Abstract
“Novichok” refers to a new group of nerve agents called the A-series agents. Their existence came to light in 2018 after incidents in the UK and again in 2020 in Russia. They are unique organophosphorus-based compounds developed during the Cold War in a program called Foliant in the USSR. This review is based on original chemical entities from Mirzayanov's memoirs published in 2008. Due to classified research, a considerable debate arose about their structures, and hence, various structural moieties were speculated. For this reason, the scientific literature is highly incomplete and, in some cases, contradictory. This review critically assesses the information published to date on this class of compounds. The scope of this work is to summarize all the available and relevant information, including the physicochemical properties, chemical synthesis, mechanism of action, toxicity, pharmacokinetics, and medical countermeasures used to date. The environmental stability of A-series agents, the lack of environmentally safe decontamination, their high toxicity, and the scarcity of information on post-contamination treatment pose a challenge for managing possible incidents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The word “Novichok” entered the public consciousness through “the Salisbury poisonings” in March 2018 (Borger 2018; Philp 2018; Dodd et al. 2023). The term per se represents a relatively broad class of neurotoxic compounds. The group is listed among the organophosphorus (OP) compounds developed in the 1970s as a part of the Russian program Foliant (Mirzayanov 2008; Vásárhelyi and Földi 2007). The program was designed to produce new single components or binary agents from commonly available industrial chemicals. Such sources of chemicals should avoid raising suspicion of control organs. At the same time, emphasis was also placed on producing compounds more toxic and volatile than the existing Russian VX (a.k.a. VR, RVX, R-33, or VX) (Mirzayanov 2008). The Foliant program was supervised by Vladislav Gorodilov, who supposedly died from “Novichok” poisoning. After his death, the program was led by Peter Kirpichev, an official of the State Research Institute of Organic Chemistry and Technology (GOSNIIOKhT) in the Saratov region (Reiter and Gevorkyan 2018; Kanygin 2018; Sobchak 2020). The first information on these new OP compounds was announced by Vil Mirzayanov and Lev Fedorov in Russian newspapers at the beginning of the 1990s (quote) (Fedorov and Mirzayano 1992; Von Hippel 1993; Smithson et al. 1995).
“At the State Research Institute of Organic Chemistry and Technology (GOSNIIOKhT), a new chemical agent was created. In terms of its insidiousness (“combat characteristics”), it significantly exceeded the well-known VX; its damage is practically incurable. In any case, people who were once exposed to this chemical agent have remained disabled.”
A quote from the article “Poisoned Politics” first mentioned the research, production, and testing of chemical agents, weekly newspaper “Moscow News”, Sep 20, 1992, #38 (633) (Fedorov and Mirzayano 1992).
Interestingly, the name "Novichok" has never been officially included in the research program and appeared after the studies had been completed. The term refers only to binary agents that were effectively weaponized and tested. For example, Novichok-5 and -7 were binary agents synthesized from the base structure of A-232 and A-234, respectively (Mirzayanov 2008; Chai et al. 2018). This is in contrast with Pitschmann (2016), who misleadingly stated that “Novichok” is termed one of the subprojects of the Foliant special program. Hence, the term could have been allegedly used for the A-series compounds (non-binary) as well as their precursors and binary forms. Other details were uncovered in interviews with Vladimir Uglev, a prominent scientist involved in the secret research. Uglev claimed to be the co-author of A-232/Novichok-5 and confirmed the existence of its binary version (Waller 1997; Hoffman 1998). He also said that the name "Novichok" was common in the West only, but its Russian designers did not use it (Reiter and Gevorkyan 2018; Wintour 2018). Fedorov (1995) disputed the term "Novichok" in his book. Additionally, alleged “Novichok” poisoning survivor Vladimir Fedorov, a former GOSNIIOKhT technology section employee, confirmed the compound's code designation A-xxx (Sobchak 2020). Therefore, the designation A-series for these new compounds is seen as the only one correct. Based on the data collected, the Organization for the Prohibition of Chemical Weapons (OPCW) also concluded that "Novichok" should not be considered an independent group of chemical warfare agents. A-series compounds should be treated analogously to other nerve agents (NAs), possibly forming a separate group (Table 1) (Kloske and Witkiewicz 2019; Noga and Jurowski 2023).
The structures of the A-series agents have never been officially disclosed. Several less efficient compounds developed during the Foliant program were systematically uncovered in the literature to hide the agenda of developing new NAs as a part of a pesticide research program (Kruglyak et al. 1972; Raevskiĭ et al. 1987a, b, 1990; Ivanov et al. 1990; Makhaeva et al. 1998). For the first time, the mass spectrum and structure of one of the compounds named "Novichok" were most likely recorded at the US National Institute of Standards and Technology (NIST) spectral database in 1998. The database indicated that the author of the Edgewood Center for Defense Research and Development of the United States Army provided the spectrum. This fact implies that this compound must have been synthesized in the USA and subjected to spectral analysis and possibly other research (VGTRK 2018; Executive Council 2018). Notably, the entry for this compound was deleted without any explanation in 2000. The head of the Russian Chemical Weapons Detection Laboratory revealed this fact on a Russian television program on Mar 25, 2018 (VGTRK 2018). He pointed out that Edgewood Arsenal had submitted a mass spectrometry profile for a compound called N-(O-ethyl-fluorophosphoryl)-N',N''-diethylacetamidine (corresponding to the structure designated A-234 in Mirzayanov's book). The NIST98 entry additionally referred to the Registry of Toxic Effects of Chemical Substances (RTECS) database, meaning the toxicity results were also submitted (VGTRK 2018; Executive Council 2018).
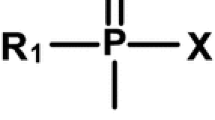
The detective story continues. Mirzayanov (2008) disclosed the exact chemical structures of A-agents in his book. He rendered A-agents as phosphonamidofluoridate and phosphoramidofluoridate compounds (Fig. 1).
Molecular structure of A-series compounds proposed by Mirzayanov (Mirzayanov 2008) (color figure online)
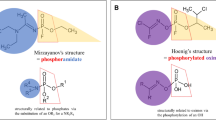
Mirzayanov noted that the synthesized unitary compounds and other OPs retained their original codenames (A-230, A-232, A-234, etc.). However, many alleged structures claiming to be "Novichoks" have been mentioned in the literature. For instance, Hoenig (2007) and Ellison (2008) published different formulas in 2007 (Fig. 2), citing a US Army source. According to these reports, the compounds contain carbonimidic alkyl monofluorophosphate substituents.
Professor Leonid Rink, another scientist who participated in implementing the Foliant program, claimed in 2018 that the structures published by Mirzayanov were the correct ones (Cockburn 2018; Wintour 2018). The phosphoramide formula also matched that in the Russian Ministry of Interior’s investigation report (Executive Council 2018; General Assembly Security Council 2018) and was indirectly verified by the US and Canada at the OPCW conference in November 2018. Both countries confirmed a match between Mirzayanov’s version of A-234 and the compound that poisoned the Skripals (Review Conference 2018a, b). However, A-series agents remain shrouded by mystery. The OPCW recognized only the compounds reported by Mirzayanov and included two new general structures and one individual compound into Schedule 1 on Dec 10, 2019, adjusting the Chemical Weapons Convention (CWC) Annex on Chemicals for the first time (Costanzi and Koblentz 2019). However, further modifications of CWC may follow. Constanzi and Koblentz (2021) noted that the joint proposal had not covered guanidine-bearing fluorophosphates such as A-262.
Structures and synthesis of the a-series agents
A-series compounds have been initially derived from the V- and G-series agents (Smithson et al. 1995; Mirzayanov 2008; Halamek and Kobliha 2011; Kloske and Witkiewicz 2019; Costanzi and Koblentz 2021). Over a hundred analogs were allegedly synthesized and tested (Tucker 2006; Halamek and Kobliha 2011). Consistent with Mirzayanov, the typical nerve agent alkoxy substituent (–OR) on the central phosphorus atom is replaced in the case of A-agents by a nitrogen substituent (Pitschmann 2014; Franca et al. 2019). The first compound, substance-84/code designation A-230, is a sarin derivative, with an acetamidine moiety replacing the O-isopropyl group. After A-230, Peter Kirpichev and his group synthesized and tested A-232, A-234, A-242, and A-262 (Mirzayanov 2008). Notably, most A-series agents follow the A-230 design. A-232 and A-234 structures are A-230 methoxy and ethoxy analogs, respectively, with acetamidine moieties (Fig. 1, blue color). Other analogs, namely, A-242 and A-262, are guanidine analogs (Fig. 1, red color). Agents A-230 and A-242 belong to the group of phosphonates, while agents A-232, A-234, and A-262 are phosphates (Costanzi and Koblentz 2021). In his book, Mirzayanov (2008) published a simple schematic synthesis of A-234 from direct binary precursors based on the route used to provide V- and G-series agents (Fig. 3).
The synthesis of RVX and A-232 binary forms by Mirzayanov (2008)
Still, the discussion on the synthesis must be cautious due to many conflicting structures, potential misuse, and extraordinarily little information available in the literature. Many authors refer only to Mirzayanov and add proposed chemistry schemes of synthesis without any detailed information on the synthesis-specific conditions (Halamek and Kobliha 2011; Chai et al. 2018; Nepovimova and Kuca 2018; Kloske and Witkiewicz 2019; Franca et al. 2019; Vicar et al. 2021). The exception can be found in the work of Hosseini and colleagues, publishing a study related to this topic in 2016. Iranian scientists prepared five A-agent derivatives using micro-scale conditions (Hosseini et al. 2016). However, none of their structures precisely matches the A-series compounds Mirzayanov uncovered. The structures reported by Hosseini et al. (2016) were close to A-242. The only difference was the presence of methyl substituents on the nitrogen atom instead of ethyl groups. The synthetic approach of this compound was feasible by the controlled reaction of methylphosphonyl difluoride and N,N,N',N'-tetraethylguanidine or N,N,N',N'-tetramethylguanidine (Fig. 4).
The final structure shown is O-alkyl N-(bis(dimethylamino)methylidene)-P-methylphosphonamidate, and the intermediate has a bis(dimethylamino)methylene moiety; DCM dichloromethane, TEA triethylamine; according to (Hosseini et al. 2016, 2021; Eskandari et al. 2022; Carvalho-Silva et al. 2023; Noga and Jurowski 2023)
The same group released two more studies related to chemistry. They described the microsynthesis of selenophosphorus compounds as close analogs to A-series agents in 2021 (Hosseini et al. 2021). A year later, they disclosed new data on synthesizing A-230, A-232, A-234, and six other compounds using previously published procedure (Eskandari et al. 2022). Based on the work of Hosseini et al. (2016) and another publication (Ledgard 2006), it is possible to deduce the synthesis route of compounds reported by Mirzayanov from commercially available building blocks. All other studies working with A-series agents continue to bypass the publishing of their procedures by just referring to a military provider or unspecified "in-house" methods. The Brazilian group reported a reaction scheme similar to that of the Iranian group for the microsynthesis of the A-242 analog (Carvalho-Silva et al. 2023).
Physicochemical properties of A-series agents
The availability of physicochemical properties information on A-series from public sources is scarce. Mirzayanov (2008) reported only limited data. According to his book, A-230, A-232, and A-234 are liquids. A-230 crystallizes at temperatures below − 10 °C. A-232 and A-234 are more stable, so they can be used in winter, unlike the A-230 variant (without the solvent N,N-dimethylformamide to prevent crystallization). A-232 possesses higher volatility but is less stable to moisture than A-230 (and VX). Agents A-242 and A-262 should be solids. Mirzayanov (2008) also estimated the hydrolysis half-life of A-234 at pH 6.5–7.4 to be moderate, i.e., 10–30 days. Several studies describing A-agent properties only referred to Mirzayanov's observations (Halamek and Kobliha 2011; Pitschmann 2014).
Nepovimova and Kuca (2018) published a complex overview of A-series agent properties, including boiling point, density, state, behavior at low temperatures, volatility, and moisture stability. However, the review referred to unsubstantiated data and, for instance, speculated on the low environmental stability. And notably, the facts from the Salisbury poisoning investigation suggest the opposite (Peplow 2018).
Similarly, Franca et al. (2019) estimated melting point, boiling point, vapor pressure, and solubility in their review a year later. In the same study, logP was calculated, indicating a high lipophilic character of the compounds, contributing to high permeation into the body. Other molecular modeling studies discussed various parameters, covering different structural, electric, spectral, thermodynamic, thermal, and hydrolytic properties (reviewed in Table 2). These results represent a helpful starting point for laboratory experiments, which should validate them. For instance, Bhakhoa et al. (2019) modeled hydrolysis under neutral conditions (among other parameters). They identified two active electronegative centers (phosphorus atom/P4 and the hybridized carbon atom/sp2), with fragmentation being more probable on the carbon atom. As another example, Otsuka and Miyaguchi (2021) calculated hydrolysis in an alkaline environment, indicating that such conditions would yield easier hydrolysis than neutral conditions. Both studies were later contradicted by experimental work by Lee et al. (2021). Thus, the hydrolytic degradation calculated based on the reaction mechanism and activation energy may not always be reliable. Yet, effective hydrolysis is significant, especially when dealing with decontamination.
Experimental data on the physical and chemical properties of A-agents are limited to spontaneous hydrolysis, degradation in acidic or alkaline conditions, and enzymatic degradation. Harvey et al. (2020) published the first results on some A-series agents' stability and hydrolysis rate in 2020. The study showed that the hydrolysis was 2–3 and 0–2 orders of magnitude slower than for the G- and V-series agents, respectively, confirming the stability of A-series agents in the environment. The study also disclosed the activation energies for A-230, A-232, and A-234 and kinetic values for organophosphorus acid anhydrolase (OPAA) hydrolysis, resulting in 2–3 orders lower magnitude than of the G-series agents and 2 orders higher magnitude than of the V-agents. Nevertheless, their results were based on a 10-min measurement, evaluating only released fluorides.
Lee et al. (2021) studied the degradation modes of A-234 under three different pH conditions (pH 3.5, 7.2, 9.4). Regardless of pH, the main fragmentation product was ethylhydrogen (1-(diethylamino)ethylidene)phosphoramidate, while N,N-diethylethanimidamide and ethylhydrogenphosphorofluoridate were minor products (Fig. 5). It implies that the phosphorus atom can be designated as the hotspot for degradation, being the major active electropositive center.
The study also showed hydrolysis of A-agents was more effective under acidic conditions than neutral or alkaline ones. Such a phenomenon was explained by the increased positive partial charge of the phosphorus atom due to the easily protonated nitrogen atom of the acetamidine group at low pH of 3.5 (Lee et al. 2021). The study aimed to simulate natural environmental conditions, avoiding solutions with extremely low or high pH values. De Koning et al. (2022) confirmed low stability of A-230 in pH 4.5 because it was completely degraded within 16 h. A-232 and A-234 appeared stable. However, their hydrolysis in acidic, neutral, and alkaline conditions (pH 10) was measured only for 1 h. Similarly, Jacquet et al. (2021) confirmed the high stability of A-230, A-232, and A-234 in pH 9.5, conducting only a 1-h study.
In contrast, Jung et al. (2023) focused on degradation by strong acids and bases such as NaOH and HCl for decontamination. They confirmed that extreme alkaline and acid solutions could effectively hydrolyze A-232 and A-234. The stabilities of A-series agents derived from different experiments are summarized in Table 3.
Jacquet et al. (2021) additionally tested the catalytic effectivity of two engineered phosphotriesterase (PTE) enzymes developed for rapid hydrolytic detoxification of OPs. Both enzymes could degrade all three agents, with the mixture of the two enzymes being the most effective. Interestingly, A-232 and A-234 hydrolysis was a one-step reaction, while the hydrolysis of A-230 underwent two steps. Later, de Koning et al. (2022) effectively decomposed A-agents using the MOF-808 Zr metal–organic framework under alkaline conditions. This material is a highly efficient and regenerative catalyst, and the degradation was carried out in two steps. The initial degradation rate of A-230 and A-232 was fast, while considerably slower in the case of A-234. Catalytic degradation is summarized in Table 4.
Mechanism of action
The symptoms of intoxication and the effectiveness of anticholinergics confirm that the A-series mechanism of action is associated with acetylcholinesterase (AChE, E.C. 3.1.1.7) inhibition. The first data based on modeling started to appear after the Salisbury incident. Carlsen (2019) assessed the probability of cholinomimetic effects using the Prediction of Activity Spectra for Substance (PASS) prediction tool. All five numbered A-series compounds were positive for this biological activity. However, such prediction is highly limited by using only 2D structure for the calculation and not including molecular energy levels (Parasuraman 2011). Bhakhoa et al. (2019) modeled the reaction between A-234 and the AChE enzyme. The study utilized methanol as the simplest model for the active serine site of the enzyme, which is burdened by several limitations. Jeong and Choi (2019) performed a thermodynamic study, but they evaluated the reaction of serine with the compounds proposed by Hoenig (2007) and Ellison (2008) only. A research group from the University of California, San Diego, published the most relevant information on the interaction between AChE and A-series molecules. Luedtke et al. (2021) and Radić (2021a, 2023) used X-ray structural data of recombinant human AChE (hAChE) inhibited by A-234 uploaded into the Protein Data Bank. They conducted a computational study of different OP–hAChE conjugates, including those of A-230, A-232, and A-234. They pinpointed that even bulkier structures like A-series agents fit into the hAChE active site without significant steric hindrance onto the hAChE backbone or side chains and can form stabilizing hydrophobic or electrostatic interactions with the choline-binding site. Such stabilization could render them more resistant to nucleophilic reactivation with oxime antidotes (Blumenthal et al. 2021; Luedtke et al. 2021; Radić 2021, 2023). They also presented A-234–hAChE conjugate very vividly in virtual reality on YouTube (Radic 2021b).
Crystal structures of hAChE inhibited by A-230, A-232, and A-234 in complex with the HI-6 reactivator are now available in the Protein Data Bank (Bester et al. 2020a, b, c, d). The distance between the oxime reactivation warhead of HI-6 and the central phosphorus atom of A-234 is 12.2 Å (PDB ID: 6NTG), implying no possibility for a nucleophilic attack and thus no or negligible reactivation ability of HI-6 (Fig. 6A). The similar distance between the phosphorus atom of A-230 and the oxime moiety of HI-6 (PDB ID: 6NTN), and A-232 and the oxime moiety of HI-6 (PDB ID: 6NTM) equal 10.1 and 10.0 Å, respectively, also presuming no reactivation process (not shown). For clarity, we also provide the hAChE–A-234-inhibited complex (Fig. 6B).
Crystal structure of the hAChE–A-234-inhibited complex with oxime reactivator HI-6 (A; PDB ID: 6NTG) and hAChE–A-234-inhibited adduct (B; PDB ID: 6NTL). A-234 is rendered by green carbon atoms, catalytic triad residues by yellow carbon atoms, and essential amino acid residues involved in interacting with the A-234 agent in blue carbon atoms. The oxime reactivator is displayed in orange (A), captured in two orientations, and stacked at the enzyme’s peripheral anionic site. The hydrogen bond interactions are shown as dashed lines with distances measured in Å. Figures were created by Pymol v. 2.4.1. (Bester et al. 2020c, d) (color figure online)
Only one study has established the analytical data on the reactivation and aging of A-agent-inhibited AChE, using only an A-242 surrogate and non-human AChE. Santos et al. (2022a) synthesized N,N,N',N'-tetraethyl-N''-(methyl(4-nitrophenoxy)phosphoryl)guanidine and evaluated the efficacy of pralidoxime, trimedoxime, obidoxime, and HI-6 to reactivate the surrogate-inhibited Electrophorus eel AChE in vitro. The study showed no aging after 90 min of enzyme inhibition and highlighted trimedoxime as the most promising oxime. The finding had been supported by modeling (Santos et al. 2022b).
Finally, no relevant information about interactions with other proteins, including butyrylcholinesterase (BChE, E.C. 3.1.1.8), is available. However, the A-series amine group may interact with different targets, widening the spectrum of action mechanisms that could affect the clinical picture of toxidrome.
Toxicity
Information on the toxicity of A-series compounds remains highly elusive. Mirzayanov (2008) described that the toxicity of Novichok-5 was 5–8 times higher than that of VR, while Novichok-7 was 10 times more potent than soman. He also noticed that compounds A-242 and A-262 should be highly toxic. Earlier in 1997, Bill Gertz (1997) stated that A-232 and A-234 are "as toxic as VX, as resistant to treatment as soman, and more difficult to detect and easier to manufacture than VX," referring to a classified report by the US Army’s National Ground Intelligence Center.
Many authors have speculated about the toxic properties of the A-agent or referred to unpublished information (Hoenig 2007; Karev 2009; Nepovimova and Kuca 2018; Franca et al. 2019; Carlsen 2019). According to Karev (2009), finding accurate toxicological data from Russian sources is possible. However, his seminal paper did not provide references. Franca et al. (2019) attempted to estimate LCt50 and LD50, i.e., lethal concentration in the environment and lethal doses, based on Mirzayanov and other literature. However, the study mismatched the compounds disclosed by Mirzayanov to those depicted by Hoenig (2007) and Ellison (2008). Carlsen (2019) contested Mirzayanov’s statement using computer modeling. Their study calculated the lethal doses (LD50) of five A-series compounds for the oral exposure route in rats using the Toxicity Estimation Software Tool (TEST). These data were then translated into humans. The prediction showed that the toxicity of the A-series was 5–75 times lower than that of VX. Noga et al. (2023b) conducted a similar in silico acute toxicity study of reagent A. They calculated the average per oral lethal doses for rats utilizing two software tools, the quantitative structure–activity relationship (QSAR) Toolbox and the TEST Consensus method, and then they extrapolated animal data to humans. The lethal toxicity was predicted as follows: A-232 > A-230 > A-234 > A-242 > A-262 (summarized in Table 5).
De Farias (2019) and Jeong et al. (2022a) published different results, although both studies did not provide specific values. De Farias (2019) evaluated the toxicity parameters for A-230 and A-234 using DFT calculations. He concluded that fewer conformers with high dipole moments could be associated with a higher biological/toxic activity, comparing the toxicity of both compounds to VX. Jeong et al. (2022a) defined a different order of toxicity, with A-234 being the most poisonous, followed by A-232, and A-230 designated as the least toxic. They also perceived propyl-bearing derivatives from all the A-agents as less toxic. Importantly, we must remember that acute oral OP toxicity in mammals correlates poorly with enzyme inhibitory activity, implying that toxicity cannot be assessed only by computational data (Wang et al. 2021; Bolt and Hengstler 2022). So far, no experimental data on A-agent toxicity have been published.
Publicly available experimental data on symptoms of poisoning are now almost non-existent. In 2019, the U.S. government published medical management guidelines on the A-series agents, claiming bronchoconstriction and seizure activity had been a prominent feature of their toxicity in animals (Chemical hazards emergency medical management (CHEMM) (2019). Nevertheless, other experimental data remained classified. Exposed victims represent another valuable source of information. These sources include one original article, police reports, and direct interviews with victims in the newspaper. We could find five confirmed or highly probable incidents involving A-series agents. The first victim of intoxication was a scientist working in the Foliant program. Other events can be classified as deliberate poisonings of four people. Besides them, seven other victims displayed significant signs of intoxication. More than 14 other people were possibly exposed to A-agents, but they either showed minor symptoms or no data were given. Each case of A-agent intoxication is listed in Table 6. Newspaper articles also indicate that there may have been more incidents. For instance, they suggest field accidents, testing new OP compounds on soldiers, or a link to the assassinations of Muslim Chechen leaders in 2002 (Ibn al-Khattab) and 2013 (Dokka Umarov). However, no solid evidence exists (Nepovimova and Kuca 2018; Rozhdestvensky 2018; Knight 2018, TV Rain 2021; Dzutsati 2021).
The cases in Table 6 indicate that the typical A-agent exposure route was transdermal intoxication. Such a route could be perceived as desired in assassinations for slowing the onset of symptoms. Inhalation was the primary route of exposure in the first victim reported in 1987. He was accidentally exposed when a hood vent malfunctioned, releasing a small amount of concentrated A-232 into the air. Oral poisoning did not play a role in the presented cases, although Mirzayanov emphasized that ingestion and inhalation would be the most probable routes of poisoning (BBC News 2020).
Several factors influence the onset of OP intoxications, including dose, exposure time, route of poisoning, and therapeutic intervention. (Marrs et al. 2007; Ciottone 2018; Costanzi et al. 2018). The inhalation route is associated with the rapid onset of symptoms. According to a briefing note released by Public Health England, toxidrome develops within minutes to hours after exposure, usually less than 6 h. The note also recommends investigating any illness occurring within 12 h after potential contact with suspect material or contaminated location (PHE 2018). On the other hand, low-dose exposure may have a latency of up to several days, as shown in a victim from 2015. Low-dose exposure most likely played a significant role in this case.
The symptoms exhibited by the cases have been typical of OP poisoning arising from overstimulation of muscarinic and nicotinic receptors. Clinicians usually refer to DUMBLES abbreviating defecation/diaphoresis, urination, miosis, bronchospasm and bronchorrhea, lacrimation, emesis, and salivation. Another term, SLUDGE, covers only "wet signs," including salivation, lacrimation, urination, diaphoresis, gastrointestinal discomfort, and emesis (Saalbach 2023). Severe hypothermia was reported in one victim intoxicated in 2020 (Steindl et al. 2021). However, the symptom can be seen in up to 50% of OP poisonings (Moffatt et al. 2010; Mozafari et al. 2016; Wang et al. 2021). Mydriasis was observed only in the first victim, possibly developed upon prevailing nicotinic receptor overstimulation. The briefing note reviewing the Salisbury and Amesbury incidents emphasizes that blurred vision with either miosis or mydriasis is the best descriptor. The death of the intoxicated victims occurred between 1 to 8 days after exposure. In surviving patients, hospitalization took approximately 29 days (16–72 days). But, the small number of casualties and lack of information about the agent and the dose used do not allow for drawing any clear conclusions. The long-term prognosis is similarly uncertain because most of the incidents are recent. However, the victim from 1987 reported disabling neurological and neuropsychological symptoms, indicating that A-series agents may cause delayed neuropathy (Mirzayanov 2008; Noga and Jurowski 2023).
Toxicokinetics
Reliable information on toxicokinetics is also minimal. Incidents involving A-series agents verify inhalation and transdermal routes of intoxication. The calculated partition coefficient and vapor pressure mentioned above support both observations (Bhakhoa et al. 2019; Franca et al. 2019; Carlsen 2019; Vieira et al. 2021; Jeong et al. 2022a). Bhakhoa et al. (2019) and Carlsen (2019) provided more detailed computational models. Bhakhoa et al. (2019) modeled the lipophilicity, solubility, topological polar surface area, and skin permeability of A-234 using the SwissADME tool. They indicated high human gastrointestinal absorption and good skin and blood–brain barrier permeability. However, they assessed A-234 as less skin permeant than VX but still crossing the barriers quickly. Carlsen (2019) used various QSAR models, including the finite-dose skin permeation calculator and the ACD/Percepta platform, to estimate skin permeation, first-pass metabolism after oral administration, oral bioavailability, time for maximum plasma concentration, elimination rate constant, and elimination half-life. They predicted slower human skin permeation and a reduced amount of all five numbered A-series agents permeating human skin compared to VX. They also noted that slower skin permeation could lead to a prolonged recovery. First-pass metabolism after oral administration was estimated at approximately 35–50%, the time for maximum plasma concentration was about 55–70 min, and elimination half-lives were 3.5–4 h (comparable to VX). Nevertheless, published animal data confirming such calculations do not exist.
Diagnostics and retrospective detection
Detection and biomonitoring currently represent the most robust lines of research on A-series agents (Bolt and Hengstler 2022). The specific determination of the poison is essential for diagnosis and selecting adequate therapy. Considering the laboratory capabilities of current hospitals and the acuteness of poisoning progression, doctors could only diagnose non-specific cholinesterase inhibition based on the developing cholinergic toxidrome and decreased BChE levels in the patient´s serum (Steindl et al. 2021; Haslam et al. 2022). We could expect reports from designated toxicological laboratories with a latency of days (Steindl et al. 2021). Such laboratories can determine the agent from biomedical and environmental samples, but this information is late and has mainly forensic value. According to Mirzayanov, it may be possible to affiliate the agent with a particular laboratory by determining the so-called promotor, i.e., the third component (catalyst or stabilizer) added to binary substances (Lenta 2018).
Iranian scientists were the first to publish analytical data on A-series compounds (Hosseini et al. 2016, 2021; Eskandari et al. 2022). They used the microsynthesis mentioned above to produce deuterated analogs of A-series agents and performed mass spectrometric (MS) analysis via electron ionization and positive electrospray ionization methods. They observed that the ionization process of the studied compounds induced several fragmentation pathways, including McLafferty rearrangement, hydrogen rearrangement, and intramolecular electrophilic aromatic substitution in some cases. Later, they revealed product ion mass spectra of A-230, A-232, A-234, other A-series analogs, and selenophosphorus compounds that they provided to the Central Analytical Database maintained by the OPCW (OCAD) to improve MS detection. The database assists in verification and on-/off-site analysis (Hosseini et al. 2016, 2021; Eskandari et al. 2022). Vibrational spectra modeled by Tan et al. (2019) and nuclear magnetic resonance (NMR) characteristics reported by Jeong et al. (2022b) and Jung et al. (2023) can also be exploited.
The first manuscript utilizing optical detection of chemical warfare agents has already been published. Bauer et al. (2023) used three commercially available handheld forensic light sources to identify contaminated surfaces. They showed that blue light (445 nm peak emission) most effectively visualized auxochromes, including the P(O)N = arrangement in A-series compounds. Although non-specific (not providing a stand-alone identification) and limited to ambient light conditions, a person wearing wavelength filter goggles could rapidly screen surface contamination on-site. Another fast on-site detection method has been developed by Termeau et al. (2023). They presented a rapid, portable, and specific colorimetric detection of A-series compounds based on simple contact with a detection paper. The chemosensor is glass fibers impregnated with hydrazone derivatives. The sharp color contrast is easily observable almost immediately with the naked eye. Simultaneously, the detection apparatus does not respond to other interfering compounds, including other chemical warfare agents.
Another six studies have been published, focusing on identifying A-series compounds in blood and urine (Fig. 7). Jeong et al. (2021) and Noort et al. (2021) applied the nonapeptide method in spiked blood. The technique exploits selective isolation of human BChE from plasma followed by enzymatic cleavage with pepsin, producing the nonapeptide fragment from the active site modified with the stable NA adduct in the case of exposure. Both teams then used precursor ion scanning combined with high-resolution mass spectrometry (HRMS), providing information on the molecular structure of the adduct moiety without reference samples. Jeong et al. (2021) analyzed A-232 and A-234, while Noort et al. (2021) studied A-230, A-232, and A-234. Consistently, they confirmed high protonation of A-series molecules due to the presence of several nitrogen atoms in their structure and unique MS2 fragmentations. Lee et al. (2022) studied the ability of A-234 to react with human serum albumin (HSA) using nano-liquid chromatography (nano-LC)–MS/MS. OP and OP-like compounds can modify HSA at up to 12 sites. However, according to the results, A-234 binds only to Tyr411. Mirbabaei et al. (2022) successfully detected and quantified A-234 in plasma and urine samples by four different GC–MS/MS and LC–MS/MS approaches. They directly measured A-234 released from deactivated proteins using potassium fluoride in plasma and conducted the nonapeptide method and analysis of albumin covalent adducts. In the urine samples, they focused on the targeted detection of the agent in its original form due to the high resistance of A-234 to hydrolysis. The study renders detection limits and calibration curves with other analytical parameters for each method. By contrast, Yamaguchi et al. (2022) and Otsuka et al. (2022) aimed at hydrolytic degradation products of all six A-series compounds reported by Mirzayanov in spiked urine. The degradation products were directly synthesized. Using derivatization, Yamaguchi et al. (2022) validated a novel 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride LC–MS/MS method. They detected various phosphoric and phosphonic acids, but the technique was ineffective for the A-242 degradation product. The latter study showed that HILIC–MS/MS analysis is more sensitive to guanidine analogs (A-242 or A-262) (Otsuka et al. 2022). Nevertheless, we must consider the limitations imposed by the fact that the in vivo degradation pathways and pharmacokinetics remain unknown.
Finally, Bruin-Hoegée et al. (2022) and Lee et al. (2023) released an environmental analysis of A-agents. Lee et al. (2023) developed a method based on solid-phase extraction for unknown samples collected from suspect sites. They applied and validated the LC–MS method to analyze the A-234 agent degradation product in water, sand, and soil matrices. Bruin-Hoegée et al. (2022) detected A-234 protein adducts in basil, bay laurel, and stinging nettle leaves using LC–MS/MS. According to their results, biomarkers could be found in the living and dried plants even three months after the exposure, providing a long investigation window.
Decontamination and therapy
The care of victims affected by A-series agents should not generally differ from that applied to victims of other OP intoxications. The management of patients relies on decontamination, evacuation, proper supportive care, and specific treatment. For all procedures, the stability of A-series agents in the environment (Harvey et al. 2020; Jeong et al. 2021; Jacquet et al. 2021; de Koning et al. 2022; Jung et al. 2023) emphasizes that rescuers, medical caregivers, and other personnel in contact with the victim must wear suitable personal protective equipment to avoid unprotected contact with potentially contaminated surfaces and mitigate any potential cross-contamination. The victim from 2018 (a 41-year-old man; Table 6) was a policeman poisoned by touching a contaminated door handle, wearing only forensic gear (Osborne 2018).
Decontamination is another critical issue. Decontamination based on absorption has not been tested. By contrast, Jung et al. (2023) demonstrated that A-234 was decontaminated by 0.5–1 M mixtures of Oxone® (KHSO5·½KHSO4·½K2SO4), Ca(OCl)2, KOH, NaOH, and HCl within 30 min. Oxone® and Ca(OCl)2 were also successfully tested against A-232. However, such solutions are highly caustic, which could limit their practical use. Additionally, the decontamination efficiency of Oxone® decreased when applied to contaminated sand. Other data on the efficacy of emergency service decontamination mixtures are not publicly available. Only general information and recommendations published by various scientific teams or governments are available. For details, see CHEMM: chemm.nlm.nih.gov; US Army Medical Research Institute of Chemical Defense: ccc.apgea.army.mil; NHS (National Health Service): england.nhs.uk; PHE (Public Health England): publishing.service.gov.uk; and NATO, AMedP-7.1 (Allied joint medical publication): coemed.org or nso.nato.int.
Information on the treatment can be derived only from one publication. Since case reports on incidents from Salisbury and Amesbury are missing, German scientists documented the therapy of the victim poisoned in 2020 (Table 6). Although the therapeutic intervention during the first two days remains unclear, Steindl et al. (2021) assumed that supportive care, particularly intubation with mechanical ventilation, had been most likely the critical element, preventing severe hypoxia and leading to the patient´s favorable outcome. In OP poisonings, death is typically caused by respiratory failure resulting from bronchospasms, bronchorrhea, central respiratory depression, and respiratory muscle weakness/paralysis (Robb and Baker 2023). The importance of intubation and mechanical ventilation is exceptionally high in cases of delayed diagnosis, which may happen in A-series agent intoxications due to misdiagnosing (Morris 2021; Haslam et al. 2022). In particular, if the toxidrome is not fully expressed, miosis, reduced consciousness/unconsciousness, and respiratory depression may be mismatched with an opiate overdose. Escalating doses of naloxone (opiate antidote) can help exclude the diagnosis (Schiller et al. 2023). Supportive therapy of A-series agent poisoning may also include analgosedation, myorelaxation, crystalloids, antipyretics, and antibiotics.
Specific treatment is based on three pillars, including atropine, oxime reactivators, and neuroprotective agents. Atropine or other anticholinergics are administered to control symptoms such as bradycardia, bronchoconstriction, and bronchorrhea (so-called 3Bs). The recommended initial dose of atropine ranges from 2 to 6 mg, depending on the severity of intoxication, and can be doubled in at least 5-min intervals until atropinization happens (adequate blood pressure, heart rate ≥ 80/minute, and clear lungs). Additional dosing is titrated depending on the patient’s clinical response. Specific atropine consumption in A-series agent victims has not been disclosed. Steindl et al. (2021) only mentioned that the patient had received atropine for 12 days. Haslam et al. (2022) pointed out that victims from Salisbury had consumed the hospital-wide supply within 24 h. Atropine can also have diagnostic value. Therapeutic response to high-dose atropine highly suggests OP poisoning (Morris et al. 2019), which was the case of the man from Amesbury. By contrast, the second victim (a 46-year-old man) received small doses of atropine for the eye fundus examination, improving his clinical condition. However, this finding did not prompt the therapy for OP poisoning (Kislinskaya 2001).
Oxime reactivators help restore AChE physiological functions by attacking the OP–AChE complex, releasing the active enzyme. Oxime reactivators may also directly counteract nicotinic- and muscarinic-mediated side effects (Milatović and Jokanović 2009; Soukup et al. 2012; Worek et al. 2020; Gorecki et al. 2022). However, the therapeutic window for oximes after OP exposure can be narrow due to the aging of the OP–AChE conjugate (Worek et al. 2016). The German medical team administered 250 mg of obidoxime bolus (i.v.), followed by a continuous application of 750 mg. After one day, the reactivator administration was discontinued because there was no sign of reactivation or the slightest improvement in neuromuscular function (Steindl et al. 2021). This observation corresponds with preliminary data from Salisbury, suggesting that pralidoxime (i.v.) at 30 mg/kg did not reactivate AChE inhibited by the A-234 (Eddleston and Chowdhury 2021). Nevertheless, British clinicians who treated the victims implied that high doses of pralidoxime helped stabilize cardiac parameters and renal function, possibly through non-targeted interactions with extrasynaptic cholinergic receptors (CHEMM 2019; Hatfill 2019). On the other hand, high oxime doses may impair liver functions (Marrs et al. 2007; Pejchal et al. 2008; Horn et al. 2023). Steindl et al. (2021) observed elevated transaminases and γ-glutamyl transferase several days after they stopped the oxime therapy. However, it is difficult to determine whether this was related to obidoxime, the poisoning, or both.
Neuroprotective agents are necessary to prevent or control seizures as the risk of seizures significantly increases in patients with OP intoxication, and untreated seizures can lead to death (Chuang et al. 2019). Additionally, excessive neuronal activity can induce brain damage and contribute to long-term neurological complications (Pulkrabkova et al. 2023). Benzodiazepines are considered the first-line drugs. Midazolam and diazepam have been approved by the Food and Drug Administration (FDA) for OP poisoning therapy (Jett and Spriggs 2020). Benzodiazepines can also be indicated for OP-induced agitation and delirium (Hui 2018). Interestingly, Steindl et al. (2021) supplemented analgosedation (sufentanil and propofol) with midazolam to support neuroprotection, even though propofol and midazolam have a similar mode of action (Patki and Shelgaonkar 2011).
Finally, fresh frozen plasma, iron, and folate were indicated in the last A-series agent victim (Steindl et al. 2021). Fresh frozen plasma restores BChE levels. BChE can act as a stoichiometric scavenger during the early phase of poisoning (Allard et al. 2022). The patient received six units of fresh frozen plasma on day 6. The administration was prompted by persistently reduced enzyme activity, possibly indicating ongoing redistribution of NA from the lipid tissue into the bloodstream. However, the effectiveness of the infusion, immediately increasing BChE activity, did not confirm this suspicion. On the other hand, the AChE activity was restored much later (after 21 days), suggesting de novo synthesis of the enzyme. From this point of view, the key strategy is to ensure vital functions until the AChE activity is restored; in the case of A-series agents, this means “resynthesized,” as there is no proof that currently available oximes are capable or reactivation of the enzyme. I.v. iron and p.o. folate supplementation helped recover reduced erythrocytes and hemoglobin (Steindl et al. 2021). Other drugs recommended for OP poisoning, including NMDAR and other glutamatergic inhibitors, neurosteroids, magnesium sulfate, lipid emulsion, and antioxidants, may help stabilize the victim or even improve the prognosis (Hoegberg and Gosselin 2017; Vanova et al. 2018; Pulkrabkova et al. 2023). However, preclinical experiments confirming their efficacy are necessary.
Conclusion
The little information available on the A-series agents indicates that the reviewed compounds represent a unique subgroup of NAs. The situation is further complicated by the emergence of other alternative names, such as fourth-generation agents (FGAs) (Konopski 2009; Halamek and Kobliha 2011) or non-traditional agents (NTAs) (Meselson and Robinson 2005). Unification of nomenclature will, therefore, play an important role. Another problem is the public data availability on their properties, structures, and toxicities. Such information is minimal, primarily based on computational studies or classified. These agents present several unique challenges regarding toxicity, such as detection, persistence, decontamination, treatment, and the potential for delayed onset of symptoms. There is no proof that marketed oximes can reactivate inhibited AChE. Therefore, only symptomatic treatment, consisting of parasympatholytic and neuroprotective agents, is in hand. If such poisoning is recognized, supplementation by BChE-containing plasma may lead to scavenging of the poison. In any other case, ensuring the vital function is crucial until the replenishment of the AChE pool. Nevertheless, more research will be necessary to overcome these challenges. However, given the hazardous nature and legislative constraints, current research is conducted in a limited number of laboratories. It is also uncertain to what extent the results will be shared. Therefore, new data will most probably emerge very slowly.
References
Allard JL, Shields KA, Munro TP, Lua LHL (2022) Strategies for developing a recombinant butyrylcholinesterase medical countermeasure for Organophosphorus poisoning. Chem Biol Interact 363:109996. https://doi.org/10.1016/j.cbi.2022.109996
Bauer G, Wildauer A, Povoden G et al (2023) Crime scene Novichok—optical detection of fourth-generation agents (FGAs) using handheld forensic light sources. Forensic Sci 3:231–244. https://doi.org/10.3390/forensicsci3020017
BBC News (2018a) Russian spy poisoning: Sergei Skripal “improving rapidly.” In: BBC. https://www.bbc.com/news/uk-43671958. Accessed 11 Jul 2023
BBC News (2018b) Ex-spy Sergei Skripal discharged after poisoning. Lond. In: BBC. https://www.bbc.com/news/uk-44165718. Accessed 11 Jul 2023
BBC News (2020) Navalny “poisoned”: What are Novichok agents and what do they do? In: BBC. https://www.bbc.com/news/world-europe-43377698. Accessed 11 Jul 2023
Bellingcat Investigation Team (2019a) Third Skripal Suspect Linked to 2015 Bulgaria Poisoning. In: Bellingcat. Accessed 11 Jul 2023. In: Bellingcat. https://www.bellingcat.com/news/uk-and-europe/2019/02/07/third-skripal-suspect-linked-to-2015-bulgaria-poisoning/
Bellingcat Investigation Team (2019b) The Dreadful Eight: GRU’s Unit 29155 and the 2015 Poisoning of Emilian Gebrev. In: Bellingcat. https://www.bellingcat.com/news/uk-and-europe/2019/11/23/the-dreadful-eight-grus-unit-29155-and-the-2015-poisoning-of-emilian-gebrev/Accessed 11 Jul 2023
Bester SM, Guelta MA, Height JJ, Pegan SD (2020a) RCSB PDB—6NTN: Crystal Structure of Recombinant Human Acetylcholinesterase Inhibited by A-230 in Complex with the Reactivator, HI-6. https://doi.org/10.2210/pdb6NTN/pdb
Bester SM, Guelta MA, Height JJ, Pegan SD (2020b) RCSB PDB - 6NTM: Crystal Structure of Recombinant Human Acetylcholinesterase Inhibited by A-232 in Complex with the Reactivator, HI-6. https://doi.org/10.2210/pdb6NTM/pdb
Bester SM, Guelta MA, Height JJ, Pegan SD (2020c) RCSB PDB - 6NTG: Crystal Structure of Recombinant Human Acetylcholinesterase Inhibited by A-234 in Complex with Reactivator, HI-6. https://doi.org/10.2210/pdb6NTG/pdb
Bester SM, Guelta MA, Height JJ, Pegan SD (2020d) RCSB PDB - 6NTL: Crystal Structure of Recombinant Human Acetylcholinesterase Inhibited by A-234. https://doi.org/10.2210/pdb6NTL/pdb
Bhakhoa H, Rhyman L, Ramasami P (2019) Theoretical study of the molecular aspect of the suspected novichok agent A234 of the Skripal poisoning. R Soc Open Sci 6:181831. https://doi.org/10.1098/rsos.181831
Blumenthal DK, Cheng X, Fajer M et al (2021) Covalent inhibition of hAChE by organophosphates causes homodimer dissociation through long-range allosteric effects. J Biol Chem 297:101007. https://doi.org/10.1016/j.jbc.2021.101007
Bolt HM, Hengstler JG (2022) Recent research on Novichok. Arch Toxicol 96:1137–1140. https://doi.org/10.1007/s00204-022-03273-7
Borger J (2018) Spy poisoning: allies back UK and blast Russia at UN security council. In: The Guardian. https://www.theguardian.com/world/2018/mar/14/uk-spy-poisoning-russia-tells-un-it-did-not-make-nerve-agent-used-in-attack. Accessed 11 Jul 2023
Carlsen L (2019) After salisbury nerve agents revisited. Mol Inform 38:1800106. https://doi.org/10.1002/minf.201800106
Carvalho-Silva T, Modesto-Costa L, Borges CVN et al (2023) Synthesis, experimental and molecular dynamics simulation of the ESI-CID spectrum of the nerve agent Novichok analog O-2-methoxyethyl N- [bis(dimethylamino)methylidene]-P-methylphosphonamidate. Int J Mass Spectrom 490:117087. https://doi.org/10.1016/j.ijms.2023.117087
Chai PR, Hayes BD, Erickson TB, Boyer EW (2018) Novichok agents: a historical, current, and toxicological perspective. Toxicol Commun 2:45–48. https://doi.org/10.1080/24734306.2018.1475151
CHEMM (2019) Fourth generation agents (FGA): Medical Management Guidelines. In: CHEMM HHS. https://chemm.hhs.gov/nerveagents/FGA_Medical_Management_Guidelines_508.pdf. Accessed 11 July 2023
ChemSpider CSID:64808785 (2018a) A-230 (Nerve agent). In: ChemSpider. http://www.chemspider.com/Chemical-Structure.64808785.html. Accessed 11 Jul 2023
ChemSpider CSID:64808786 (2018b) A-232 (Nerve agent). In: ChemSpider. http://www.chemspider.com/Chemical-Structure.64808786.html. Accessed 11 Jul 2023
ChemSpider CSID:64808787 (2018c) A-234 (Nerve agent). In: ChemSpider. http://www.chemspider.com/Chemical-Structure.64808787.html. Accessed 11 Jul 2023
Chernicharo FCS, Modesto-Costa L, Borges I Jr (2021) Simulation of the electron ionization mass spectra of the Novichok nerve agent. J Mass Spectrom 56:e4779. https://doi.org/10.1002/jms.4779
Chuang C-S, Yang K-W, Yen C-M et al (2019) Risk of seizures in patients with organophosphate poisoning: a nationwide population-based study. Int J Environ Res Public Health 16:3147. https://doi.org/10.3390/ijerph16173147
Ciottone GR (2018) Toxidrome recognition in chemical-weapons attacks. N Engl J Med 378:1611–1620. https://doi.org/10.1056/NEJMra1705224
Cockburn H (2018) Soviet-era scientists contradict Moscow’s claims Russia never made Novichok nerve agent. The Independent. https://www.independent.co.uk/news/world/europe/novichok-nerve-agent-poison-soviet-russia-salisbury-attack-leonid-rink-vladimir-uglev-the-bell-a8265626.html. Accessed 11 Jul 2023
Costanzi S, Koblentz GD (2019) Controlling Novichoks after salisbury: revising the chemical weapons convention schedules. Nonproliferation Rev 26:599–612. https://doi.org/10.1080/10736700.2019.1662618
Costanzi S, Koblentz GD (2021) Strengthening controls on Novichoks: a family-based approach to covering A-series agents and precursors under the chemical-weapons nonproliferation regime. Nonproliferation Rev 28:95–113. https://doi.org/10.1080/10736700.2021.2020010
Costanzi S, Machado J-H, Mitchell M (2018) Nerve agents: what they are, how they work, how to counter them. ACS Chem Neurosci 9:873–885. https://doi.org/10.1021/acschemneuro.8b00148
Counter Terrorism Policing (2018) Salisbury & Amesbury investigation update. https://www.counterterrorism.police.uk/salisbury/. Accessed 11 Jul 2023
de Bruin-Hoegée M, Lamriti L, Langenberg JP et al (2022) Verification of exposure to chemical warfare agents through analysis of persistent biomarkers in plants. Anal Methods 15:142–153. https://doi.org/10.1039/D2AY01650H
De Farias R (2019) The number of conformers explains the high toxicity of novichok agents. Pharm Chem J 6:24–26
de Koning MC, Vieira Soares C, van Grol M et al (2022) Effective degradation of novichok nerve agents by the zirconium metal-organic framework MOF-808. ACS Appl Mater Interfaces 14:9222–9230. https://doi.org/10.1021/acsami.1c24295
Dimitrov M (2019) Bulgaria Admits ‘Skripal’ Suspect was in Country in 2015. In: Balk. Insight. https://balkaninsight.com/2019/02/11/bulgaria-admits-skripal-suspect-was-in-country-in-2015/. Accessed 11 Jul 2023
Dobrynin S (2018) Novichok victims. The history of poison. Salisbury poisoning case. In: Radio Lib. https://www.svoboda.org/a/29096697.html. Accessed 11 Jul 2023
Dodd V, Harding L, MacAskill E (2023) Sergei Skripal: former Russian spy poisoned with nerve agent, say police. In: The Guardian. https://www.theguardian.com/uk-news/2018/mar/07/russian-spy-police-appeal-for-witnesses-as-cobra-meeting-takes-place. Accessed 11 Jul 2023
Dzutsati V (2021) Poisonings of Activists in the North Caucasus: A Low Threshold for Chemical Weapons Use Inside Russia? In: Eurasia Dly. Monit. https://jamestown.org/program/poisonings-of-activists-in-the-north-caucasus-a-low-threshold-for-chemical-weapons-use-inside-russia/. Accessed 11 Jul 2023
Eddleston M, Chowdhury FR (2021) Organophosphorus poisoning: the wet opioid toxidrome. Lancet 397:175–177. https://doi.org/10.1016/S0140-6736(20)32749-5
Ellison D (2008) Handbook of chemical and biological warfare agents. CRC Press, London
Ellison DH (2017) Emergency action for chemical and biological warfare agents, 2nd edn. Routledge, New York
Eskandari M, Faraz SM, Hosseini SE et al (2022) Fragmentation pathways of chemical weapons convention-related organophosphorus Novichok agents: The electron ionization and electrospray ionization tandem mass spectroscopy and DFT calculation studies. Int J Mass Spectrom 473:116794. https://doi.org/10.1016/j.ijms.2021.116794
Executive Councile (2018) Russian Federation: Statement by G.V. Kalamanov Deputy Minister of Industry and Trade of the Russian Federation and I.V. Rybalchenko Professor, Doctor of Chemical Sciences, Expert of the Ministry of Defence of the Russian Federation. In: OPCW. https://www.opcw.org/sites/default/files/documents/EC/M-57/en/ecm57nat04_e_.pdf. Accessed 11 Jul 2023
Fedorov L (1995) Russia’s undeclared chemical war : politics against ecology. Center for Ecological Policy, Moscow
Fedorov L, Mirzayano V (1992) Poisoned politics. Mosc. News
Felshtinsky Y, Pribylovsky V (2010) Poisoning of Ivan Kivilidi. In: Corporation. Russia and the KGB under President Putin. Terra, Moscow
Franca TCC, Kitagawa DAS, de Cavalcante ASF et al (2019) Novichoks: the dangerous fourth generation of chemical weapons. Int J Mol Sci 20:1222. https://doi.org/10.3390/ijms20051222
General Assembly Security Council (2018) General and complete disarmament: implementation of the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction. In: OSN. https://digitallibrary.un.org/record/1630241/files/A_72_841%26S_2018_371-EN.pdf?version=1. Accessed 11 Jul 2023
Gertz B (1997) Russia dodges chemical arms ban. In. Wash. Time. https://www.globalsecurity.org/wmd/library/news/russia/1997/msg00043c.htm. Accessed 11 Jul 2023
Gorecki L, Soukup O, Korabecny J (2022) Countermeasures in organophosphorus intoxication: pitfalls and prospects. Trends Pharmacol Sci 43:593–606. https://doi.org/10.1016/j.tips.2022.04.008
Halamek E, Kobliha Z (2011) Potential chemical warfare agents. Chem Listy 105:323–333
Harvey SP, McMahon LR, Berg FJ (2020) Hydrolysis and enzymatic degradation of Novichok nerve agents. Heliyon 6:e03153. https://doi.org/10.1016/j.heliyon.2019.e03153
Haslam JD, Russell P, Hill S et al (2022) Chemical, biological, radiological, and nuclear mass casualty medicine: a review of lessons from the Salisbury and Amesbury Novichok nerve agent incidents. Br J Anaesth 128:e200–e205. https://doi.org/10.1016/j.bja.2021.10.008
Hatfill SJ (2019) Chemical warfare: nerve agents. J Am Phys Surg 24:19–24
Hoegberg LCG, Gosselin S (2017) Lipid resuscitation in acute poisoning: after a decade of publications, what have we really learned? Curr Opin Anesthesiol 30:474. https://doi.org/10.1097/ACO.0000000000000484
Hoenig SL (2007) Compendium of chemical warfare agents. Springer, New York
Hoffman D (1998) Soviets Reportedly Built Weapon Despite Pact. Wash Post Co A36
Horn G, Kranawetvogl T, John H et al (2023) Human HepaRG liver spheroids: cold storage protocol and study on pyridinium oxime-induced hepatotoxicity in vitro. Chem Biol Interact 369:110285. https://doi.org/10.1016/j.cbi.2022.110285
Hosseini SE, Saeidian H, Amozadeh A et al (2016) Fragmentation pathways and structural characterization of organophosphorus compounds related to the chemical weapons convention by electron ionization and electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.7757
Hosseini SE, Mousavi Faraz S, Naseri MT et al (2021) Structural characterization of chemical weapons convention-related phosphonoselenoates by electron ionization and electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.9209
Hui D (2018) Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time and right indication. Curr Opin Support Palliat Care 12:489–494. https://doi.org/10.1097/SPC.0000000000000395
Imrit YA, Bhakhoa H, Sergeieva T et al (2020) A theoretical study of the hydrolysis mechanism of A-234; the suspected novichok agent in the Skripal attack. R Soc Chem Adv 10:27884–27893. https://doi.org/10.1039/D0RA05086E
Ivanov II, Sokolov VB, Epishina TA, Martynov IV (1990) O-substituted alkylchloroformoximes as substrates and inhibitors of cholinesterases. Dokl Akad Nauk SSSR 310:1253–1255
Jacquet P, Rémy B, Bross RPT et al (2021) Enzymatic decontamination of G-Type, V-Type and Novichok nerve agents. Int J Mol Sci 22:8152. https://doi.org/10.3390/ijms22158152
Jeong K, Choi J (2019) Theoretical study on the toxicity of ‘Novichok’ agent candidates. R Soc Open Sci 6:190414. https://doi.org/10.1098/rsos.190414
Jeong W-H, Lee J-Y, Lim K-C, Kim H-S (2021) Identification and study of biomarkers from Novichok-inhibited butyrylcholinesterase in human plasma. Molecules 26:3810. https://doi.org/10.3390/molecules26133810
Jeong K, Lee J-Y, Woo S et al (2022a) Vapor pressure and toxicity prediction for novichok agent candidates using machine learning model: preparation for unascertained nerve agents after chemical weapons convention schedule 1 update. Chem Res Toxicol 35:774–781. https://doi.org/10.1021/acs.chemrestox.1c00410
Jeong K, Ryu TI, Hwang S-R et al (2022b) Precisely predicting the 1H and 13C NMR chemical shifts in new types of nerve agents and building spectra database. Sci Rep 12:20288. https://doi.org/10.1038/s41598-022-24647-y
Jett DA, Spriggs SM (2020) Translational research on chemical nerve agents. Neurobiol Dis 133:104335. https://doi.org/10.1016/j.nbd.2018.11.020
Jung H, Heo J, Park N et al (2023) Elimination of A-234 from the environment: effect of different decontaminants. J Hazard Mater 451:131150. https://doi.org/10.1016/j.jhazmat.2023.131150
Kanygin P (2018) “Novichok” is too much for one Skripal".In: Novaya Gaz. https://novayagazeta.ru/articles/2018/03/23/75908-demonstrativnoe-ubiystvo-mozhno-bylo-organizovat-prosche. Accessed 11 Jul 2023
Karev SA (2009) The problems of chemical disarmament and the ways of their solution. Ministry of Education and Science of the Russian Federation Penza State University Department of “Ecology and life safety”, Penza
Kim H, Yoon UH, Ryu TI et al (2022) Calculation of the infrared spectra of organophosphorus compounds and prediction of new types of nerve agents. New J Chem 46:8653–8661. https://doi.org/10.1039/D2NJ00850E
Kislinskaya L (2001) When doctors are powerless. In: Sovershenno Sekretno. https://www.sovsekretno.ru/articles/politika/kogda-vrachi-bessilny/. Accessed 11 Jul 2023
Kloske M, Witkiewicz Z (2019) Novichoks—the A group of organophosphorus chemical warfare agents. Chemosphere 221:672–682. https://doi.org/10.1016/j.chemosphere.2019.01.054
Knight A (2018) Novichok Victim Dies: Did the Kremlin Really Lose Control of its Deadliest Poisons? In: Dly. Beast. https://www.thedailybeast.com/novichok-victim-dies-did-the-kremlin-really-lose-control-of-its-deadliest-poisons. Accessed 11 Jul 2023
Konopski L (2009) Historia broni chemicznej. Bellona, Warszawa
Kruglyak LY, Malekin S, Martynov l (1972) Phosphorylated oximes XII. Reactions of 2-halophospholanes with dichlorofluoronitrosomethane. Zh Obshch Khim 42:811–814
Ledgard JB (2006) The laboratory history of chemical warfare agents, 2nd edn. Lulu, Morrisville
Lee JY, Lim KC, Kim HS (2021) Characterization and study on fragmentation pathways of a novel nerve agent, ‘Novichok (A234)’, in aqueous solution by liquid chromatography-Tandem Mass spectrometry. Molecules 26:1059. https://doi.org/10.3390/molecules26041059
Lee JH, Jang WE, Park JH et al (2022) Identification of organophosphate modifications by high-resolution mass spectrometry. Bull Korean Chem Soc 43:444–449. https://doi.org/10.1002/bkcs.12478
Lee JY, Shin JY, Kim HS (2023) Optimization of solid-phase extraction of a degradation product of Novichok (A234) and its application to environmental samples. J Anal Toxicol. https://doi.org/10.1093/jat/bkac028
Lenta (2018) Revealed a way to determine the creator of “Novichok.” In: Lenta. https://lenta.ru/news/2018/03/21/novichok_secret/. Accessed 11 Jul 2023
Luedtke S, Bojo C, Li Y et al (2021) Backbone conformation shifts in X-ray structures of human acetylcholinesterase upon covalent organophosphate inhibition. Crystals 11:1270. https://doi.org/10.3390/cryst11111270
Lyagin I, Efremenko E (2019) Theoretical evaluation of suspected enzymatic hydrolysis of Novichok agents. Catal Commun 120:91–94. https://doi.org/10.1016/j.catcom.2018.11.019
Makhaeva GF, Filonenko IV, Yankovskaya VL et al (1998) Comparative studies of O, O-dialkyl-O-chloromethylchloroformimino phosphates: interaction with neuropathy target esterase and acetylcholinesterase. Neurotoxicology 19:623–628
Marrs TT, Maynard RL, Sidell F (2007) Chemical warfare agents: toxicology and treatment, 2nd edn. Wiley, New York
Meselson M, Robinson JP (2005) HSP. In: Harv Sussex Program. http://hsp.sussex.ac.uk/new/_uploads/bulletin/CBWCB68.pdf. Accessed 11 Jul 2023
Milatović D, Jokanović M (2009) Chapter 65—Pyridinium oximes as cholinesterase reactivators in the treatment of OP poisoning. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents. Academic Press, San Diego, pp 985–996
Mirbabaei F, Mohammad-Khah A, Naseri MT et al (2022) Unambiguous identification and determination of A234-Novichok nerve agent biomarkers in biological fluids using GC-MS/MS and LC-MS/MS. Anal Bioanal Chem 414:3429–3442. https://doi.org/10.1007/s00216-022-03964-1
Mirzayanov VS (2008) State secrets: an insider’s chronicle of the Russian chemical weapons program. Outskirts Press, Parker
Moffatt A, Mohammed F, Eddleston M et al (2010) Hypothermia and fever after organophosphorus poisoning in humans—a prospective case series. J Med Toxicol 6:379–385. https://doi.org/10.1007/s13181-010-0012-y
Morris S (2021) Dawn Sturgess novichok death inquest to look at role of Russian state. In. The Guardian. https://www.theguardian.com/uk-news/2021/mar/30/dawn-sturgess-novichok-death-inquest-to-look-at-role-of-russian-state. Accessed 11 Jul 2023
Morris S, Bannock C, Bannock SM a C, Morris S (2019) Revealed: anti-nerve agent drug was used for first time in UK to save novichok victim. In. The Guardian. https://www.theguardian.com/uk-news/2019/jul/08/revealed-anti-nerve-agent-drug-was-used-for-first-time-in-uk-to-save-novichok-victim. Accessed 11 Jul 2023
Motlagh NM, Rouhani M, Mirjafary Z (2020) Aminated C20 fullerene as a promising nanosensor for detection of A-234 nerve agent. Comput Theor Chem 1186:112907. https://doi.org/10.1016/j.comptc.2020.112907
Mozafari N, Talaie H, Shoaei SD et al (2016) Survey on hypothermia and hyperthermia in poisoned patients in a unique referral hospital, Tehran, Iran. Iran Red Crescent Med J 18:e35483. https://doi.org/10.5812/ircmj.35483
Nakano Y, Imasaka T, Imasaka T (2019) Generation of a nearly monocycle optical pulse in the near-infrared region and its use as an Ionization source in mass spectrometry. Anal Chem 92:7130–7138. https://doi.org/10.1021/acs.analchem.0c00542
Nepovimova E, Kuca K (2018) Chemical warfare agent NOVICHOK—mini-review of available data. Food Chem Toxicol 121:343–350. https://doi.org/10.1016/j.fct.2018.09.015
Noga M, Jurowski K (2023) What do we currently know about Novichoks? The state of the art. Arch Toxicol 97:651–661. https://doi.org/10.1007/s00204-022-03437-5
Noga M, Michalska A, Jurowski K (2023a) The prediction of hydrolysis and biodegradation of Novichoks using in silico toxicology methods. Sci Total Environ 890:164241. https://doi.org/10.1016/j.scitotenv.2023.164241
Noga M, Michalska A, Jurowski K (2023b) Application of toxicology in silico methods for prediction of acute toxicity (LD50) for Novichoks. Arch Toxicol 97:1691–1700. https://doi.org/10.1007/s00204-023-03507-2
Noort D, Fidder A, van der Riet-van OD et al (2021) Verification of Exposure to novichok nerve agents utilizing a semitargeted human butyrylcholinesterase nonapeptide assay. Chem Res Toxicol 34:1926–1932. https://doi.org/10.1021/acs.chemrestox.1c00198
Osborne S (2018) Former Russian spy and daughter were poisoned on their front door. In: The Independent. https://www.independent.co.uk/news/uk/crime/sergei-skripal-salisbury-poison-nerve-agent-russia-daughter-attack-novichok-front-door-home-a8278631.html. Accessed 11 Jul 2023
Otsuka M, Miyaguchi H (2021) Theoretical evaluation of the hydrolysis of conventional nerve agents and novichok agents. Chem Phys Lett 785:139116. https://doi.org/10.1016/j.cplett.2021.139116
Otsuka M, Yamaguchi A, Miyaguchi H (2022) Analysis of degradation products of Novichok agents in human urine by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Forensic Toxicol. https://doi.org/10.1007/s11419-022-00656-4
Parasuraman S (2011) Prediction of activity spectra for substances. J Pharmacol Pharmacother 2:52–53. https://doi.org/10.4103/0976-500X.77119
Patki A, Shelgaonkar VC (2011) A Comparison of equisedative infusions of propofol and midazolam for conscious sedation during spinal anesthesia—a prospective randomized study. J Anaesthesiol Clin Pharmacol 27:47–53
Pejchal J, Osterreicher J, Kuca K et al (2008) The influence of acetylcholinesterase reactivators on selected hepatic functions in rats. Basic Clin Pharmacol Toxicol 103:119–123. https://doi.org/10.1111/j.1742-7843.2008.00249.x
Peplow M (2018) Nerve agent attack on spy used ‘Novichok’ poison. Chem Eng News 96:3–3. https://doi.org/10.1021/cen-09612-leadcon
PHE (2018) Briefing note for emergency departments—Management of suspected Novichok poisonings. In: PHE. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/738497/ED_briefing_note_nerve_agents.pdf. Accessed 11 Jul 2023
Philp C (2018) Salisbury poison ‘made at Russia’s Porton Down.’In: The Times. https://www.thetimes.co.uk/article/salisbury-poison-made-at-russia-s-porton-down-7p7kfcs3c. Accessed 11 Jul 2023
Pitschmann V (2014) Overall view of chemical and biochemical weapons. Toxins 6:1761–1784. https://doi.org/10.3390/toxins6061761
Pitschmann V (2016) Chemická válka ve věku atomu a DNA, 1st edn. Naše vojsko, Praha
Pulkrabkova L, Svobodova B, Konecny J et al (2023) Neurotoxicity evoked by organophosphates and available countermeasures. Arch Toxicol 97:39–72. https://doi.org/10.1007/s00204-022-03397-w
Radić Z (2021a) Shifts in backbone conformation of acetylcholinesterases upon binding of covalent inhibitors, reversible ligands and substrates. Crystals 11:1557. https://doi.org/10.3390/cryst11121557
Radic Z (2021b) Ca shifts video. In: YouTube. https://youtu.be/CDZilvzTf6o Accessed 11 Jul 2023
Radić Z (2023) Connectivity between surface and interior in catalytic subunits of acetylcholinesterases inferred from their X-ray structures. J Neurochem. https://doi.org/10.1111/jnc.15802
Raevskiĭ OA, Chapysheva NV, Ivanov AN et al (1987a) Effect of alkyl substituents in phosphorylated oximes. Zh Obshch Khim 57:2720–2723
Raevskiĭ OA, Grigor’ev VY, Solov’ev VP et al (1987b) Electron-donor functions of ethyl methylchloroformimino methylphosphonate. Zh Obshch Khim 57:2073–2078
Raevskiĭ OA, Chistiakov VV, Agabekian RS et al (1990) Formation of models of the interaction between organophosphate compound structure and their ability to inhibit cholinesterase. Bioorg Khim 16:1509–1522
Rashid MAM, Lee B, Kim KH, Jeong K (2023) Theoretical prediction on the hydrolysis rate of the new types of nerve agents: a density functional study. Toxicol Rep 10:27–31. https://doi.org/10.1016/j.toxrep.2022.12.001
Reiter S, Gevorkyan N (2018) The scientist who developed “Novichok”: “Doses ranged from 20 grams to several kilos.” In: The Bell. https://thebell.io/en/the-scientist-who-developed-novichok-doses-ranged-from-20-grams-to-several-kilos/. Accessed 11 Jul 2023
Review Conference (2018a) Statement by HE Ambassador Kenneth D. Ward Permanent Representative of the United States of America to the OPCW at the Fourth Special Session of the Conference of the States Parties to Review the Operation of the Chemical Weapons Convention. In: OPCW https://www.opcw.org/sites/default/files/documents/2018/11/rc4nat07%28e%29.pdf. Accessed 11 Jul 2023
Review Conference (2018b) Statement of Canada to the Fourth Review Conference Delivered by Ambassador Sabine Nölke, Permanent Representative. In: OPCW. https://www.opcw.org/sites/default/files/documents/2018b/11/Canada%20-%20EN%20FR.pdf. Accessed 11 Jul 2023
Ridley D (2019) The Inquest touching upon the death of Dawn Kelly Sturgess. In: Wiltshire Council. https://www.wiltshire.gov.uk/media/3904/Scope-ruling-Sturgess-20-December-2019/pdf/Scope-ruling-sturgess-20-december-20191.pdf. Accessed 11 Jul 2023
Robb EL, Baker MB (2023) Organophosphate Toxicity [Updated 2022 May 1]. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. https://www.ncbi.nlm.nih.gov/books/NBK470430/
Roth A, McCarthy T (2018) ‘It’s got me’: the lonely death of the Soviet scientist poisoned by novichok. In: The Guardian. https://www.theguardian.com/world/2018/mar/22/andrei-zheleznyakov-soviet-scientist-poisoned-novichok. Accessed 11 Jul 2023
Rozhdestvensky I (2018) Victims of “Novichka”. How the poisonous substance from Salisbury was created and tested on humans. In: Argument. https://argumentua.com/stati/zhertvy-novichka-kak-sozdavali-i-ispytyvali-na-lyudyakh-otravlyayushchee-veshchestvo-iz-solsbe. Accessed 11 Jul 2023
Saalbach KP (2023) Chapter 24—therapeutic treatment of nerve agent toxicity. In: Das S, Thomas S, Das PP (eds) Sensing of deadly toxic chemical warfare agents, nerve agent simulants, and their toxicological aspects. Elsevier, Amsterdam, pp 569–585
Sajid H, Khan S, Ayub K, Mahmood T (2021) Effective adsorption of A-series chemical warfare agents on graphdiyne nanoflake: a DFT study. J Mol Model 27:117. https://doi.org/10.1007/s00894-021-04730-3
Santos MC, Botelho FD, Gonçalves AS et al (2022a) Theoretical assessment of the performances of commercial oximes on the reactivation of acetylcholinesterase inhibited by the nerve agent A-242 (novichok). Food Chem Toxicol 165:113084. https://doi.org/10.1016/j.fct.2022.113084
Santos MC, Botelho FD, Gonçalves AS et al (2022b) Are the current commercially available oximes capable of reactivating acetylcholinesterase inhibited by the nerve agents of the A-series? Arch Toxicol 96:2559–2572. https://doi.org/10.1007/s00204-022-03316-z
Schiller EY, Goyal A, Mechanic OJ (2023) Opioid Overdose [Updated 2022 Sep 19]. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. https://www.ncbi.nlm.nih.gov/books/NBK470415/
Science’s news staff (2020) Poisoning of Putin opponent renews spotlight on deadly Russian chemical weapon. In: Science. https://www.science.org/content/article/poisoning-putin-opponent-renews-spotlight-deadly-russian-chemical-weapon. Accessed 11 Jul 2023
Security Council (2018) Security Council Seventy-third year 8237th meeting Wednesday, 18 April 2018, 3 p.m. New York. In. United Nations. https://www.securitycouncilreport.org/atf/cf/%7B65BFCF9B-6D27-4E9C-8CD3-CF6E4FF96FF9%7D/s_pv_8237.pdf. Accessed 11 Jul 2023
Shleinov R (2018a) Rejection of “Novichka.” In. Novaya Gazhttps://novayagazeta.ru/articles/2018/03/22/75896-rezhim-novichka . Accessed 11 Jul 2023
Shleinov R (2018b) “Novichok” has already killed. In. Novaya Gaz https://novayagazeta.ru/articles/2018/04/02/76026-otritsanie-novichka. Accessed 11 Jul 2023
Smithson A, Mirzayanov V, Lajoie R, Krepov M (1995) Chemical weapons disarmament in Russia: problems and prospects. In: Stimson. https://www.stimson.org/1995/chemical-weapons-disarmament-russia-problems-and-prospects/. Accessed 11 Jul 2023
Smolentseva N (2020) The creator of “Novichok” about the poisoning of Navalny.In: Dtsch. Welle. https://www.dw.com/ru/sozdatel-novichka-ob-otravlenii-navalnogo/a-54815311. Accessed 11 Jul 2023
Sobchak X (2020) WARNING: VICTIMS OF THE “NEW”. The Poisoned Homeland of Secret Poison. Special issue from the closed city. In: YouTube. https://youtu.be/sG0KM83Tx_M?t=1296. Accessed 11 July 2023
Soukup O, Jun D, Tobin G, Kuca K (2012) The summary on non-reactivation cholinergic properties of oxime reactivators: the interaction with muscarinic and nicotinic receptors. Arch Toxicol 87:711–719. https://doi.org/10.1007/s00204-012-0977-1
Stanley A (1995) Moscow Journal; To the Business Risks in Russia, Add Poisoning. In: NY Times. https://www.nytimes.com/1995/08/09/world/moscow-journal-to-the-business-risks-in-russia-add-poisoning.html. Accessed 11 Jul 2023
Steindl D, Boehmerle W, Körner R et al (2021) Novichok nerve agent poisoning. Lancet 397:249–252. https://doi.org/10.1016/S0140-6736(20)32644-1
Stone R (2018) U.K. attack puts nerve agent in the spotlight by Richard Stone. Science 359:1314–1315. https://doi.org/10.1126/science.359.6382.1314
Stone R (2020) How German military scientists likely identified the nerve agent used to attack Alexei Navalny. Sci AAAS. https://doi.org/10.1126/science.abe6561
Tan YB, Tay IR, Loy LY et al (2019) A scheme for ultrasensitive detection of molecules with vibrational spectroscopy in combination with signal processing. Molecules 24:776. https://doi.org/10.3390/molecules24040776
Technical Secretariat (2018) Note by the technical secretariat (technical assistance visit TAV/02/18). In: OPCW. https://www.opcw.org/sites/default/files/documents/S_series/2018/en/s-1612-2018_e___1_.pdf. Accessed 11 Jul 2023
Technical secretariat (2018) Note by the technical secretariat (technical assistance visit TAV/03/18). In: OPCW. https://www.opcw.org/sites/default/files/documents/2018/09/s-1671-2018%28e%29.pdf. Accessed 11 Jul 2023
Technical Secretariat (2020) Note by the technical secretariat (technical assistance visit TAV/01/20). In: OPCW. https://www.opcw.org/documents/2020/10/s19062020/note-technical-secretariat-summary-report-activities-carried-out. Accessed 11 Jul 2023
Termeau L, Penlou S, Carella A (2023) Selective colorimetric detection of novichok agents with hydrazone chemosensors. ACS Sens 8:1510–1517. https://doi.org/10.1021/acssensors.2c02505
The Insider, Bellingcat (2020) Samples of analyzes of Gebrev, who was poisoned by Novichok, disappeared from the Finnish laboratory. This will affect the investigation. In: The Insider. https://theins.ru/politika/234395. Accessed 11 Jul 2023
Tucker JB (2006) War of nerves: chemical warfare from world war I to Al-Qaeda, 1st edn. Pantheon Books, New York
TV Rain (2021) Mysterious deaths and poisonings. Data on the trips of Navalny’s poisoners published. In: YouTube. https://www.youtube.com/watch?v=o4Mt92ulyME. Accessed 11 Jul 2023
Vale JA, Marrs TC, Maynard RL (2018) Novichok: a murderous nerve agent attack in the UK. Clin Toxicol 56:1093–1097. https://doi.org/10.1080/15563650.2018.1469759
Vanova N, Pejchal J, Herman D et al (2018) Oxidative stress in organophosphate poisoning: role of standard antidotal therapy. J Appl Toxicol 38:1058–1070. https://doi.org/10.1002/jat.3605
Vásárhelyi G, Földi L (2007) History of Russia’s chemical weapons. Acad Appl Res Mil Sci 6:135–146
VGTRK (2018) Evening with Vladimir Solovyov. In: Ross. 1. https://smotrim.ru/video/1772350 Accessed 11 Jul 2023
Vicar D, Princ I, Masek I, Mika O (2021) Nuclear, radiological and chemical weapons, radiation and chemical accidents. Tomas Bata University, Zlin
Vieira LA, Almeida JSFD, França TCC, Borges I (2021) Electronic and spectroscopic properties of A-series nerve agents. Comput Theor Chem 1202:113321. https://doi.org/10.1016/j.comptc.2021.113321
Von Hippel F (1993) Russian whistleblower faces jail. Bull at Sci 49:7–8. https://doi.org/10.1080/00963402.1993.11456309
Waller MJ (1997) The Chemical Weapons Coverup. In: Wall Str. J. https://www.wsj.com/articles/SB855787549252167000. Accessed 11 Jul 2023
Wang L, Ding J, Shi P et al (2021) Ensemble machine learning to evaluate the in vivo acute oral toxicity and in vitro human acetylcholinesterase inhibitory activity of organophosphates. Arch Toxicol 95:2443–2457. https://doi.org/10.1007/s00204-021-03056-6
Wintour P (2018) Boris Johnson compares Russian World Cup to Hitler’s 1936 Olympics. In: The Guardian. https://www.theguardian.com/football/2018/mar/21/boris-johnson-compares-russian-world-cup-to-hitlers-1936-olympics. Accessed 11 Jul 2023
Worek F, Jenner J, Thiermann H (2016) Chemical warfare toxicology: volume 2: management of poisoning, 2nd edn. Royal Society of Chemistry, Cambridge
Worek F, Thiermann H, Wille T (2020) Organophosphorus compounds and oximes: a critical review. Arch Toxicol 94:2275–2292. https://doi.org/10.1007/s00204-020-02797-0
Yamaguchi A, Miyaguchi H, Tokeshi M (2022) Dimethoxytriadinylation LC–MS/MS of Novichok A-series degradation products in human urine. Anal Chem 94:4658–4665. https://doi.org/10.1021/acs.analchem.1c04634
Yar M, Hashmi MA, Khan A, Ayub K (2021) Carbon nitride 2-D surface as a highly selective electrochemical sensor for V-series nerve agents. J Mol Liq 311:113357. https://doi.org/10.1016/j.molliq.2020.113357
Acknowledgements
This work has been supported by the Long term development plan (Faculty of Military Health Sciences)—Medical issues of WMD II (DZRO-FVZ22-ZHN II), by the Long term development plan (Nuclear, Biological and Chemical Defence Institute)—Research of methods and technologies for protection against the effects of weapons of mass destruction and industrial hazardous substances II (DZRO-UOPZHN22-PROTECT II), by the project of Czech Science Foundation No 22-12859S (D. Jun), by the Ministry of Education, Youth, and Sports of the Czech Republic, Inter-Excellence program, No. LUAUS23275, and by the Ministry of Education, Youth, and Sports (SV/FVZ202302).
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The manuscript was written through the contributions of all authors. All the authors have approved the final version of the manuscript. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Opravil, J., Pejchal, J., Finger, V. et al. A-agents, misleadingly known as “Novichoks”: a narrative review. Arch Toxicol 97, 2587–2607 (2023). https://doi.org/10.1007/s00204-023-03571-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03571-8