Abstract
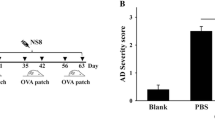
Contamination with fumonisins produced by Fusarium spp. is rapidly growing in both developing and developed countries. The purpose of this study was to determine whether oral exposure to fumonisin contributed to the development of allergic diseases. We initially examined the immunotoxic potential of short-term, oral administration of fumonisin B1 (FB1, 1 mg/kg) and fumonisin B2 (FB2, 1 mg/kg), both naturally occurring fumonisins, using a BALB/c mouse model of allergic contact dermatitis and Dermatophagoides farina-induced asthma. Using an NC/nga mouse model of atopic dermatitis (AD), we evaluated the adverse effects of subchronic oral exposure to low concentrations of FB2 (2 or 200 μg/kg). Finally, we explored the influence of FB2 on regulatory T cell proliferation and function in mesenteric lymph nodes after 1-week oral exposure to FB2 in BALB/c mice. Oral exposure to FB2 markedly exacerbated the symptoms of allergy, including skin thickness, histological evaluation, immunocyte proliferation, and proinflammatory cytokine production, although no change was observed following exposure to FB1. Furthermore, oral exposure to low concentrations of FB2 considerably exacerbated the AD scores, skin thickness, transepidermal water loss, histological features, and proinflammatory cytokine production. The aggravated allergic symptoms induced by oral exposure to FB2 could be attributed to the direct inhibition of IL-10 production by regulatory T cells in mesenteric lymph nodes. Our findings indicate that the recommended maximum fumonisin level should be reconsidered based on the potential for allergy development.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Aihara R, Ookawara T, Morimoto A et al (2020) Acute and subacute oral administration of mycotoxin deoxynivalenol exacerbates the pro-inflammatory and pro-pruritic responses in a mouse model of allergic dermatitis. Arch Toxicol 94(12):4197–4207. https://doi.org/10.1007/s00204-020-02875-3
Alizadeh AM, Rohandel G, Roudbarmohammadi S et al (2012) Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac J Cancer Prev 13(6):2625–2628. https://doi.org/10.7314/apjcp.2012.13.6.2625
Boonen J, Malysheva SV, Taevernier L, Diana Di Mavungu J, De Saeger S, De Spiegeleer B (2012) Human skin penetration of selected model mycotoxins. Toxicology 301(1–3):21–32. https://doi.org/10.1016/j.tox.2012.06.012
Caldas ED, Silva AC (2007) Mycotoxins in corn-based food products consumed in Brazil: an exposure assessment for fumonisins. J Agric Food Chem 55(19):7974–7980. https://doi.org/10.1021/jf0712898
Cawood ME, Gelderblom WC, Alberts JF, Snyman SD (1994) Interaction of 14C-labelled fumonisin B mycotoxins with primary rat hepatocyte cultures. Food Chem Toxicol 32(7):627–632. https://doi.org/10.1016/0278-6915(94)90006-x
Danicke S, Carlson L, Heymann AK et al (2023) Inactivation of zearalenone (ZEN) and deoxynivalenol (DON) in complete feed for weaned piglets: efficacy of ZEN hydrolase ZenA and of sodium metabisulfite (SBS) as feed additives. Mycotoxin Res. https://doi.org/10.1007/s12550-023-00486-2
Fukuyama T, Ehling S, Cook E, Baumer W (2015) Topically administered janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther 354(3):394–405. https://doi.org/10.1124/jpet.115.223784
Fukuyama T, Martel BC, Linder KE, Ehling S, Ganchingco JR, Baumer W (2018) Hypochlorous acid is antipruritic and anti-inflammatory in a mouse model of atopic dermatitis. Clin Exp Allergy 48(1):78–88. https://doi.org/10.1111/cea.13045
Gelderblom WC, Jaskiewicz K, Marasas WF et al (1988) Fumonisins–novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol 54(7):1806–1811. https://doi.org/10.1128/aem.54.7.1806-1811.1988
Gordon RJ, Hungerford NL, Laycock B, Fletcher MT (2020) A review on Pimelea poisoning of livestock. Toxicon 186:46–57. https://doi.org/10.1016/j.toxicon.2020.07.023
Gruber-Dorninger C, Jenkins T, Schatzmayr G (2019) Global mycotoxin occurrence in feed: a ten-year survey. Toxins (Basel). https://doi.org/10.3390/toxins11070375
Guilliams M, Ginhoux F, Jakubzick C et al (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14(8):571–578. https://doi.org/10.1038/nri3712
Han M, Breckenridge HA, Kuo S et al (2022) M2 Macrophages promote IL-33 expression, ILC2 expansion and mucous metaplasia in response to early life rhinovirus infections. Front Immunol 13:952509. https://doi.org/10.3389/fimmu.2022.952509
Justice JP, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott MR (2001) IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol 280(2):L363–L368. https://doi.org/10.1152/ajplung.2001.280.2.L363
Kishimoto R, Kato N, Koike M, Iwashita N, Takagi Y, Fukuyama T (2021) Topical treatment with mastic (resin from Pistacia lentiscus) elicits anti-inflammatory and anti-pruritic responses by modulating keratinocyte activation in a mouse model of allergic dermatitis. Phytomedicine 91:153679. https://doi.org/10.1016/j.phymed.2021.153679
Komuro M, Nagane M, Endo R et al (2022a) Glucosylceramide in T cells regulates the pathology of inflammatory bowel disease. Biochem Biophys Res Commun 599:24–30. https://doi.org/10.1016/j.bbrc.2022.02.004
Komuro M, Nagane M, Fukuyama T et al (2022) Sphingomyelin maintains the cutaneous barrier via regulation of the STAT3 pathway. FASEB J 36(4):e22111. https://doi.org/10.1096/fj.202100721RR
Kulcsar S, Kovesi B, Balogh K et al (2023) The co-occurrence of T-2 toxin, deoxynivalenol, and fumonisin B1 activated the glutathione redox system in the EU-limiting doses in laying hens. Toxins (Basel). https://doi.org/10.3390/toxins15050305
Lahiri S, Futerman AH (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci 64(17):2270–2284. https://doi.org/10.1007/s00018-007-7076-0
Laughter MR, Maymone MBC, Mashayekhi S et al (2021) The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017. Br J Dermatol 184(2):304–309. https://doi.org/10.1111/bjd.19580
Makino E, Fukuyama T, Watanabe Y et al (2019) Subacute oral administration of folic acid elicits anti-inflammatory response in a mouse model of allergic dermatitis. J Nutr Biochem 67:14–19. https://doi.org/10.1016/j.jnutbio.2019.01.009
Marsella R, De Benedetto A (2017) Atopic dermatitis in animals and people: an update and comparative review. Vet Sci. https://doi.org/10.3390/vetsci4030037
Mishra S, Srivastava S, Dewangan J, Divakar A, Kumar Rath S (2020) Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: a survey. Crit Rev Food Sci Nutr 60(8):1346–1374. https://doi.org/10.1080/10408398.2019.1571479
Missmer SA, Suarez L, Felkner M et al (2006) Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect 114(2):237–241. https://doi.org/10.1289/ehp.8221
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173. https://doi.org/10.1146/annurev.iy.07.040189.001045
Munoz-Solano B, Gonzalez-Penas E (2023) Co-occurrence of mycotoxins in feed for cattle, pigs, poultry, and sheep in navarra, a region of Northern Spain. Toxins (Basel). https://doi.org/10.3390/toxins15030172
Murphy RC, Lai Y, Liu M et al (2023) Distinct epithelial-innate immune cell transcriptional circuits underlie airway hyperresponsiveness in asthma. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202209-1707OC
Norred WP, Plattner RD, Dombrink-Kurtzman MA, Meredith FI, Riley RT (1997) Mycotoxin-induced elevation of free sphingoid bases in precision-cut rat liver slices: specificity of the response and structure-activity relationships. Toxicol Appl Pharmacol 147(1):63–70. https://doi.org/10.1006/taap.1997.8272
Ookawara T, Aihara R, Morimoto A et al (2020) Acute and subacute oral toxicity of deoxynivalenol exposure in a Dermatophagoides farinae induced murine asthma model. Toxicol Sci. https://doi.org/10.1093/toxsci/kfaa168
Ookawara T, Aihara R, Morimoto A et al (2021) Acute and subacute oral toxicity of deoxynivalenol exposure in a dermatophagoides farinae-induced murine asthma model. Toxicol Sci 179(2):229–240. https://doi.org/10.1093/toxsci/kfaa168
Rheeder JP, Marasas WF, Vismer HF (2002) Production of fumonisin analogs by Fusarium species. Appl Environ Microbiol 68(5):2101–2105. https://doi.org/10.1128/AEM.68.5.2101-2105.2002
Rossi GA, Ballarini S, Salvati P, Sacco O, Colin AA (2022) Alarmins and innate lymphoid cells 2 activation: a common pathogenetic link connecting respiratory syncytial virus bronchiolitis and later wheezing/asthma? Pediatr Allergy Immunol 33(6):e13803. https://doi.org/10.1111/pai.13803
Rubtsov YP, Rasmussen JP, Chi EY et al (2008) Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28(4):546–558. https://doi.org/10.1016/j.immuni.2008.02.017
Sofi MH, Tian L, Schutt S et al (2022) Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway. Leukemia 36(7):1907–1915. https://doi.org/10.1038/s41375-022-01581-6
Spergel JM, Paller AS (2003) Atopic dermatitis and the atopic march. J Allergy Clin Immunol 112(6 Suppl):S118–S127. https://doi.org/10.1016/j.jaci.2003.09.033
Stover K, Fukuyama T, Young AT et al (2016) Topically applied manganese-porphyrins BMX-001 and BMX-010 display a significant anti-inflammatory response in a mouse model of allergic dermatitis. Arch Dermatol Res 308(10):711–721. https://doi.org/10.1007/s00403-016-1693-0
Tajiki-Nishino R, Makino E, Watanabe Y, Tajima H, Ishimota M, Fukuyama T (2018) Oral administration of bisphenol a directly exacerbates allergic airway inflammation but not allergic skin inflammation in mice. Toxicol Sci 165(2):314–321. https://doi.org/10.1093/toxsci/kfy132
Tajima H, Tajiki-Nishino R, Watanabe Y, Fukuyama T (2019) Direct activation of aryl hydrocarbon receptor by benzo[a]pyrene elicits T-helper 2-driven proinflammatory responses in a mouse model of allergic dermatitis. J Appl Toxicol. https://doi.org/10.1002/jat.3782
Tham EH, Leung ASY, Yamamoto-Hanada K et al (2023) A systematic review of quality and consistency of clinical practice guidelines on the primary prevention of food allergy and atopic dermatitis. World Allergy Organ J 16(4):100770. https://doi.org/10.1016/j.waojou.2023.100770
van der Westhuizen L, Shephard GS, Rheeder JP, Somdyala NI, Marasas WF (2008) Sphingoid base levels in humans consuming fumonisin-contaminated maize in rural areas of the former Transkei, South Africa: a cross-sectional study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(11):1385–1391. https://doi.org/10.1080/02652030802226195
Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH Jr (1991) Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem 266(22):14486–90
Watanabe Y, Tajiki-Nishino R, Tajima H, Fukuyama T (2019) Role of estrogen receptors alpha and beta in the development of allergic airway inflammation in mice: a possible involvement of interleukin 33 and eosinophils. Toxicology 411:93–100. https://doi.org/10.1016/j.tox.2018.11.002
World Health O (2017) Evaluation of certain contaminants in food. World Health Organ Tech Rep Ser 1002:1–166
Yang B, Wilkie H, Das M et al (2023) The IL-4Ralpha Q576R polymorphism is associated with increased severity of atopic dermatitis and exaggerates allergic skin inflammation in mice. J Allergy Clin Immunol 151(5):1296-1306 e7. https://doi.org/10.1016/j.jaci.2023.01.011
Yoshinari T, Watanabe M, Hara-Kudo Y (2022) Cross-genus inhibitory activity of polyoxins against aflatoxin production by Aspergillus parasiticus and fumonisin production by Fusarium fujikuroi. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnac048
Zentai A, Szeitzne-Szabo M, Mihucz G, Szeli N, Szabo A, Kovacs M (2019) Occurrence and risk assessment of fumonisin B(1) and B(2) mycotoxins in maize-based food products in hungary. Toxins (Basel). https://doi.org/10.3390/toxins11120709
Acknowledgements
We thank Editage (www.editage.jp) for English language editing.
Funding
A part of this study was funded by the grant-in-aid for scientific research of The Tojuro Iijima Foundation for Food Science and Technology.
Author information
Authors and Affiliations
Contributions
Conceptualization: TF. Methodology: MA, HY, AM, NI, YT, MN, TY, and TF. Formal analysis and investigation: MA and TF. Writing—original draft preparation: MA and TF. Writing—review and editing: MA, HY, AM, NI, YT, MN, TY, and TF. Funding acquisition: TF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no actual or potential competing financial interests.
Ethical approval
All aspects of in vivo study were conducted in accordance with the Animal Care and Use Program of Azabu University (Approval No. 220316-46).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
204_2023_3579_MOESM1_ESM.jpg
Supplementary file1 Supplemental Fig. 1 Experimental protocol used to generate mouse models of allergic contact dermatitis (A), asthma (B), and atopic dermatitis (C) (JPG 1615 KB)
204_2023_3579_MOESM2_ESM.jpg
Supplementary file2 Supplemental Fig. 2 Impact of oral exposure to FB1 and FB2 on the reproducibility of a mouse model of allergic contact dermatitis. Oral exposure to FB2 significantly increases the ear swelling response (A). Representative histological image of affected skin in each group (B). Number of CD11c+CD40+ DCs (C), CD3+CD4+ T cells (D), and CD19+IgE+ B cells (E) in the auricular LNs. There is no significant change in serum levels of IgE (F). FB2 exposure upregulated IL-4 (G) and IL-13 (H) production in LNs. Bar = 100 μm. Each result is presented as the mean ± standard error of the mean (SEM). FB1, fumonisin B1; FB2, fumonisin B2; DCs, dendritic cells; LNs, lymph nodes; IL, interleukin (JPG 1282 KB)
204_2023_3579_MOESM3_ESM.jpg
Supplementary file3 Supplemental Fig. 3 Exposure to fumonisin B2 (FB2) did not alter the cytokine production by stimulated THP-1 (A), human keratinocytes (HaCaT) (B), or human bronchial epithelium (BEAS-2B) (C). Each result is presented as the mean ± standard error of the mean (SEM) (JPG 629 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ando, M., Yamaguchi, H., Morimoto, A. et al. Chronic oral exposure to low-concentration fumonisin B2 significantly exacerbates the inflammatory responses of allergies in mice via inhibition of IL-10 release by regulatory T cells in gut-associated lymphoid tissue. Arch Toxicol 97, 2707–2719 (2023). https://doi.org/10.1007/s00204-023-03579-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03579-0