). Furthermore, the existence of a huge number of splice variants was described, a common phenomenon of TRPM1 and TRPM3. Analysis of signals from Northern hybridization studies revealed the presence of at least four different transcripts coding for TRPM1 proteins (1.3, 1.8, 4.5, 5.4 kb mRNA). The transcription of the individual mRNA species depends on melanoma cell lines and differentiation states. The available EST profiling of human EST indicates the presence of TRPM1 mRNA during all stages of embryonal development whereas in tissues of adults TRPM1 mRNA is restricted to skin and eye. Although melastatin is the first member of the TRPM family, little is known about its functional properties and cellular functions.
Like for TRPM1, the transcription of the TRPM3 gene results in a vast number of different mRNA species. In Northern Blot analyses of mouse brain at least three transcripts of different lengths were detectable (Grimm et al. 2003), whereas Lee et al. (2003) cloned six variants from human kidney, which vary in short deletions and insertions indistinguishable by Northern Blot analysis. The variability of TRPM3 transcripts is further enhanced by the presence of two or probably three alternative start positions and two different C-terminal ends. At this time, it is unclear whether mRNA species of all possible variations are transcribed and result in missense proteins, which are subsequently degraded prior to translocation to the plasma membrane, or whether the different TRPM3 mRNA species are transcribed in a cell type-specific manner. TRPM3 protein has been shown to be expressed in human brain and human kidney, whereas in mouse kidney TRPM3 is undetectable.
The different lengths of TRPM3 mRNAs and the apparent molecular masses of the TRPM3 proteins probably result in different activation mechanism. The long variant encoded by 1545 amino acids is probably activated by store-depletion (Lee et al. 2003), whereas the short variant encoded by 1325 amino acids forms a channel protein of approximately 150 kDa which mediates calcium entry upon extracellular application of hypotonic solution (Fig. 2; Grimm et al. 2003). The TRPM3-mediated hypotonicity-induced calcium entry is blocked by the nonselective blockers of cation channels, i.e. lanthanum and gadolinium ions, whereas the channel blocker SK&F-96365 is ineffective (see Table 1). The expression of TRPM3 in kidney and the activation by hypotonicity argue for the function of TRPM3 in renal osmo-homeostasis. Recently, we were able to characterize TRPM3 as the first cation channel activated by sphingosine (Fig. 2; Grimm et al. 2005). In transiently transfected HEK293 cells, sphingosine and its precursor dihydrosphingosine induce TRPM3 currents. Other metabolites of the sphingolipid pathway like ceramides and sphingosine-1-phosphate, described as ligand of G-protein-coupled-receptors, were ineffective in activating TRPM3 (Grimm et al. 2005). The responses to sphingosine are restricted to TRPM3, while sphingosine was ineffective in HEK293 cells expressing TRPC3, TRPC4, TRPC5, TRPV4, TRPV5, TRPV6 or TRPM2. The sphingosine-induced, TRPM3-mediated calcium entry is not affected by store-depletion nor by compounds inhibiting protein kinase C. Therefore, a direct activation of TRPM3 by sphingosine is very likely. Intracellular sphingosine concentration depend on de novo synthesis, degradation of sphingolipids by ceramidases, activity of sphingosine kinases and sphingosine-1-phosphatases and degradation of sphingosine by sphingosine lyases (Futerman and Hannun 2004). It is unclear which pathway results in sphingosine concentrations necessary to induce TRPM3 currents. In summary, TRPM1 remains a mysterious cation channel involved in proliferation and differentiation of melanocytes. Its relative TRPM3 is involved in renal osmo-homeostasis and is integrated in sphingolipid-mediated signal transduction and is the first cation channel activated by sphingosine.
TRPM6 and TRPM7
TRPM6 and TRPM7 are the closest relatives of TRPM1 and TRPM3 (see Fig. 1). The four proteins share many identical amino acids within their N-terminal sequences, the transmembrane domains, the putative pore and a small cytosolic domain following the sixth transmembrane domain. However, the C-termini are different between both groups. Whereas TRPM6 and TRPM7 share a protein kinase domain at their C-termini, the function of the long C-terminal ends of TRPM1 and TRPM3 is unclear. Delineated from the chimeric structure of a pore-forming transmembrane domain and a putative C-terminal domain with enzymatic activity, TRPM6 and TRPM7 were described as chanzymes or channel kinases (Drennan and Ryazanov 2004; Chubanov et al. 2005).
Within the human kinome, both channel kinases can be classified as atypical alpha protein kinases, which have homologies with the elongation factor 2 kinase and the heart, lymphocyte and muscle alpha kinases (Manning et al. 2002). Introduction of mutations in the alpha kinase domain results in loss of function, therefore it is believed that the protein kinase domain is involved in the activation process (Runnels et al. 2001). However, the relationship between TRPM7 channel activity and kinase activity remain unclear.
Experiments characterizing the heterologously expressed kinase domain of TRPM7 showed that the protein fragment is able to mediate autophosphorylation and to phosphorylate prototypic kinase substrates like myelin basic protein and histone H3 (Ryazanova et al. 2004). Furthermore, it was shown that annexine I is phosphorylated by the kinase domain of TRPM7 (Dorovkov and Ryazanov 2004). The kinase activity of TRPM7 depends on ATP and requires magnesium (optimum 4–10 mM) (Dorovkov and Ryazanov 2004). On the other hand, TRPM7 is inhibited by magnesium concentrations ∼0.6 mM (Nadler et al. 2001; Runnels et al. 2001). The discrepancies in sensitivity to the magnesium concentration could result from differences in the experimental approach (bacterial vs. eukaryotic expression system and holoprotein vs. protein fragment) or from spatially distributed magnesium concentrations, which may differ between cytosolic concentrations and local concentrations next to the magnesium-transducing mouth of the pore. Despite these discrepancies, it is accepted that TRPM7 is modulated by the intracellular magnesium concentration and by ATP, forms a pore permeable for divalent cations (permeability Zn2+∼Ni2+≫Ba2+>Co2+>Mg2+≥Mn2+≥Sr2+≥Cd2+≥Ca2+), is blocked by magnesium, polycations like spermine, 2-aminophenoxyborate (2-APB), Mn (III) tetrakis (4–benzoic acid) porphyrin chloride (MnTBAP), lanthanum and gadolinium ions (see Table 1; Nadler et al. 2001; Runnels et al. 2001; Hermosura et al. 2002; Aarts et al. 2003; Kerschbaum et al. 2003).
The ubiquitously expressed TRPM7 is discussed to be involved in the regulation of cellular magnesium homeostasis and to be hormonally regulated via G-protein-coupled receptors, however, the activation process is controversially discussed. The group of David Clapham (Runnels et al. 2001, 2002) characterized TRPM7 as a phospholipase C-interacting TRP channel (TRP-PLIK) and provided evidence for an activation mechanism dependent on the phosphoinositol-4,5-bisphosphate (PIP2) concentration and phospholipase C activity (Fig. 3). Delineated from their data, TRPM7 is activated by a reduced PIP2 concentration resulting from breakdown of phosphoinositides by phospholipase C and inhibited by the activity of phosphoinositide kinases restoring the plasma membrane PIP2 concentration. The group of Andrea Fleig and Reinhold Penner (Takezawa et al. 2004) suggested an alternative activation pathway via a GPCR-induced, cAMP-dependent phosphorylation of TRPM7 (see Fig. 3). In their experiments, they found differential modulation of TRPM7 activity after β-adrenergic stimulation or muscarinic activation of the cells. Whereas β-adrenergic resulted in increased TRPM7 activity, muscarinic stimulation inhibited TRPM7 currents in a pertussis toxin-dependent manner. Further characterization showed that the TRPM7 activity depends on the intracellular cAMP concentration and a functional cAMP-dependent protein kinase. The variety of proposed activation mechanisms was enhanced by the description of the involvement of TRPM7 in neurotoxic death (see Fig. 3; Aarts et al. 2003). Cellular calcium overload mediated by NMDA receptors in brain ischemia is thought to be a major trigger causing neuronal cell death. However, the pathogenesis is very complex and additionally triggered by increased concentrations of reactive oxygen species (ROS) during the reperfusion phase. In cultured neurons, the down-regulation of TRPM7 by siRNA was associated with reduced anoxic cell death and decreased ROS production, arguing for a model in which a triggering increased intracellular calcium concentration is mediated by NMDA receptors during ischemia and by TRPM7 during the reperfusion phase. It is unclear whether TRPM7 is modulated by the different activation mechanism synergistically or independently.
Whereas the ubiquitously expressed TRPM7 is involved in the regulation of cellular magnesium homeostasis, TRPM6 is selectively expressed in intestinal and renal epithelia and involved in the body magnesium homeostasis (Konrad et al. 2004). Mutations in the TRPM6 gene are responsible for the hereditary disease of familial hypomagnesemia with secondary hypocalcemia (Schlingmann et al. 2002; Walder et al. 2002). The mutations found in patients often result in truncated proteins or in variants with deficits in the translocation process to the plasma membrane (Chubanov et al. 2004). Heterologously expressed TRPM6 was found to form a magnesium- and calcium-permeable cation channel, which is regulated by magnesium and blocked by ruthenium red in a voltage-dependent manner (see Table 1; Voets et al. 2004). In summary, TRPM6 is involved in intestinal uptake and renal reabsorption of magnesium, whereas TRPM7 regulates cellular magnesium homeostasis.
TRPM4 and TRPM5
TRPM4 and TRPM5 both form calcium-activated sodium channels impermeable for calcium and mediate depolarization of the plasma membrane (Fig. 4; Launay et al. 2002; Hofmann et al. 2003; Nilius et al. 2003; Prawitt et al. 2003). Whereas expression of TRPM4 has been described to occur ubiquitously, TRPM5 is selectively expressed in stomach, intestine and cells of the taste buds, suggesting an involvement of TRPM5 in taste transduction (Launay et al. 2002; Perez et al. 2002). Down-regulation of TRPM4 in cerebral vascular smooth muscle cells results in attenuated myogenic constriction, arguing for the regulation of cerebral blood flow by TRPM4-dependent plasma membrane depolarization (Earley et al. 2004; Guinamard et al. 2004). TRPM4 is activated by intracellular calcium, modulated by voltage and inhibited by ATP, ADP, AMP and intracellular application of polyamines like spermine (see Table 1; Nilius et al. 2003, 2004a). Decavanadate, an agent interfering with the ATP-binding sites of ATP-dependent transport proteins, selectively blocked TRPM4, but was ineffective in blocking TRPM5 currents (Nilius et al. 2004b). Surprisingly, decavanadate does not infer with ATP in blocking TRPM4, arguing for two independent binding sites. In summary, TRPM4 and TRPM5 form calcium-activated sodium channels mediating plasma membrane depolarization.
TRPM2 and TRPM8
TRPM2 is the third chanzyme within the TRP family, forming a calcium-permeable cation channel fused to an enzymatic domain with ADP-ribose pyrophosphatase activity at the cytosolic C-termini of the protein (Cahalan 2001; Perraud et al. 2001; Heiner et al. 2005). The sequence of the enzymatic domain was identified by sequence similarities to Nudix hydrolases, enzymes cleaving mononucleotide and dinucleotide polyphosphates (Fig. 5; Perraud et al. 2001). The mutT signature is the common feature of the very diverse protein family. Proteins with the mutT signature are probably house-keeping enzymes removing mutagenic nucleotide derivates like 8-oxo dGTP, dinucleotide polyphosphates or inositol polyphosphates (Bessman et al. 1996; Guranowski 2000).
The Nudix domain of TRPM2 cleaves ADP-ribose, a breakdown product of NAD and cyclic ADP-ribose, representing an intracellular second messenger stimulating calcium release mediated by ryanodine receptors. While ADP-ribose is hydrolyzed by TRPM2, it also activates TRPM2 and induces TRPM2 currents during infusion of ADP-ribose by the patch pipette. The activity of TRPM2 depends on the presence of intracellular calcium and is induced by extracellular application of hydrogen peroxide (see Fig. 5; Wehage et al. 2002; McHugh et al. 2003). The activation of TRPM2 by hydrogen peroxide is probably linked to the activity of the poly(ADP-ribose) polymerase, an enzyme transferring multiple ADP-ribose groups to proteins. Oxidative stress and other stimuli causing DNA damage enhance poly(ADP-ribose) polymerase activity (Davidovic et al. 2001). The large and branched structure of the poly(ADP-ribose) modifications are reduced prior to protein degradation to mono(ADP-ribose) by the poly(ADP-ribose) glycohydrolase, a process releasing ADP-ribose. Evidence for this intracellular pathway resulting in TRPM2 activation has been confirmed by the use of inhibitors of poly(ADP-ribose) polymerase, which were able to interfer with the hydrogen peroxide-induced TRPM2 activation (see Fig. 5; Fonfria et al. 2004).
Whereas TRPM2 is insensitive to lanthanum and gadolinium ions, it was recently shown that TRPM2 currents are blocked by flufenamic acid, a compound known to block a great variety of channel proteins (Hill et al. 2004a). Flufenamic acid has been characterized as an open-channel blocker of TRPM2. Activity of flufenamic acid depends on the pH with enhanced effect at acidic conditions. Flufenamic acid will allow analyzing TRPM2-mediated cellular effects in cells of the immune system, where TRPM2 is expressed especially in cells of the monocytic lineage including various cultured macrophage cell lines, peripheral blood monocytes and neutrophils (Perraud et al. 2001; Sano et al. 2001; Hara et al. 2002). TRPM2 expression was detected in pancreas and cell lines derived form pancreatic islet cells (Inamura et al. 2003). In brain, TRPM2 is mainly expressed in the immune cells of the brain, the microglia (Kraft et al. 2004). TRPM2 sensitivity to hydrogen peroxide depends on the activation state of the microglia (Kraft et al. 2004). This suggests that TRPM2 is involved in the regulation of intracellular calcium depending on the developmental state of the microglia. In summary TRPM2 is a hydrogen peroxide-activated cation channel involved in the host-defense system of the body.
The next relative to TRPM2 is TRPM8, a channel protein activated by a quite different mechanism. The cDNA of TRPM8 was isolated from prostate cancer cells, and the function of TRPM8 was initially linked to progression of cancer cells (Tsavaler et al. 2001). The physiological role of TRPM8 as a cold receptor of the body was revealed by an expression cloning approach to identify a menthol receptor from trigeminal neurons (McKemy et al. 2002; Peier et al. 2002). The isolated cDNA codes for TRPM8 and forms a calcium-permeable cation channel. In TRPM8-expressing cells, application of menthol, icilin or other cooling agents induce TRPM8 currents, which are comparable to activation of TRPM8 by temperatures lower than 28°C. Furthermore, TRPM8 is activated by many other odorant agents isolated from plants, e.g., linalool, geraniol, and hydroxycitronellal (Behrendt et al. 2004). Like TRPV1, TRPM8 is inhibited by capsazepine, N-(4-tert. butyl-phenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1 (2H)-carboxamide (BCTC) and a thio-derivative of BCTC. TRPM8 expression is detected in prostate and other tissues of the urogenital tract, but high TRPM8 expression is specifically found in a subset of pain- and temperature-sensing neurons (McKemy et al. 2002; Peier et al. 2002; Stein et al. 2004). In summary, TRPM8 displays a cold receptor of the body, and its activation can be modulated by many cooling compounds and odorants.
ANKTM1
Noxious cold is transduced by ANKTM1, another channel protein with many properties of TRP channels, which is therefore classified as TRPA1. Like TRPM8, which was initially found in prostate cancer cells, ANKTM1 was initially identified in a cancer cell line (Jaquemar et al. 1999). ANKTM1 was characterized as a protein with six putative transmembrane domains and many N-terminal ankyrin repeats. As the Drosophila mechanosensor channel protein NOMPC also carries 29 ankyrin repeats, the accumulation of 18 ankyrin repeats in the N terminus of ANKTM1 made it likely that ANKTM1 is involved in mechanotransduction. This has recently been confirmed (Corey et al. 2004). ANKTM1 is highly expressed in the hair cell epithelia of the cochlea, which are responsible for mechanotransduction. Down-regulation of highly expressed ANKTM1 resulted in a loss of function and reduced ANKTM1 currents. On the other hand, ANKTM1 is expressed in a subset of pain- and temperature-sensing neurons, where ANKTM1 transduces noxious cold (Story et al. 2003; Bandell et al. 2004; Jordt et al. 2004). Like TRPM8, ANKTM1 is activated by icilin and cold temperatures (lower than 17°C), whereas ANKTM1 is insensitive to menthol and can be blocked by ruthenium red (see Table 1; Story et al. 2003). Furthermore, ANKTM1 is activated by a wide range of naturally occurring compounds isolated from plants. ANKTM1 can be stimulated by pungent compounds of mustard, cinnamon, wintergreen, ginger and clove containing allyl- or benzyl-isothyiocyanate, cinnamaldehyde, methyl salicylate, gingerol and eugenol, respectively (see Table 1; Bandell et al. 2004). In summary, pungent agents activate ANKTM1, a channel involved in mechanotransduction and sensation of noxious cold.
Outlook
The mysteries of TRPM function slowly solve resulting in many unexpected functions, such as involvement in magnesium homeostasis and in plasma membrane depolarization, cold sensation or osmo-regulation. This process of understanding is accelerated by the use of pharmacological tools and will probably end in a picture of channels proteins regulated by highly different activation mechanisms. The phenomenon of dually activated cation channels has already been described for other TRP channel proteins e.g., for TRPA1 involved in cold- and mechanosensation, for TRPV4 activated by heat, small lipid compounds and cell-swelling or hypo-osmolarity. Dual regulation will probably become a common property of TRPM channels as well.
References
Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115:863–877
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R (2004) Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 141:737–745
Bessman MJ, Frick DN, O’Handley SF (1996) The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J Biol Chem 271:25059–25062
Cahalan MD (2001) Cell biology. Channels as enzymes. Nature 411:542–543
Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA 101:2894–2899
Chubanov V, Mederos y Schnitzler M, Wäring J, Plank A, Gudermann T (2005) Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes. Naunyn-Schmiedebergs Arch Pharmacol (in press)
Clapham DE, Montell C, Schultz G, Julius D (2003) International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev 55:591–596
Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 13:13
Davidovic L, Vodenicharov M, Affar EB, Poirier GG (2001) Importance of poly(ADP-ribose) glycohydrolase in the control of poly(ADP-ribose) metabolism. Exp Cell Res 268:7–13
Deeds J, Cronin F, Duncan LM (2000) Patterns of melastatin mRNA expression in melanocytic tumors. Hum Pathol 31:1346–1356
Dietrich A, Mederos y Schnitzler M, Kalwa H, Storch U, Gudermann T (2005) Functional characterization and physiological relevance of the TRPC3/6/7subfamily of cation channels. Naunyn-Schmiedebergs Arch Pharmacol (in press)
Dorovkov MV, Ryazanov AG (2004) Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 12:12
Drennan D, Ryazanov AG (2004) Alpha-kinases: analysis of the family and comparison with conventional protein kinases. Prog Biophys Mol Biol 85:1–32
Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW (1998) Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 58:1515–1520
Earley S, Waldron BJ, Brayden JE (2004) Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95:922–929
Fang D, Setaluri V (2000) Expression and up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun 279:53–61
Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S (2004) TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol 143:186–192
Futerman AH, Hannun YA (2004) The complex life of simple sphingolipids. EMBO Rep 5:777–782
Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278:21493–21501
Grimm C, Kraft R, Schultz G, Harteneck C (2005) Activation of the melastatin-related cation channel TRPM3 by d-erythro-sphingosine. Mol Pharmacol 67:798–805
Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P (2004) Functional characterization of a Ca2+-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol 558:75–83
Guranowski A (2000) Specific and nonspecific enzymes involved in the catabolism of mononucleoside and dinucleoside polyphosphates. Pharmacol Ther 87:117–139
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9:163–173
Harteneck C, Plant TD, Schultz G (2000) From worm to man: three subfamilies of TRP channels. Trends Neurosci 23:159–166
Heiner I, Radukina N, Eisfeld J, Kühn F, Lückhoff A (2005) Regulation of TRM2 channels in neutrophil granulocytes by ADP-ribose: a promising pharmacological target. Naunyn-Schmiedebergs Arch Pharmacol (in press)
Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A (2002) Dissociation of the store-operated calcium current ICRAC and the Mg2+-nucleotide-regulated metal ion current MagNuM. J Physiol 539:445–458
Hill K, Benham CD, McNulty S, Randall AD (2004a) Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 47:450–460
Hill K, McNulty S, Randall AD (2004b) Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn-Schmiedebergs Arch Pharmacol 370:227–237
Hofmann T, Chubanov V, Gudermann T, Montell C (2003) TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol 13:1153–1158
Hunter JJ, Shao J, Smutko JS, Dussault BJ, Nagle DL, Woolf EA, Holmgren LM, Moore KJ, Shyjan AW (1998) Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1). Genomics 54:116–123
Inamura K, Sano Y, Mochizuki S, Yokoi H, Miyake A, Nozawa K, Kitada C, Matsushime H, Furuichi K (2003) Response to ADP-ribose by activation of TRPM2 in the CRI-G1 insulinoma cell line. J Membr Biol 191:201–207
Jaquemar D, Schenker T, Trueb B (1999) An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem 274:7325–7333
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Kerschbaum HH, Kozak JA, Cahalan MD (2003) Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J 84:2293–2305
Konrad M, Schlingmann KP, Gudermann T (2004) Insights into the molecular nature of magnesium homeostasis. Am J Physiol Renal Physiol 286:F599–F605
Kraft R, Grimm C, Grosse K, Hoffmann A, Sauerbruch S, Kettenmann H, Schultz G, Harteneck C (2004) Hydrogen peroxide and ADP-ribose induce TRPM2-mediated calcium influx and cation currents in microglia. Am J Physiol Cell Physiol 286:C129–C137
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407
Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, Huang M, Lin JH, Feder JN, Janovitz EB, Levesque PC, Blanar MA (2003) Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J Biol Chem 278:20890–20897
Liu D, Liman ER (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 100:15160–15165
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298:1912–1934
McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ (2003) Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 278:11002–11006
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58
Montell C, Rubin GM (1989) Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2:1313–1323
Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX (2002) A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 9:229–231
Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A (2001) LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411:590–595
Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V (2003) Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 278:30813–30820
Nilius B, Prenen J, Janssens A, Voets T, Droogmans G (2004a) Decavanadate modulates gating of TRPM4 cation channels. J Physiol 26:26
Nilius B, Prenen J, Voets T, Droogmans G (2004b) Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflügers Arch 448:70–75
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715
Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5:1169–1176
Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411:595–599
Plant T, Schaefer M (2005) Receptor-operated cation channels formed by TRPC4 and TRPC5. Naunyn-Schmiedebergs Arch Pharmacol (in press)
Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R (2003) TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]I. Proc Natl Acad Sci USA 100:15166–15171
Runnels LW, Yue L, Clapham DE (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291:1043–1047
Runnels LW, Yue L, Clapham DE (2002) The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol 4:329–336
Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG (2004) Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem 279:3708–3716
Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K (2001) Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293:1327–1330
Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31:166–170
Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, Patel AS, Nelson JB, Futrell WJ, Yoshimura N, Chancellor MB, De Miguel F (2004) Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol 172:1175–1178
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A (2004) Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci USA 101:6009–6014
Tsavaler L, Shapero MH, Morkowski S, Laus R (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61:3760–3769
Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG (2004) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279:19–25
Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31:171–174
Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A (2002) Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277:23150–23156
Zufall F (2005) The TRPC2 ion channel and pheromone sensing in the accessoryolfactory system. Naunyn-Schmiedebergs Arch Pharmacol (in press)
Acknowledgements
Our own work reviewed here was supported by the Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie, and Sonnenfeld-Stiftung.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harteneck, C. Function and pharmacology of TRPM cation channels. Naunyn-Schmiedeberg's Arch Pharmacol 371, 307–314 (2005). https://doi.org/10.1007/s00210-005-1034-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-005-1034-x