Abstract
The development of inhaled drugs for respiratory diseases is frequently impacted by lung pathology in non-clinical safety studies. To enable design of novel candidate drugs with the right safety profile, predictive in vitro lung toxicity assays are required that can be applied during drug discovery for early hazard identification and mitigation. Here, we describe a novel high-content imaging-based screening assay that allows for quantification of the tight junction protein occludin in A549 cells, as a model for lung epithelial barrier integrity. We assessed a set of compounds with a known lung safety profile, defined by clinical safety or non-clinical in vivo toxicology data, and were able to correctly identify 9 of 10 compounds with a respiratory safety risk and 9 of 9 compounds without a respiratory safety risk (90% sensitivity, 100% specificity). The assay was sensitive at relevant compound concentrations to influence medicinal chemistry optimization programs and, with an accessible cell model in a 96-well plate format, short protocol and application of automated imaging analysis algorithms, this assay can be readily integrated in routine discovery safety screening to identify and mitigate respiratory toxicity early during drug discovery. Interestingly, when we applied physiologically-based pharmacokinetic (PBPK) modelling to predict epithelial lining fluid exposures of the respiratory tract after inhalation, we found a robust correlation between in vitro occludin assay data and lung pathology in vivo, suggesting the assay can inform translational risk assessment for inhaled small molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhaled drug delivery is an effective treatment paradigm for respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), due to direct access to the intended target organ and the potential to minimize systemic exposure and consequent side effects (Ruge et al. 2013). The development of innovative inhaled medicines is often impacted by toxicity in the respiratory tract in non-clinical species, leading to attrition or dose limitations in clinic, typically after the selection of novel drug candidates (David et al. 2014). As part of the industry shift from observational to predictive toxicology, a proactive discovery safety strategy aims to identify and mitigate these safety risks early during drug discovery, and thereby enable the design and selection of drug candidates with the right safety profile (Hornberg et al. 2014a; Hornberg and Mow 2014; Morgan et al. 2018). This relies heavily on in vitro methods that are predictive for in vivo toxicity, clinical safety and compatible with the design-make-test-analyze (DMTA) cycle during lead optimization campaigns (Hornberg et al. 2014b; Johansson et al. 2019). A plethora of high-content screening (HCS) methodologies have been developed and deployed to successfully identify for example cardio-, hepato-, neuro-, and nephrotoxicity (Li and Xia 2019; Persson and Hornberg 2016). However, the development of suitable in vitro assays to predict inhaled toxicity has been a challenge, which is due in part to the different routes of administration, inhaled versus systemic but also the complexity of the human lung. The lung is composed of approximately 50 different cell types at various stages of differentiation within the lung epithelia and involves a complex structure combining air conducting and gas exchanging regions (Berube et al. 2010; Hiemstra et al. 2018). The primary target within the lung for inhaled toxicants is the epithelial layer (Hiemstra et al. 2018). The tissues of the conducting airways are lined by a layer of pseudostratified columnar epithelium containing ciliated, goblet, club and basal cells. This epithelial layer has a protective function, but also moistens inhaled air (Vielle et al. 2019). The alveolar space is composed primarily of alveolar type I and II epithelial cells. Type I alveolar cells form a single layer which allows rapid gas exchange with the bloodstream while type II produces surfactants and gives rise to new alveolar cells (Knudsen and Ochs 2018). In order to prevent the passage of external detritus into the bloodstream, these epithelial cells form a tight cell-to-cell junction that acts as a barrier (Overgaard et al. 2012). The integrity of the epithelial barrier in the lung is often compromised in respiratory diseases, which can be studied in vitro using complex air–liquid interface (ALI) cultures of primary human epithelial cells (Aghapour et al. 2018; Gon and Hashimoto 2018; Hiemstra et al. 2018; Pell et al. 2021; Upadhyay and Palmberg 2018). We have previously shown that measurements of trans-epithelial electrical resistance (TEER) and cell viability in such a physiologically relevant trans-well model are highly predictive for respiratory toxicity of inhaled drugs (Balogh Sivars et al. 2018). The ALI model has, however, limitations for applicability in early drug discovery decision-making. Due to its complexity and consequent resource intensity, the ALI model does not have sufficient throughput to test large numbers of compounds to inform dose response and structure–activity relationships. In addition, we have previously shown that TEER measurements in ALI models appear sensitive only at a high concentration (400 µM, single dose level) which presents issues with precipitation for poorly soluble inhaled small molecules. Compounds are therefore typically scored categorically as toxic or non-toxic, whereas lead optimization campaigns rely on quantitative information, such as half-maximal inhibitory concentration (IC50) values in a relevant concentration range. The maintenance of these physiological barriers, often measured via TEER, including the air–blood, blood–brain and intestinal barriers, is supported primarily by junctional proteins (Hashimoto and Campbell 2020; Rouaud et al. 2020; Slifer and Blikslager 2020). We hypothesized that high-content imaging of tight junction proteins in a lung epithelial 2D culture would enable a scalable alternative to the ALI cultures, amenable for use during the drug discovery phase and for quantitative risk assessment.
Here we developed a novel high-content screening assay, based on the quantification of the tight junction protein occludin in A549 cells, as a surrogate marker for lung epithelial barrier integrity and consequent pulmonary toxicity. We validated the predictivity of the assay using 19 inhaled small molecules with known lung safety profiles, based on non-clinical and clinical data and demonstrated how the assay could be applied to quantitative risk assessment. This method provides a cheap, fast and highly predictive way to screen early discovery phase compounds for identification of undesirable safety profiles to guide chemistry design.
Materials and methods
Cell culture
A549 (ATCC), an alveolar epithelial cell line, and CALU-3 (ATCC), an epithelial cell line, were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS). 16HBE bronchial epithelial cells, kindly provided by Carl Staples (University of Southampton), were cultured in DMEM supplemented with 10% FBS and 1% non-essential amino acids). All culture media materials were purchased from Thermo Fisher Scientific. For imaging experiments, cells were seeded at 10,000 cells per well in 96-well CellCarrier imaging plates (Perkin Elmer) and cultured for 72 h to allow tight junction formation. All experiments were carried out between passages 3 and 15.
Compound treatment
All tested compounds were synthesized internally and provided in 100% DMSO. Compounds were diluted in appropriate media to a 9-point concentration range from 100 µM down to 15 nM with a final concentration of 1% DMSO. Cigarette smoke extract (CSE) was produced by passing smoke from 5 University of Kentucky research cigarettes with filters removed through PBS with an air pump at a rate 0.07 L/min. CSE was diluted in appropriate media to a concentration range of 12% to 0.01%. TNF-α and TGF-β (Thermo Fisher Scientific) were diluted in appropriate media to a concentration range of 50 ng/ml to 0.02 ng/ml. All treatments were for 24 h and terminated by removing media and fixing with 4% paraformaldehyde (PFA). Vehicle (1% DMSO) was included for each plate and all treatments were carried out in duplicate on each plate. All experiments were independently repeated at least 3 times.
Immunofluorescence imaging
Following treatment, cells were washed twice in PBS and fixed in 4% PFA for 20 min at room temperature and permeabilized using 0.5% Triton-X for 15 min at room temperature. Cells were then blocked using 5% goat serum in PBS at room temperature for 1 h. Primary antibodies were applied as per optimized conditions based on manufacturers recommended dilutions overnight at 4 °C. Cells were washed twice in PBS prior to application of secondary antibodies at a dilution of 1:5000 for 1 h at room temperature. Cells were washed twice with PBS and subsequently stained with a mixture of Hoechst and Cell Mask Deep Red at 1:1000 in PBS. Finally, cells were washed twice in PBS and either stored at 4 °C or imaged immediately. Plates were imaged using a MolecularDevices IXC. Each well was imaged at 40 × magnification with 16 images taken per well which provided 32 images per condition for each biological replicate. Cell viability was assessed through direct counts of nuclei.
Image analysis
Analysis modules were written using the custom module editor of MetaXpress version 6.5.4.532. Nuclei were segmented and enumerated using the embedded count nuclei module. Cellular membrane outlines and areas of cellular junctions were identified using the Cell Mask and anti-occludin antibody staining, respectively. Briefly, due to the propensity of this dye to stain all cellular structures, the Cell Mask image was inverted to better highlight the plasma membrane staining. The resulting image was watershed using the previously identified nuclei. This watershed image was then again inverted to give a 1 pixel outline of all cell membranes. In order to fully capture the area of cell membrane responsible for tight junctions, this membrane area was expanded using the Grow Objects function to incorporate a space of 10 pixels on either side of the identified membrane. This thickened outline mask was then applied to the cell mask image and the area of staining was quantified. For each image, the area of membrane staining is reported as the total for all cells within the entire image. Tight junction staining was assessed in a similar manner; however, image inversion was not required due to the significantly more specific staining pattern. To quantify the amount of occludin staining at the membrane, the final step of the module overlaid the thickened membrane mask with the thickened junction mask. The occludin staining fluorescence area within this image overlay was quantified as a measure of tight junction presence and compared between experimental conditions to assess drug-induced effects on tight junctions. Within each image, all nuclei were counted and expressed as a mean value of all 16 images for each well. Loss of cell viability was determined as a drop in total cell nuclei counts. Area of membrane and area of occludin staining in µm2 per image were expressed as the mean of all 16 images normalized to the number of cells counted.
Physiologically based pharmacokinetic modelling
A whole-body rat-specific PBPK model, which places emphasis on pulmonary drug disposition, was implemented in MATLAB R2017a (MathWorks Inc., Natick, MA, USA) (Boger et al. 2016; Boger and Friden 2019). The lung was divided into 24 airway generations and one extra-thoracic region according to the morphometry presented by (Lee and Wexler 2011). The structural model is illustrated in supplementary Fig. 1, where airway generation 1 refers to the trachea. Details regarding calculations of the regional surface areas as well as mucociliary clearance and volumes of the epithelial lining fluid (ELF), epithelium and sub-epithelium are provided in (Boger et al. 2016; Boger and Friden 2019). Each airway generation is divided into three compartments: (1) ELF, (2) epithelium and (3) sub-epithelium (supplementary Fig. 1). The airway generations either belong to the tracheobronchial- (generation 1–16) or the alveolar region (generation 17–24). Drug particle dissolution (if applicable) in the ELF was modelled by the Nernst–Brunner equation (Nernst 1904; Noyes and Whitney 1897). In addition, the model assumed the absence of any non-specific binding in the ELF compartment. Perfusion-rate limited distribution was assumed to apply for all tissues in the PBPK model. An additional tissue-binding compartment was introduced in each epithelium and sub-epithelium compartment with model optimized values for kin and kout representing the distribution in and out of a volume-less ‘deep’ compartment. The permeability in the alveolar region was set at tenfold, the value for the bronchiolar region. The tracheobronchial region is perfused by the bronchial blood flow and the alveolar region by the entire cardiac output. The local bronchial blood flow to a tracheobronchial generation was calculated according to (Boger et al. 2016; Boger and Friden 2019). The local blood flow to the alveolar generations was assumed to be constant in terms of flow per tissue volume. All ordinary differential equations (ODEs) used in the model are provided in (Boger et al. 2016; Boger and Friden 2019).
The PBPK model was first fitted to available plasma and lung concentrations after intratracheal dosing with 70 and 30% deposition in the tracheobronchial- and alveolar region, respectively (Boger and Friden 2019). The resultant optimized values for the main PBPK model parameters whilst setting constant the values for the systemic PK parameters of CL and Vss can be found in supplementary Table 1. The optimized PBPK model was subsequently used to simulate the unbound compound concentrations in the epithelium lining fluid for all tracheobronchial generations (Gen. 1–16) after dry powder inhalation.
Nonclinical toxicology study and histopathological assessment
Inhalation dosing of the small molecule AZ5 was achieved using a dry powder aerosol, generated using a Wright Dust Feed (WDF) mechanism. The exposure system comprised an aerosol conditioning pre-chamber and a snout-only inhalation exposure chamber, to which Han Wistar rats were attached via restraining tubes, air supply and extract lines. The duration of dosing was 30 min, once per day for a period of 4 weeks. The achieved dose levels were 0.361, 2.20 or 13.8 mg/kg/day (target dose levels of 0.3, 2 or 15 mg/kg/day, respectively), using aerosol concentrations of 16.2, 99.0 or 621 µg/L. The mass median aerodynamic diameters (MMAD) were within the respirable range (1 to 3 µm) at all dose levels. During the course of the study, blood samples were taken on days 1 and 28, immediately post-dose and up to 23.5 h post-dose to measure plasma exposures. Compound levels in plasma were used in the PBPK modelling for calculating ELF concentrations.
At study termination, all animals underwent a full necropsy examination and selected tissues were collected for histopathological examination. Tissues collected from the respiratory tract included nasal cavity, larynx, trachea, carina, extrapulmonary bronchus and lung. Tissues were collected into 10% neutral-buffered formalin, processed and embedded in paraffin and sectioned on a microtome according to standard methods. Sections were stained with haematoxylin and eosin and examined by light microscopy.
Data analysis and statistics
IC50 values for each in vitro treatment were calculated using GraphPad prism version.8.4.2. Sensitivity, specificity and accuracy (overall percentage of correct predictions) of the assay were calculated according to Cooper statistics (Cooper et al. 1979). Statistical significance of validation compound concentrations was calculated as a Student’s t test in GraphPad prism version.8.4.2 from three biological replicates.
Results
Measurement of junctional staining and treatment-driven perturbations in epithelial cells
To establish the screening platform, we aimed to select an appropriate combination of a tight junction marker and a cell line that would be compatible with high-throughput image analysis as well as respond to relevant stimuli. We characterized antibodies for four junctional proteins (occludin, ZO-1, β-catenin, E-cadherin) across three commonly used respiratory epithelial cell lines (A549, CALU-3, 16HBE). Of the cell lines explored, CALU-3 cells were shown to have the greatest degree of variability in their growth pattern with a tendency to grow in 3D rather than in a single monolayer. Three-dimensional cultures can influence staining and imaging analysis in a multi-well format, introducing variability. For CALU-3, this resulted in irreproducible, poor-quality images across all markers tested and therefore not of sufficient quality for further assay development. 16HBE cells displayed a robust growth pattern with well-defined junctional borders for occludin and ZO-1. However, these borders did not overlap consistently with cell edges as detected by the cell mask staining, which hampered attempts to quantify changes. A549 cells however, had a consistent growth pattern, achieving a single monolayer over similar incubation times. Whilst the ZO-1, β-catenin and E-cadherin staining for these cells was either not reproducible or lacked the expected staining pattern, occludin displayed the expected “cobblestone” staining pattern (Fig. 1a). This allowed for robust quantification of the junctional area stained per image (Fig. 1b) and the alignment of junctional markers to the cell membrane region (Fig. 1c).
Characterization and measurement of junctional staining in multiple epithelial cell lines. A A549, 16HBE and CALU-3 stained for Occludin, ZO-1, β-catenin and E-cadherin. Scale bar represents 200 µm. B Area of membrane staining detected for each antibody was measured within each cell line. Results are expressed as the mean of the area (µm2) (n = 96, error bars represent SD). C Area of junctional staining was aligned with the area of membrane staining to ascertain the most robust cell line and stain combination. Results are expressed as the % alignment between both staining regions
Next, we determined whether occludin staining in A549 cells could detect perturbations of the cell-to-cell junctional barrier. Cells were treated with known modulators of lung epithelial barrier integrity: CSE, Cadmium chloride, TNF-α, TGF-β and a small molecule IKK2 inhibitor (Balogh Sivars et al. 2018; Schilpp et al. 2021). Image analysis detected a significant decrease in the area of junctional staining present at the cell membrane in treated wells compared to DMSO controls, in a concentration-dependent manner (Fig. 2). The staining was not only associated with cell loss, which was clearly demonstrated using CSE. This indicated the screening system could indeed detect induced changes to the cell barrier staining pattern.
Perturbation of barrier phenotype with known inducers of lung epithelial barrier impairment accurately measured as a reduction in junctional occludin staining. A Imaging of occludin staining in cells treated with (i) DMSO and (ii) CSE show a significant breakdown in the staining pattern following treatment. B Treatment of A549 cells with a combination of (i) TNFα/TGFβ (ng/ml), (ii) CSE (%), (iii) CdCl2 (μM), and (iv) an internal small molecule with defined in vivo lung pathology reduced the area of junctional staining in A549 cells across concentrations independent of changes in cell number
Prediction of lung irritancy for inhaled small molecules
In order to establish the assays predictive performance for drug-induced lung toxicity, a set of 19 inhaled small molecules were tested across a 9-point concentration range up to 100 µM, for their ability to induce changes to the occludin marker in the junctional area. The compound set included a mix of 10 compounds that had previously demonstrated irritancy in the respiratory tract (group 1), at relevant dose levels in pivotal toxicology studies in pre-clinical species or in the clinic, as well as 9 compounds that had a suitable lung safety profile to support clinical development or market registration (group 2) (Table 1).
Occludin area staining was calculated for all compounds across a screening assay compatible concentration range. Values were normalized to total cell number and to DMSO controls. Linear regression analysis was used to generate individual IC50 values for compound ranking. For 9 of the 10 group 1 compounds, a clear effect on occludin staining could be detected and dose response curves could be established with IC50 values in the range 2–50 µM (Fig. 3a). The data also show the assay can rank compounds in terms of their response, which is highly advantageous in order to inform compound selection and chemical design. Indeed AZ1, AZ2 and AZ7 represent alternative chemistries with the same target with known differences in their in vivo safety profiles (data not shown) the severity of which aligns with the assay IC50. In contrast, some of the 9 group 2 compounds, namely AZ14 and AZ18 had only minor effects on Occludin levels at the highest concentration. Hence, IC50 curves could not be established for these compounds (Fig. 3b), clearly indicating the assay can distinguish between compounds with lung irritancy potential and those without.
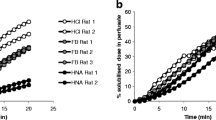
To explore the assay’s predictive ability across concentrations, Student’s t-test statistical analysis was performed on each concentration level. It clearly demonstrated that significant changes between respiratory irritants and non-irritants were detected at concentrations as low as 1 µM (Fig. 4a). When compared to previously published lung epithelial models (Balogh Sivars et al. 2018), the occludin imaging assay in A549 cells had similar predictivity using a comparable validation compound set with 90% sensitivity and 100% specificity. However, that sensitivity was achieved at a lower concentration range (Fig. 4b). Additionally, due to the 96-well plate format, the simple cell system and quick assay protocol, the assay has sufficient throughput to easily obtain full dose–response curves.
The occluding HCI assay could predict lung irritancy at lower concentrations compared to TEER measurements in ALI culture. A Significant differences between compounds with (group1) and without (group 2) a respiratory safety risk were seen across the entire assay concentration range. B In comparison with ALI-based TEER measurements, the imaging assay provides granularity between compounds with varying IC50 values in a relevant concentration range to support medicinal chemistry optimization programs
Alignment of predicted ELF concentrations and assay IC50 with observed pathological findings
With the development of the assay and validation of its predictivity, we established a tool to identify the hazard of lung toxicity for inhaled small molecules and to support the generation of structure–activity relationships required to optimize chemical series to remove that hazard. Next, we sought to examine if the assay had applicability for quantitative risk assessment. As a proof of concept, we assessed how the safety profile of an inhaled small molecule candidate drug from our internal compound collection could be quantitatively predicted by the occludin imaging assay. In a dry powder inhalation toxicology study with AZ5 in rats, we observed pathological changes consistent with airway irritation, with areas of cilia loss, epithelial erosion and ulceration (Fig. 5a). These pathological changes to the rat lung were restricted to the upper tracheobronchial (TB) areas, where compound concentrations are typically higher than in the lower areas of the respiratory tract. At the highest dose, 19 out of 20 animals displayed either minimal (10) or mild (9) tracheal epithelial alteration (loss of cilia and flattening of the epithelial cells). Eight animals experienced minimal (4), mild (3) and moderate (1) epithelial alteration at the carina, and 6 animals had a minimal severity mixed inflammatory cell infiltrate. In the lung, epithelial alterations were also found in the bronchi of 15 animals classified as minimal (12) or mild (3) severity, whilst 5 animals experienced minimal (3) or mild (2) bronchial erosion and one had minimal severity bronchiolo-alveolar metaplasia/hyperplasia. Aggregates of alveolar macrophages, minimal (8) or mild (5) were found in 13 animals.
In vitro assay data align with predicted ELF Cmax in regions with and without lung pathology from a rat dry powder inhalation study. A H&E (i) images display areas of progressive loss of cilia (ii) and epithelial erosion (iii) in the bronchial bifurcation regions and are representative of areas within the upper tracheobronchial region where lesions were commonly located. B PBPK modelling predicted compound concentration in the ELF across the generations of the lung over time. C Cmax concentrations from generations 1 to 6 (red), 7 to 16 (blue), and 17 to 24 (green) are plotted onto the imaging assay IC50 curve for the same compound. Results are expressed as fold change over control. D Graphic shows the calculated Cmax concentrations in each lung region
Plasma exposures were fed into a PBPK model, which incorporates various compound-specific properties, such as distribution, solubility, dissolution of the dry powder, and aspects of the lung tissue architecture, such as cell type permeability (Boger et al. 2016), to calculate the ELF concentrations of the compound in the various areas (generations) of the respiratory tract over time following inhalation (Fig. 5b). This yielded relevant maximum exposure averages for the upper TB, lower TB and alveolar regions of the respiratory tract (Fig. 5d). The occludin results from the in vitro model were correlated to in vivo findings by mapping the modelled ELF concentrations onto the dose response curve. Interestingly, we found that by mapping the modelled ELF concentrations onto the occludin dose–response curve, we could show that pathological changes in vivo were only observed in areas of the respiratory tract that were exposed to drug concentrations (14 µM) similar to that of the in vitro IC50 (27 µM) (Fig. 5c). Equally, no pathological changes were observed in areas of the respiratory tract that were exposed to concentrations (3.2 & 0.02 µM Cmax) that did not elicit an effect in the occludin assay. This suggests that a combination of PBPK modelling and this novel in vitro assay can inform translational and quantitative risk assessment for inhaled small molecules.
Discussion
Pharmaceutical R&D productivity requires a proactive discovery safety strategy to identify and mitigate safety risks early to bring forward candidate drugs with the Right Safety profile (Hornberg and Mow 2014; Morgan et al. 2018). To inform decision-making during drug discovery, robust in vitro assays are required that are compatible with integration in the DMTA cycle namely being cost-effective, have sufficient throughput, and with readouts that are predictive for (non)clinical safety (Blass 2021; Hornberg et al. 2014b; Johansson et al. 2019; McKim 2010; Sanders et al. 2017; Szymanski et al. 2012). Currently, a range of organ-specific assays exist, which use a single cell type rather than attempting to fully recapitulate the complexity of the organ they represent, several of which are applicable to early drug screening, for example those aimed towards the cardiovascular system, liver and kidney (Gustafsson et al. 2014; Persson et al. 2013; Pognan et al. 2023; Pointon et al. 2017; Sjogren et al. 2018). Options to screen inhaled drugs for lung toxicity however have lagged behind. Some bronchial cell-based assays have shown some promise when grown in ALI conditions, such as our predictive TEER-based ALI assay for the detection of lung toxicants (Balogh Sivars et al. 2018) and an imaging-based assay correlating junctional protein staining with TEER measurements as a primary readout of lung barrier integrity (Pell et al. 2021). Recently published breathing lung-on-chip models have also shown significant promise as an in vitro tool to study inhalation toxicity (Sengupta et al. 2022). However, these assays come with significant limitations in terms of throughput, cost, and their ability to stratify compounds within a chemical series. Relevant comparative assay details are listed in Table 2. A549 cells have been widely used as a cell model to study toxicological responses, such as cytotoxicity, oxidative stress and inflammatory responses (Barosova et al. 2021). However, TEER values from these cells when elevated to ALI conditions are far lower than alternative alveolar cell lines such as the lentivirus immortalized hAELVi cells. Indeed hAELVi cells have also been shown to possess a more type I phenotype than the type II of the A549 cells and thus representing a larger proportion of the alveolar population (Kuehn et al. 2016). In an effort to develop an assay which can be rapidly deployed for screening within a DMTA cycle, we focused on measuring a single endpoint from a widely studied cell line which, whilst not recapitulating the full complexity of the lung alveolar space allowed for robust measurement of a physiologically relevant parameter such as junctional protein morphology. Here, we showed that quantification of occludin staining in A549 cells in a simple 2D format can detect lung toxicants/irritants with 90% sensitivity and 100% specificity after 24 h compound administration. This is likely driven by the central role played by junctional proteins in maintaining an effective epithelial barrier in the lung. Occludin is a classic tight junction protein which functions to link cells to adjacent cells and to the internal cytoskeletal structures (Kojima et al. 2013). It has been shown to have a clear link to barrier maintenance, ATP production and gene regulation (Castro et al. 2018), whilst studies with occludin knock-out mice have also shown chronic inflammation amongst other pathologies, indicating not only a wide role for occludin within cellular homeostasis but also a pivotal link to inflammation and pathological processes (Saitou et al. 2000; Sugita and Kabashima 2020).
To develop an imaging-based assay measuring localization of a tight junction protein in a simple 2D culture, growth conditions of the chosen cell line were found to be of utmost importance. Specifically, in the development of this assay, it was found that the morphological characteristics of the bronchial cell lines 16HBE and Calu3 were incompatible with the requirements of the imaging algorithm. In order for robust measurement of the occludin staining pattern, it was necessary to use a whole cell stain to allow for determination of cell edges independent of the junctional marker to narrow the region of measurement to those edges. In the case of Calu3, these cells displayed a highly overlapping growth pattern, tending to form multilayered colonies rather than a uniform monolayer of cells. 16HBE, on the other hand, grew in a highly consistent monolayer fashion but displayed an undulating pattern of cell-to-cell contact rather than a liner line. These inherent conditions posed a challenge when it came to determining the degree of overlap of the junctional marker with the defined cell edge. Alternative seeding densities and culture time points were explored (data not shown). However, A549 cells were consistently found to provide the most robust basis to enable screening assay development.
In order to apply in vitro toxicology assays to quantitative risk assessment, it is essential to incorporate drug exposure information and establish how in vitro concentrations relate to clinical exposure (Bell et al. 2018). While there are challenges to scale compound concentrations and elicited effects in in vitro assays to plasma drug exposure and adverse events in the clinic, it has been possible to use in vitro assay data for quantitative risk assessment of some organ toxicities (Albrecht et al. 2019; Archer et al. 2018; Hengstler et al. 2020; Kappenberg et al. 2020; O'Brien et al. 2006; Persson et al. 2013; Sjogren et al. 2018). Quantitative risk assessment has been more challenging for inhaled drugs because, unlike for orally administered drugs, the systemic plasma exposure at a given inhaled dose does not accurately reflect the relevant exposure in the lung (Frohlich 2019; Kassinos et al. 2021). To address this gap, we applied an existing PBPK modelling approach, which mathematically describes respiratory tract physiology and the fate of deposited drug dose, and accurately predicts PK, target site exposure, and interestingly, spatial heterogeneity in target site concentrations within the lung (Boger et al. 2016; Boger and Friden 2019). This approach allowed us to directly correlate actual compound concentrations from the in vitro assay with calculated compound exposure in the ELF in a toxicity study and demonstrate that the pathological changes consistent with airway irritation were restricted to areas of the respiratory tract that reached concentrations that aligned with the IC50 of the imaging assay. While recent studies have shown the applicability of modelling to in vitro to in vivo extrapolation (IVIVE) for toxic vapours from agrichemicals (Moreau et al. 2022) and environmental contaminants such as endocrine disrupters (Xie et al. 2023), to our knowledge, this is the first-time in vitro toxicity data have been quantitatively linked to exposure levels in the lung for compound risk assessment in drug development.
The propensity of a compound to cause to toxicity may depend on primary target pharmacology, off-target secondary pharmacology and chemical reactivity, which originate from its chemical structure and physicochemical properties, and can manifest in many different downstream effects in the cell, including direct damage to organelles or DNA, membrane permeability, impaired cellular signalling or metabolism. In general, assessing cell health parameters has proven an effective trade-off between, on the one hand, ensuring high sensitivity (compared to more generic cell survival or cytotoxicity assessment) and, on the other hand, providing robust, accessible screening options with throughput (compared to more specific but therefore less-sensitive molecular endpoints). Here, the validation compound set encompassed diverse physicochemical properties, therapeutic targets, off-target pharmacology, and a range of pathologies across multiple pre-clinical tox species, including epithelial erosion, atrophy, oedema, hyperplasia and inflammation. In summary, we present a novel imaging-based assay that can be applied to identify and mitigate the risk for lung toxicity of inhaled compounds during early drug discovery, and for quantitative risk assessment in drug development. This will enable acceleration of the identification of inhaled drug candidates with the right safety profile without the need for animal studies.
References
Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH (2018) Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol 58(2):157–169. https://doi.org/10.1165/rcmb.2017-0200TR
Albrecht W, Kappenberg F, Brecklinghaus T et al (2019) Prediction of human drug-induced liver injury (DILI) in relation to oral doses and blood concentrations. Arch Toxicol 93(6):1609–1637. https://doi.org/10.1007/s00204-019-02492-9
Archer CR, Sargeant R, Basak J, Pilling J, Barnes JR, Pointon A (2018) Characterization and validation of a human 3D cardiac microtissue for the assessment of changes in cardiac pathology. Sci Rep 8(1):10160. https://doi.org/10.1038/s41598-018-28393-y
Balogh Sivars K, Sivars U, Hornberg E et al (2018) A 3D human airway model enables prediction of respiratory toxicity of inhaled drugs in vitro. Toxicol Sci 162(1):301–308. https://doi.org/10.1093/toxsci/kfx255
Barosova H, Meldrum K, Karakocak BB et al (2021) Inter-laboratory variability of A549 epithelial cells grown under submerged and air-liquid interface conditions. Toxicol in Vitro. https://doi.org/10.1016/j.tiv.2021.105178
Bell SM, Chang X, Wambaugh JF et al (2018) In vitro to in vivo extrapolation for high throughput prioritization and decision making. Toxicol in Vitro 47:213–227. https://doi.org/10.1016/j.tiv.2017.11.016
Berube K, Prytherch Z, Job C, Hughes T (2010) Human primary bronchial lung cell constructs: the new respiratory models. Toxicology 278(3):311–318. https://doi.org/10.1016/j.tox.2010.04.004
Blass BE (2021) Chapter 4: in vitro screening systems. In: Blass BE (ed) Basic principles of drug discovery and development (Second Edition). Academic Press, pp 185–256
Boger E, Friden M (2019) Physiologically based pharmacokinetic/pharmacodynamic modeling accurately predicts the better bronchodilatory effect of inhaled versus oral salbutamol dosage forms. J Aerosol Med Pulm Drug Deliv 32(1):1–12. https://doi.org/10.1089/jamp.2017.1436
Boger E, Evans N, Chappell M et al (2016) Systems pharmacology approach for prediction of pulmonary and systemic pharmacokinetics and receptor occupancy of inhaled drugs. CPT Pharmacometrics Syst Pharmacol 5(4):201–210. https://doi.org/10.1002/psp4.12074
Castro V, Skowronska M, Lombardi J et al (2018) Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J Cereb Blood Flow Metab 38(2):317–332. https://doi.org/10.1177/0271678X17720816
Cooper JA 2nd, Saracci R, Cole P (1979) Describing the validity of carcinogen screening tests. Br J Cancer 39(1):87–89. https://doi.org/10.1038/bjc.1979.10
David C, Dearg B, Robert A et al (2014) Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discovery 13(6):419. https://doi.org/10.1038/nrd4309
Frohlich E (2019) Biological obstacles for identifying in vitro-in vivo correlations of orally inhaled formulations. Pharmaceutics 11:7. https://doi.org/10.3390/pharmaceutics11070316
Gon Y, Hashimoto S (2018) Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int 67(1):12–17. https://doi.org/10.1016/j.alit.2017.08.011
Gustafsson F, Foster AJ, Sarda S, Bridgland-Taylor MH, Kenna JG (2014) A correlation between the in vitro drug toxicity of drugs to cell lines that express human P450s and their propensity to cause liver injury in humans. Toxicol Sci 137(1):189–211. https://doi.org/10.1093/toxsci/kft223
Hashimoto Y (1862) Campbell M (2020) Tight junction modulation at the blood-brain barrier: current and future perspectives. Biochim Biophys Acta BBA Biomembranes. https://doi.org/10.1016/j.bbamem.2020.183298
Hengstler JG, Sjogren AK, Zink D, Hornberg JJ (2020) In vitro prediction of organ toxicity: the challenges of scaling and secondary mechanisms of toxicity. Arch Toxicol 94(2):353–356. https://doi.org/10.1007/s00204-020-02669-7
Hiemstra PS, Grootaers G, van der Does AM, Krul CAM, Kooter IM (2018) Human lung epithelial cell cultures for analysis of inhaled toxicants: lessons learned and future directions. Toxicol in Vitro 47:137–146. https://doi.org/10.1016/j.tiv.2017.11.005
Hornberg JJ, Mow T (2014) How can we discover safer drugs? Future Med Chem 6(5):481–483. https://doi.org/10.4155/fmc.14.15
Hornberg JJ, Laursen M, Brenden N et al (2014a) Exploratory toxicology as an integrated part of drug discovery. Part I: why and how. Drug Discov Today 19(8):1131–1136. https://doi.org/10.1016/j.drudis.2013.12.008
Hornberg JJ, Laursen M, Brenden N et al (2014b) Exploratory toxicology as an integrated part of drug discovery. Part II: Screening strategies. Drug Discov Today 19(8):1137–1144. https://doi.org/10.1016/j.drudis.2013.12.009
Johansson J, Larsson MH, Hornberg JJ (2019) Predictive in vitro toxicology screening to guide chemical design in drug discovery. Curr Opin Toxicol 15:99–108. https://doi.org/10.1016/j.cotox.2019.08.005
Kappenberg F, Brecklinghaus T, Albrecht W et al (2020) Handling deviating control values in concentration-response curves. Arch Toxicol 94(11):3787–3798. https://doi.org/10.1007/s00204-020-02913-0
Kassinos S, Bäckman P, Conway J, Hickey AJ, Hickey AJJ (2021) Inhaled Medicines : Optimizing Development Through Integration of in Silico, in Vitro and in Vivo Approaches. Academic Press 2021
Knudsen L, Ochs M (2018) The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol 150(6):661–676. https://doi.org/10.1007/s00418-018-1747-9
Kojima T, Go M, Takano K et al (2013) Regulation of tight junctions in upper airway epithelium. Biomed Res Int. https://doi.org/10.1155/2013/947072
Kuehn A, Kletting S, de Souza C-W et al (2016) Human alveolar epithelial cells expressing tight junctions to model the air-blood barrier. Altex 33(3):251–260. https://doi.org/10.14573/altex.1511131
Lee D, Wexler AS (2011) Particle deposition in juvenile rat lungs: a model study. J Aerosol Sci 42(9):567–579. https://doi.org/10.1016/j.jaerosci.2011.06.004
Li S, Xia M (2019) Review of high-content screening applications in toxicology. Arch Toxicol 93(12):3387–3396. https://doi.org/10.1007/s00204-019-02593-5
McKim JM Jr (2010) Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance. Comb Chem High Throughput Screen 13(2):188–206. https://doi.org/10.2174/138620710790596736
Moreau M, Fisher J, Andersen ME et al (2022) NAM-based prediction of point-of-contact toxicity in the lung: a case example with 1,3-dichloropropene. Toxicology. https://doi.org/10.1016/j.tox.2022.153340
Morgan P, Brown DG, Lennard S et al (2018) Impact of a five-dimensional framework on R and D productivity at AstraZeneca. Nat Rev Drug Discov 17(3):167–181. https://doi.org/10.1038/nrd.2017.244
Nernst W (1904) Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Zeitschrift für Physikalische Chemie 47U(1):52–55. https://doi.org/10.1515/zpch-1904-4704
Noyes AA, Whitney WR (1897) The rate of solution of solid substances in their own solutions. J Am Chem Soc 19(12):930–934. https://doi.org/10.1021/ja02086a003
O’Brien PJ, Irwin W, Diaz D et al (2006) High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol 80(9):580–604. https://doi.org/10.1007/s00204-006-0091-3
Overgaard CE, Mitchell LA, Koval M (2012) Roles for claudins in alveolar epithelial barrier function. Ann N Y Acad Sci 1257:167–174. https://doi.org/10.1111/j.1749-6632.2012.06545.x
Pell TJ, Gray MB, Hopkins SJ et al (2021) Epithelial barrier integrity profiling: combined approach using cellular junctional complex imaging and transepithelial electrical resistance. SLAS Discov 26(7):909–921. https://doi.org/10.1177/24725552211013077
Persson M, Hornberg JJ (2016) Advances in predictive toxicology for discovery safety through high content screening. Chem Res Toxicol 29(12):1998–2007. https://doi.org/10.1021/acs.chemrestox.6b00248
Persson M, Loye AF, Mow T, Hornberg JJ (2013) A high content screening assay to predict human drug-induced liver injury during drug discovery. J Pharmacol Toxicol Methods 68(3):302–313. https://doi.org/10.1016/j.vascn.2013.08.001
Pognan F, Beilmann M, Boonen HCM et al (2023) The evolving role of investigative toxicology in the pharmaceutical industry. Nat Rev Drug Discov 22(4):317–335. https://doi.org/10.1038/s41573-022-00633-x
Pointon A, Pilling J, Dorval T, Wang Y, Archer C, Pollard C (2017) From the cover: high-throughput imaging of cardiac microtissues for the assessment of cardiac contraction during drug discovery. Toxicol Sci 155(2):444–457. https://doi.org/10.1093/toxsci/kfw227
Rouaud F, Sluysmans S, Flinois A, Shah J, Vasileva E (2020) Scaffolding proteins of vertebrate apical junctions: structure, functions and biophysics. Biochim Biophys Acta Biomembr. https://doi.org/10.1016/j.bbamem.2020.183399
Ruge CA, Kirch J, Lehr C-M (2013) Pulmonary drug delivery: from generating aerosols to overcoming biological barriers—therapeutic possibilities and technological challenges. Lancet Respir Med 1(5):402–413. https://doi.org/10.1016/S2213-2600(13)70072-9
Saitou M, Furuse M, Sasaki H et al (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11(12):4131–4142. https://doi.org/10.1091/mbc.11.12.4131
Sanders JM, Beshore DC, Culberson JC et al (2017) Informing the selection of screening hit series with in silico absorption, distribution, metabolism, excretion, and toxicity profiles. J Med Chem 60(16):6771–6780. https://doi.org/10.1021/acs.jmedchem.6b01577
Schilpp C, Lochbaum R, Braubach P et al (2021) TGF-beta1 increases permeability of ciliated airway epithelia via redistribution of claudin 3 from tight junction into cell nuclei. Pflugers Arch 473(2):287–311. https://doi.org/10.1007/s00424-020-02501-2
Sengupta A, Roldan N, Kiener M et al (2022) A new immortalized human alveolar epithelial cell model to study lung injury and toxicity on a breathing lung-on-chip system. Front Toxicol. https://doi.org/10.3389/ftox.2022.840606
Sjogren AK, Breitholtz K, Ahlberg E et al (2018) A novel multi-parametric high content screening assay in ciPTEC-OAT1 to predict drug-induced nephrotoxicity during drug discovery. Arch Toxicol 92(10):3175–3190. https://doi.org/10.1007/s00204-018-2284-y
Slifer ZM, Blikslager AT (2020) The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int J Mol Sci 21:3. https://doi.org/10.3390/ijms21030972
Sugita K, Kabashima K (2020) Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J Leukoc Biol 107(5):749–762. https://doi.org/10.1002/JLB.5MR0120-230R
Szymanski P, Markowicz M, Mikiciuk-Olasik E (2012) Adaptation of high-throughput screening in drug discovery-toxicological screening tests. Int J Mol Sci 13(1):427–452. https://doi.org/10.3390/ijms13010427
Upadhyay S, Palmberg L (2018) Air-liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol Sci 164(1):21–30. https://doi.org/10.1093/toxsci/kfy053
Vielle NJ, Garcia-Nicolas O, Oliveira Esteves BI, Brugger M, Summerfield A, Alves MP (2019) The human upper respiratory tract epithelium is susceptible to flaviviruses. Front Microbiol 10:811. https://doi.org/10.3389/fmicb.2019.00811
Xie R, Wang X, Xu Y, Zhang L, Ma M, Wang Z (2023) In vitro to in vivo extrapolation for predicting human equivalent dose of phenolic endocrine disrupting chemicals: PBTK model development, biological pathways, outcomes and performance. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2023.165271
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors were employees of AstraZeneca at the time of study completion and were compensated as such.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fitzpatrick, P.A., Johansson, J., Maglennon, G. et al. A novel in vitro high-content imaging assay for the prediction of drug-induced lung toxicity. Arch Toxicol (2024). https://doi.org/10.1007/s00204-024-03800-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00204-024-03800-8