Abstract
Pathogens co-evolved with ticks to facilitate blood collection and pathogen transmission. Although tick saliva was recently found to be rich in bioactive peptides, it is still elusive which saliva peptide promotes virus transmission and which pathways are invovled. Here, we used a saliva peptide HIDfsin2 and a severe fever with thrombocytopenia syndrome virus (SFTSV) both carried by the tick Haemaphysalis longicornis to elucidate the relationship between tick saliva components and tick-borne viruses. HIDfsin2 was found to promote the replication of SFTSV in a dose-dependent manner in vitro. HIDfsin2 was further revealed to MKK3/6-dependently magnify the activation of p38 MAPK. The overexpression, knockdown and phosphorylation site mutation of p38α indicated that p38 MAPK activation facilitated SFTSV infection in A549 cells. Moreover, the blockade of p38 MAPK activation significantly suppressed SFTSV replication. Differently, HIDfsin2 or pharmacological inhibition of p38 MAPK activation had no effect on a mosquito-borne Zika virus (ZIKV). All these results showed that HIDfsin2 specifically promoted SFTSV replication through the MKK3/6-dependent enhancement of p38 MAPK activation. Our study provides a new perspective on the transmission of tick-borne viruses under natural conditions, and supports that the blockade of p38 MAPK activation can be a promising strategy against the mortal tick-borne virus SFTSV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are arthropods belonging to the class Arachnida. They feed on blood through direct bites and transmit a large number of viral, bacterial and protozoan pathogens, posing a serious threat to global livestock and human health (Cabezas-Cruz et al. 2019; Wang and Cull 2022). According to the report of the Centers for Disease Control and Prevention in USA, tick-borne diseases accounted for nearly 76.5% of all vector-borne diseases from 2004 to 2016. Ticks carry a variety of viruses, and at least 160 named viruses are transmitted by ticks, such as severe fever with thrombocytopenia syndrome virus (SFTSV), tick-borne encephalitis virus (TBEV), Crimean–Congo hemorrhagic fever virus (CCHF), etc. (Bogovič et al. 2022; Hawman et al. 2021; Xie et al. 2023). Viruses create persistent infections in ticks without substantial impact, making them good vectors for carrying these viruses.
Tick–host interaction is crucial for pathogen transmission. Tick salivary gland is an important site for virus uptake and reproduction, and the complex components of the salivary gland play a key role in virus transmission (Kazimírová et al. 2017; Maqbool et al. 2022). Studies had reported that protein or peptide mixtures in salivary gland enhance virus transmission through host immune regulation rather than through direct influence on virus (Jones et al. 1989, 1990). It has been experimentally demonstrated that co-infection with TBEV and tick saliva enhances both virus transmission and infection compared with TBEV infection alone (Jones et al. 1989; Nuttall and Labuda 2004). Mice co-infected with Powassan virus and Ixodes scapularis salivary gland extracts also showed the enhanced viral replication and increased rates of neural infiltration and death, compared with the same dose of Powassan virus alone (Hermance and Thangamani 2015). In addition to the co-evolution of pathogens and ticks, similar phenomena have been observed in mosquito salivary gland proteins and mosquito-borne viruses, and bat salivary gland and coronavirus (Fang et al. 2022; Jin et al. 2018). However, the specific components of tick saliva and how they mediate tick-borne virus transmission are still unknown.
HIDfsin2 (GenBank number: ABW08118.1) is an antimicrobial peptide from the tick Haemaphysalis longicornis, which was found to have expression in the tick salivary gland (Tsuji et al. 2007). It is composed of 38 amino acid residues and contains six cysteines forming three pairs of disulfide bonds, showing typical cationic polypeptide characteristics. SFTSV is a single-stranded, negative-sense, enveloped RNA virus, whose genome comprises three segments: large (L), medium (M) and small (S) (Liu et al. 2021). Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease with extensive geographical distribution and high mortality (12–30%), mainly resulted from the bites of the tick H. longicornis carried with SFTSV. SFTS is listed as an urgent public health problem by the World Health Organization (WHO) (Zhang et al. 2013). The clinical symptoms of SFTSV infection mainly include fever, gastrointestinal disease, leukopenia, thrombocytopenia and multiple organ dysfunction (Liu et al. 2014). There are still no specific vaccines or drugs developed against SFTSV infection.
During viral infection, cell signaling pathways are activated as a mode of cellular immunity, but the virus can also use enhanced cellular activity to support its various cycles of replication (Chander et al. 2021). p38 MAPK is a serine/threonine kinase and a component of the signal transduction pathway that can be induced and activated by external stimuli, thereby participating in cell development, proliferation, migration and apoptosis, etc. (Wang et al. 2022). Previous reports showed that the activation of p38 MAPK can be induced by the infection of various viruses, such as hepatitis C virus (HCV), influenza A virus (IAV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Bouhaddou et al. 2020; Cheng et al. 2020b; Marchant et al. 2010). Meanwhile, the study found that two scropion peptides, BmKDfsin3 and SVHRP, can reduce the phosphorylation level of p38 (Cheng et al. 2020a; Wang et al. 2020), while both scorpion venom peptides, HsTx2 and BmK NT1, activate p38 MAPK (Li et al. 2021; Shen et al. 2019). These showed that scorpion-related peptides have different physiological functions. Therefore, we wanted to know whether the tick salivary peptide HIDfsin2 can affect SFTSV replication and regulate the activity of p38 MAPK.
In this study, we found that the tick salivary peptide HIDfsin2 could promote the replication of SFTSV in a dose-dependent manner in both A549 and Huh7 cells, and acted a promotional effect at the post-entry stages of SFTSV. At the same time, HIDfsin2 was discovered to activate p38 MAPK in viral host cells. The overexpression, knockdown and phosphorylation site mutation (Thr180 and Tyr182) of p38α revealed that the activation of p38 MAPK significantly facilitated SFTSV replication. Subsequently, the inhibition of p38 MAPK activation and its key upstream proteins remarkablely suppressed the replication of SFTSV. Unlike SFTSV, HIDfsin2 treatment or p38 MAPK activation inhibition had no any effect on the mosquito-borne ZIKV. In short, HIDfsin2 specifically promoted the replication of the tick-borne SFTSV by enhancing p38 MAPK activation.
Meterials and methods
Cells and viruses
Huh7 and A549 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco–Invitrogen) with 10% fatal bovine serum (FBS) (Gibco–Invitrogen) and 1% penicillin/streptomycin (Thermo Fisher). All cells were cultured at 37 °C in an incubator containing 5% CO2. The Huh7 and A549 cell lines were obtained from our laboratory.
The plasmid of ZIKV Puerto Rico (PRVABC59) strain cDNA was provided by Dr. Ren Sun from the University of California, Los Angeles, USA, and the virus particles were prepared by reverse genetic system. SFTSV was kindly provided by Professor Xuejie Yu (School of Health Sciences, Wuhan University, China).
Reagents and antiboties
The linear form of the tick salivary peptide HIDfsin2 was synthesized by Nanjing Yuanpeptide Biotech Co., Ltd. (Nanjing, China). ST2345 was synthesized by GL Biochem Co., Ltd. (Shanghai, China). IRAK1/4 inhibitor I (HY-13329) and SB203580 (HY-10256) were purchased from MedChemExpress (MCE) and dissolved in 100% dimethyl sulfoxide (DMSO). Lipopolysaccharide (LPS) was purchased from Beyotime (S1732). Cell Counting Kit (CCK-8) was purchased from Yeasen (40203ES60). The P-p38 MAPK antibody (4511), p38 MAPK antibody (9212) and P-MKK3/6 antibody (12280) were ordered from the Cell Signaling Technology (CST). The MKK3/6 (A19830) antibody and HA (AE008) antibody were purchased from ABclonal Technology. The GAPDH antibody (60004-1-lg) and β-tubulin (10094-1-AP) were the products of Proteintech (Rosemont, USA). The flag antibody (F1084) was purchased from Sigma. SFTSV NP rabbit polyclonal antibody was customized by Atagenix Biological Technology Co., Ltd. (Wuhan, China).
Cell viability assay
The cytotoxicity of the compounds to A549 and Huh7 cells was evaluated using cell CCK8. 5 × 104 cell suspension was seeded to the 96-well plates for 24 h, and then different concentrations of HIDfsin2 were added to the 96-well plates. After incubation for 72 h, the medium was discarded and 100 μL fresh DMEM medium containing 10 μL CCK8 solution was added to each well for 1.5 h. The absorbance of each well was measured at 450 nm using a BioTek microplate reader (BioTek, Winooski, VT, USA).
Quantitative real-time PCR (qRT-PCR)
According to the manufacturer’s instructions, total RNA was extracted from cells using RNAiso Plus reagent (Takara, Japan). Then, 1 μg of total RNA was reversed to cDNA by HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). The target gene amplification system contained 10 μL of 2 × ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China), 0.4 μL of upstream and downstream primers and 1 μL of cDNA template, and 8.2 μL ddH2O up to 20 μL. The qRT-PCR primers used for SFTSV were 5′-ATGTCAGAGTGGTCCAGGA-3′(upstream) and 5′-TCTCCACCTGTCTCCTTCAG-3′ (downstream). The qRT-PCR primers used for ZIKV were 5′-TTGTGGAAGGTATGTCAGGTG-3′ (upstream) and 5′-ATCTTACCTCCGCCATGTTG-3′ (downstream). The qRT-PCR primers used for GAPDH were 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ (upstream) and 5′-TCCTTGGAGG CCATGTGGGCCAT-3′ (downstream). The copy number of genes was determined by a 7500 real-time PCR system (Applied Biosystems, USA). The experimental results were analyzed by the comparative method (ΔΔCT). Experiments were performed in triplicate and data were presented as mean ± standard deviations (SD).
Western blotting
1% sodium dodecyl sulfate (SDS) with protease and phosphatase inhibitors (Topscience, Shanghai, China) was used to lyse cells. The concentration of total protein was quantified with BCA protein quantification kit (Vazyme, Nanjing, China). Samples containing 20 μg of total protein were separated by 10% or 12% SDS-PAGE. Proteins were transferred to nitrocellulose (NC) membrane, and the membrane was then blocked with 5% skim milk for 2 h at room temperature. Then primary antibodies were added and the membrane were incubated overnight at 4 °C. Subsequently, the membrane was incubated with 3% skim milk containing HRP-conjugated secondary antibodies at room temperature for 2 h. Finally, the results were analyzed by Clarity™ Western ECL Substrates (#1705060, Bio-Rad) with FuJi medical X-ray film.
CRISPR/Cas9
p38α was knocked down in A549 cells using CRISPR/Cas9 technology. sgRNA was designed by the online website of Zhang Feng’s laboratory (https://zlab.bio/guide-design-resources), and then the synthesized sgRNA was ligated to the pGL3-U6-sgRNA-PGK-puromycin plasmid. Subsequently, the constructed pGL3-U6-sgRNA-PGK-puromycin-p38α and Cas9 plasmids were co-transfected into A549 cells. After 24 h, fresh DMEM medium was replaced and the final concentrations of 1 μg/μL puromycin and 15 μg/μL blasticidin were added for screening, and the screening lasted for 5–7 days. After that, the cells were collected and the knocked-down effect of p38α was detected by Western blotting.
Oxidation and purification of peptide
The synthesized linear HIDfsin2 peptide was dissolved in 0.1 M Tris–HCl (pH = 7.8) and cyclized for 48 h at 25 °C in a shaker (80–100 rpm). Then the reduced and oxidized forms of HIDfsin2 were separated and purified by reverse-phase high-performance liquid chromatography (RP-HPLC) on a C18 column (Elite HPLC, Dalian, China, 10 × 250 mm, 5 µm, 300 A). The elution conditions were as follows: the flow rate was 4 mL/min, the mobile phase was 90% acetonitrile + 0.1% trifluoroacetic acid, eluted with 5 ~ 95% linear gradient within 60 min, and the detection was performed at 230 nm. The target peptide was collected and lyophilized for preservation. The molecular weight of the target peptide was detected by matrix-assisted laser desorption lonization–time of flight mass spectrometer (MALDI–TOF).
Plasmids and cloning
The plasmid with a full-length p38α cDNA was gifted by Prof. Jiahuai Han (Xiamen University, China). Then, p38α cDNA was cloned into pCS2 + plasmid expressing 3 × Flag. The primer sequences for p38αAA and p38αDD were as follows: 5′-ACAGATGATGAAATGGCAGGCGCCGTGGCCAC TAGGTGG-3′ (upstream) and 5′-CCACCTAGTGGCCACGGCGCCTGCCATTTCATCATCTGT-3′ (downstream) for p38αAA; 5′-ACAGATGATGAAATGGACGGCGACGTGGCCACTAGGTGG-3′ (upstream) and 5′-CCACCTAGTGGCCACGTCGCCGTCCATTTCATCATCTGT-3′ (downstream) for p38αDD.
Statistical analysis
All data and graphics were accomplished and analyzed by GraphPad Prism 8, Adobe Photoshop CS6, and UniProt website. Data represent at least three repeated independent experiments, and all experimental results are presented as means ± SD. Statistical significance was analyzed using a two-tailed unpaired Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; and ns, no significance).
Results
Oxidative refolding, purification and identification of the tick saliva peptide HIDfsin2
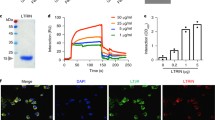
HIDfsin2 is a defensin peptide from the tick H. longicornis, which was characterized to have expression in the tick salivary gland (Tsuji et al. 2007). The reductive formation of HIDfsin2 (HIDfsin2-R) synthesized by Yuantai Biotech was folded by air oxidation in slightly alkaline Tris–HCl buffer. The peptide contained six cysteine residues forming three pairs of disulfide bonds (C1–C4, C2–C5 and C3–C6) (Fig. 1a). The oxidized product (HIDfsin2-O) was purified to homogeneity by RP-HPLC and eluted at a retention time of 18.6 min, while HIDfsin2-R was eluted at a retention time of 17.3 min (Fig. 1b), which showed that HIDsin2-O and HIDfsin2-R had different polarities. The theoretical molecular weight of HIDsin2-R was predicted to be 4130.79, and so the theoretical molecular weight of HIDfsin2-O was considered to be 4124.79 because of the formation of three disulfide bridges. MALDI–TOF mass spectrometry analysis showed that the molecular weight of the purified HIDfsin2-O was determined to be 4124.68, which was in good agreement with its theoretical value 4124.79 (Fig. 1c). The secondary structures of HIDfsin2-O and HIDfsin2-R in aqueous solution were analyzed by circular dichroism spectra, and the results showed that the structure of HIDfsin2-O had α-helix and β-strand, but not HIDfsin2-R (Fig. 1d). Furthermore, the three-dimensional structure of HIDfsin2-O was predicted by SWISS-MODEL (Fig. 1e), indicating that a stable α-helix and β-strand of HIDfsin2-O can be formed.
Oxidative refolding, purification and identification of the tick defensin peptide HIDfsin2. a Amino acid sequence of the tick defensin peptide HIDfsin2. R and O were the reduced and oxidized forms of HIDfsin2, respectively. SH represented the thiol group of cysteine. The connectivity of disulfide bonds was indicated by the solid line with S–S. The cysteine residues are shaded in yellow, and the basic residues are displayed in blue. b Oxidative refolding of chemically synthetic linear HIDfsin2. HPLC showed the difference of peak time between oxidized and reduced forms. c MALDI time of flight mass spectrometry analysis of HIDfsin2-O. d The circular dichroism spectra analyses of HIDfsin2-O and HIDfsin2-R. e Modeled three-dimensional structure of HIDfsin2-O
HIDfsin2 promoted SFTSV replication in vitro
To investigate the effect of HIDfsin2 (all subsequent HIDfsin2 was considered to be HIDfsin2-O) on the tick-borne virus SFTSV, we treated A549 or Huh7 cells with or without the peptide HIDfsin2 for 72 h in the viral infection system. The experimental results showed that with the increase of HIDfsin2 concentration from 0 μM, 5 μM and 10 μM to 20 μM in A549 cells, the expression of SFTSV NP protein was significantly promoted at both RNA (Fig. 2a) and protein (Fig. 2b) levels. The cell viability results showed that HIDfsin2 was substantially noncytotoxic and not enhanced to A549 cells under the used concentrations during experiments (Fig. 2c). The same promotion effect of HIDfsin2 on SFTSV was also observed in Huh7 cells. Huh7 cells infected with SFTSV could significantly enhance the replication of intracellular virions after HIDfsin2 treatment for 72 h (Fig. 2d and e). Similarly, HIDfsin2 did not affect the viability of Huh7 cells at the used concentrations during experiments (Fig. 2f), suggesting that the promotion effect of HIDfsin2 on SFTSV was not related with the cell growth. HIDfsin2 was previously found to have expression in the salivary gland of H. longicornis, and this tick was the transmission vector of SFTSV. Therefore, our experimental data suggested that the tick and the pathogen co-evolved in some unknown way. Altogether, these results indicate that the salivary peptide HIDfsin2 of the tick H. longicornis can promote the replication of the virus SFTSV carried by the tick H. longicornis.
HIDfsin2 promoted SFTSV replication in both A549 and Huh7 cells. a,b HIDfsin2 promoted SFTSV replication in a dose-dependent manner in A549 cells. A549 cells were preincubated with the peptide HIDfsin2 at different concentrations for 1 h and then infected with SFTSV at an MOI = 0.1. After 72 h infection, intracellular SFTSV vRNA was analyzed by qRT-PCR (a) and intracellular SFTSV NP protein level was analyzed by western blotting (b). c Effect of HIDfsin2 on the viability of A549 cells by CCK-8 assay. HIDfsin2 was dissolved in the medium, and the medium without HIDfsin2 was used as a negative control in all experiments. d, e HIDfsin2 promoted SFTSV replication in a dose-dependent manner in Huh7 cells. Huh7 cells were preincubated with the peptide HIDfsin2 at different concentrations for 1 h and then infected with SFTSV at an MOI = 0.1. At 72 h infection, intracellular SFTSV vRNA was analyzed by qRT-PCR (d) and intracellular SFTSV NP protein level was analyzed by western blotting (e). f Effect of HIDfsin2 on the viability of Huh7 cells by CCK-8 assay. HIDfsin2 was dissolved in the medium, and the medium without HIDfsin2 was used as a negative control in all experiments. Data are presented as the means ± SD from three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001)
HIDfsin2 promoted SFTSV replication by acting on the viral post-entry stage
To further determine the specific stage at which HIDfsin2 affected SFTSV life cycle, we added HIDfsin2 at different stages of viral infection, shown in the schematic diagram (Fig. 3a). The experimental results indicated that HIDfsin2 had no effect on the stage of SFTSV attachment (Fig. 3b) and the stage of SFTSV entry/fusion (Fig. 3c). However, the tick salivary peptide HIDfsin2 significantly promoted the post-entry stage of SFTSV (59%) (Fig. 3d). These data suggest that HIDfsin2 enhances the replication of SFTSV by acting on the viral post-entry phase.
HIDfsin2 enhanced the replication of SFTSV by acting on the viral post-entry stage. a Schematic of HIDfsin2 treatment during SFTSV infection. b-d Effects of HIDfsin2 on the attachment (b), entry/fusion (c), or post-entry (d) stages of SFTSV in A549 cells. A549 cells were infected with SFTSV at an MOI = 0.1 and treated with HIDfsin2 as described in the schematic diagram. All experiments were detected by qRT-PCR. HIDfsin2 was dissolved in PBS, and PBS without HIDfsin2 was used as a negative control. The internal control of all subfigures was GAPDH. Data represented the mean ± SD of at least three independent experiments. ns, no significance. *P < 0.05. **P < 0.01. ***P < 0.001
HIDfsin2 enhanced p38 MAPK activation
Some previous studies reported that the activation of p38 MAPK was closedly related to the replication of many viruses including SFTSV (Cheng et al. 2020b). To explore whether p38 MAPK activation played a role during the promotion effect of HIDfsin2 on SFTSV replication, we added different concentrations of HIDfsin2 in SFTSV-infected A549 cells and detected the change of p38 phosphorylation by western blotting. The results showed that the phosphorylation of p38 was promoted by HIDfsin2 in a dose-dependent manner during SFTSV infection (Fig. 4a). However, considering that SFTSV infection can also induce p38 phosphorylation. Thus, to further elucidate the promotion effect of HIDfsin2 on p38 phosphorylation, we detected the effect of HIDfsin2 on p38 MAPK activation in A549 cells without SFTSV infection. The results showed that the phosphorylation level of p38 did not change through western blotting (Fig. 4b), and no significant change was observed in the total amount of p38 protein at the RNA level (Fig. 4c). Interestingly, when we incubated A549 cells with the agonist LPS of the p38 MAPK signaling pathway for 2 h, and then treated them with different concentrations of HIDfsin2 for 24 h, the results of western blotting indicated that HIDfsin2 remarkedly promoted p38 phosphorylation in response to LPS (Fig. 4d), but the total p38 content in the cells remained unchanged (Fig. 4d). Moreover, HIDfsin2 did not affect the expression of p38 at the RNA level in A549 cells treated by LPS (Fig. 4e). Altogether, all these results suggest that HIDfsin2 enhances p38 MAPK activation during SFTSV infection, and the activation of p38 MAPK plays an important role in SFTSV replication.
HIDfsin2 enhanced p38 MAPK activation in A549 cells. a Effect of HIDfsin2 on p38 MAPK activation in A549 cells during SFTSV infection. A549 cells were preincubated with HIDfsin2 at different concentrations for 1 h and then infected with SFTSV at MOI = 1. After 48 h infection, intracellular P-p38, p38 and GAPDH protein levels were analyzed by western blotting. b Effect of HIDfsin2 on p38 MAPK activation and its upstream MKK3/6 in A549 cells without SFTSV infection. Cells were treated with different concentrations of HIDfsin2 for 48 h, protein samples were collected, and the content of related proteins was detected by western blotting. c Effect of HIDfsin2 on p38 MAPK mRNA expression in A549 cells without SFTSV infection. HIDfsin2 was added to A549 cells at different concentrations for 48 h. The contents of p38 mRNA were analyzed by qRT-PCR. d Enhancement of HIDfsin2 on p38 MAPK activation and its upstream MKK3/6 in A549 cells treated by LPS. A549 cells were treated with 100 ng/mL LPS for 2 h, and then HIDfsin2 was added at different concentrations for 48 h. The contents of each protein in the cells were analyzed by western blotting. e Effect of HIDfsin2 on p38 MAPK mRNA expression in A549 cells treated by LPS. A549 cells were treated with 100 ng/mL LPS for 2 h, and then HIDfsin2 was added at different concentrations for 48 h. The contents of p38 mRNA were analyzed by qRT-PCR. ns, no significance
HIDfsin2 facilitated SFTSV replication by enhancing p38 MAPK activation
Previous reports and the above results indicated that the activation of p38 MAPK was related to SFTSV replication. To further resolve the detailed relationship of p38 activation with SFTSV replication, we investigated the effects of p38α overexpression, knockdown and phosphorylation site mutation on SFTSV replication in A549 cells. First, we overexpressed p38α in A549 cells (Fig. 5a) and further found that the overexpression of p38α promoted SFTSV replication (Fig. 5b). Second, we successfully constructed the A549 cell line of p38α knockdown (p38α-KD) by CRISPR/Cas9 method (Fig. 5c), and found that the knockdown of p38α significantly reduced SFTSV replication (Fig. 5d). These results indicate that the expression of p38 promotes the replication of SFTSV and plays an important role in the life cycle of SFTSV. To further explore the effect of the p38α kinase active site on SFTSV replication, we mutated its Thr180 and Tyr182 to alanine (p38αAA) or aspartic acid (p38αDD), respectively. When p38α was overexpressed in A549 cells, a significant enhancement of SFTSV replication was detected by western blotting, but the overexpression of p38αAA or p38αDD had no any effect on SFTSV replication (Fig. 5e). Consistently, we performed similar experiments in the A549-p38αKD cell line and then found that SFTSV replication was significantly enhanced when wild-type p38α was complemented, but the rescue of p38αAA and p38αDD still had no any effect on SFTSV replication (Fig. 5f). These data showed that the promotion effect of p38α on SFTSV replication was dependent on the p38α kinase activity. The above results suggest that the activation of p38 MAPK contributes to the replication of SFTSV and HIDfsin2 facilitates SFTSV replication by enhancing p38 MAPK activation.
HIDfsin2 promoted SFTSV replication through enhancing p38 MAPK activation. a Overexpression of p38α in A549 cells. Flag-p38α was transfected into A549 cells. After 48 h, the intracellular p38 protein level was detected by western blotting. b Promotion of p38α overexpression to SFTSV replication in A549 cells. The plasmid Flag-p38α was transfected into A549 for 24 h, and then the cells were infected with SFTSV at an MOI = 0.1. At 24 h infection, intracellular SFTSV vRNA was analyzed by RT-PCR. c. Knockdown of p38α in A549 cells. p38α was knocked down in A549 cells using CRISPR/Cas9, and the protein level of p38α was detected by western blotting. d Inhibition of p38α knockdown against SFTSV replication in A549 cells. A549-p38αKD cells were infected with SFTSV at an MOI = 0.1. At 48 h infection, intracellular SFTSV vRNA was analyzed by RT-PCR. e, f Kinase activity dependence of p38α promotion on SFTSV replication. After 24 h of overexpression of p38α, p38αAA and p38αDD in A549 cells, the cells were then infected with SFTSV with MOI = 0.1 for 24 h. Intracellular protein levels were detected by western blotting (e). After 24 h of overexpression of p38α, p38αAA and p38αDD in A549-p38αKD cells, the cells were then infected with SFTSV with MOI = 0.1 for 24 h. Intracellular protein levels were detected by western blotting (f). Data represented the mean ± SD of at least three independent experiments. *P < 0.05. **P < 0.01. ***P < 0.001
Pharmacological blockade of p38 MAPK activation inhibited SFTSV replication
Next, to further verify the role of p38 MAPK activation in SFTSV replication, we introduced the interference of myeloid differentiation factor 88 (MyD88) and interleukin-1 receptor-associated kinase-1/4 (IRAK1/4), two key upstream proteins of p38 MAPK cascade. SB203580 is a selective and ATP-competitive p38 MAPK activation inhibitor. IRAK1/4 inhibitor I suppresses the phosphorylations of IRAK1 and IRAK4. ST2345 is a short peptide composed of 23 amino acids (RQIKIWFQNRRMKWKKRDVLPGT), which is recognized to block MyD88 dimerization and thus inhibit downstream signal transduction. In short, the treatment of SB203580, IRAK1/4 inhibitor I and ST2345 can all pharmacologically block p38 MAPK activation. Therefore, we used these three inhibitors to observe the effect of p38 MAPK activation on SFTSV replication. The experimental results showed that SB203580 could inhibit SFTSV in a dose-dependent manner at both RNA and protein levels (Fig. 6a and b). The treatment of IRAK1/4 inhibitor I also reduced the transcription of SFTSV vRNA in a dose-dependent manner (Fig. 6c), as well as SFTSV NP protein (Fig. 6d). Similarly, ST2345 was observed to have a concentration-dependent antiviral activity against SFTSV (Fig. 6e and f). These results indicate that the pharmacological blockade of p38 activation inhibits SFTSV replication, and the regulation of p38 activation is a promising strategy against the mortal tick-borne virus SFTSV.
Blockade of p38 MAPK activation inhibited SFTSV replication in vitro. a, b Inhibitory activity of SB203580 against SFTSV. A549 cells were preincubated with SB203580 at different concentrations for 1 h and then infected with SFTSV at an MOI = 0.1. At 72 h infection, intracellular SFTSV vRNA and protein were analyzed by qRT-PCR (a) and western blotting (b), respectively. c, d Inhibitory effect of IRAK1/4 inhibitor I on SFTSV. A549 cells were preincubated with IRAK1/4 inhibitor I at different concentrations for 1 h and then infected with SFTSV at an MOI = 0.1. At 72 h infection, intracellular SFTSV vRNA and protein were analyzed by qRT-PCR (c) and western blotting (d), respectively. e, f Inhibition of ST2345 against SFTSV in A549 cells. A549 cells were preincubated with ST2345 at different concentrations for 1 h and then infected with SFTSV at an MOI = 0.1. At 72 h infection, intracellular SFTSV vRNA and protein were analyzed by qRT-PCR (e) and western blotting (f), respectively. SB203580 and IRAK1/4 inhibitor I were dissolved in dimethyl sulfoxide (DMSO), and DMSO was used as a negative control. The peptide ST2345 was dissolved in PBS, and PBS was used as a negative control
HIDfsin2 or blocking p38 MAPK activation had no effect on ZIKV replication
HIDfsin2 is derived from the tick H. longicornis. According to our previous results, HIDfsin2 promoted the replication of the tick-borne SFTSV through enhancing p38 MAPK activation. In addition to tick-borne viruses, mosquito-borne viruses also spread rapidly in nature. Therefore, in order to study if the tick salivary peptide HIDfsin2 had a specific promotion on the replication of tick-borne viruses, we investigated the effect of HIDfsin2 on a mosquito-borne virus ZIKV. We pretreated A549 cells with HIDfsin2 and then infected cells with the mosquito-borne ZIKV. The results showed that the tick salivary peptide HIDfsin2 did not affect the replication of ZIKV at both RNA (Fig. 7a) and protein (Fig. 7b) levels. Further, we also explored whether the pharmacological blockade of p38 MAPK activation had effect on ZIKV replication in A549 cells. The results showed that the replication of ZIKV was not affected by SB203580 at both transcription (Fig. 7c) and protein (Fig. 7d) levels. Similarly, the treatment of IRAK1/4 inhibitor I showed no antiviral activity against ZIKV in A549 cells (Fig. 7e and f). Consistently, the replication of ZIKV was not affected by ST2345 at both transcription (Fig. 7g) and translation (Fig. 7h) levels. All these results suggest that the tick salivary peptide HIDfsin2 specifically promotes the replication of the tick-borne SFTSV by enhancing p38 MAPK activation.
HIDfsin2 treatment or blocking p38 activation did not affect ZIKV replication. a, b HIDfsin2 had no effect on ZIKV replication in A549 cells. A549 cells were preincubated with HIDfsin2 with different concentrations for 1 h and then infected with ZIKV at an MOI = 0.1. After 72 h infection, intracellular ZIKV RNA and protein were analyzed by qRT-PCR (a) and western blotting (b), respectively. c, d SB203580 had no effect on ZIKV replication in A549 cells. A549 cells were preincubated with SB203580 at different concentrations for 1 h and then infected with ZIKV at an MOI = 0.1. After 72 h infection, intracellular ZIKV RNA and protein were analyzed by qRT-PCR (c) and western blotting (d), respectively. e, f IRAK1/4 inhibitor I had no effect on ZIKV replication. A549 cells were preincubated with IRAK1/4 inhibitor I with different concentrations for 1 h and then infected with ZIKV at an MOI = 0.1. After 72 h infection, intracellular ZIKV RNA and protein were analyzed by qRT-PCR (e) and western blotting (f), respectively. g, h ST2345 had no effect on ZIKV replication. A549 cells were preincubated with ST2345 at different concentrations for 1 h and then infected with ZIKV at an MOI = 0.1. After 72 h infection, intracellular ZIKV RNA and protein were analyzed by qRT-PCR (g) and western blotting (h), respectively. Data represented the mean ± SD of at least three independent experiments. ns, no significance
Discussion
There are a variety of complex and biologically active substances in the salivary gland of ticks, which have anticoagulant, anti-inflammatory and immunomodulatory activities. These bioactive molecules can alter the local environment at the bite sites of ticks, and thus they are important factors for ticks to effectively transmit viral, bacterial and protozoan pathogens (Boulanger and Wikel 2021; Strobl et al. 2022). Therefore, an in-depth understanding of the interface reactions at tick bite sites is of great significance for the prevention and treatment of tick-borne diseases. Although reports on transcriptomics of tick salivary glands have gradually emerged in the public view, the relationship between specific salivary gland components and tick-borne pathogens remains unknown.
In this study, we prepared the tick salivary peptide HIDfsin2 from H. longicornis by chemical synthesis and oxidative refolding and found that HIDfsin2 specifically promoted the replication of this tick-borne SFTSV, but had no effect on the mosquito-borne ZIKV. HIDfsin2 is a cationic antimicrobial peptide consisting of 38 amino acid residues with an isoelectric point of 9.38. Previously, it was reported that the tick saliva mixture could promote virus replication, but the specific salivary gland components and the specific transmission route were both unknown. To our knowledge, the peptide HIDfsin2 was identified to be the first tick salivary component promoting the tick-borne virus transmission. In 2018, Lai group found that a mosquito salivary gland component LTRIN was able to facilitate ZIKV transmission by interacting with lymphotoxin-β receptor and then inhibiting antiviral innate immunity (Jin et al. 2018). Most recently, this group reported again that an inhibitor of leukotriene-A4 hydrolase from the venom gland of the bat Myotis pilosus promoted virus infection (Fang et al. 2022). The discovery of tick, mosquito and bat salivary components promoting their carried viruses suggested the phenomenon of convergent evolution in the venomous animals of nature. SFTSV infection can induce the activation of p38 MAPK. p38 MAPK is a member of the serine/threonine kinase family, which is closely related to the stress response and transmits signals by activating downstream key transcription factors and cytokines (Chander et al. 2021; Wang et al. 2022).
In our study, the tick salivary peptide HIDfsin2 was found to enhance the activation of p38 MAPK, dependent on MKK3/6 pathway. The overexpression, knockdown and phosphorylation site mutation of p38α showed that the activation of p38 MAPK contributed to promote the replication of SFTSV. Moreover, the tick salivary peptide HIDfsin2 had no any effect on the replication of the mosquito-borne ZIKV. Although we did not identify the direct target regulated by HIDfsin2, it was no doubt that MKK3/6-dependent p38 MAPK activation specifically played a key mechanistic role during the promotion effect of HIDfsin2 on SFTSV replication.
SFTSV is a new tick-borne bunyavirus, which was firstly isolated in 2011 (Yu et al. 2011). This virus was prevalent in central and eastern China, South Korea and Japan. People bitten by ticks carrying SFTSV will cause a new infectious disease SFTS with a mortality rate of up to 30%, which seriously endangers public health. In recent years, the number of cases with SFTSV infection has increased steadily. At present, there is no vaccine or specific therapeutic drug for the prevention and control of SFTSV infection. In our study, the pharmacological blockade of p38 MAPK activation by SB203580, IRAK1/4 inhibitor I or ST2345 treatment significantly inhibited the replication of SFTSV, suggesting that the target of p38 MAPK activation can be a promising strategy against the mortal virus SFTSV. Moreover, many chemical small molecules inhibiting p38 MAPK activation were being used to be antitumor drugs under clinical trials, which was preferably considered to be tested as anti-SFTSV drug candidates.
Conclusions
The peptide HIDfsin2 of the tick H. longicornis was identified to be the first salivary gland component promoting the tick-borne viruses. Moreover, the activation of p38 MAPK is involved in this promotion process. Our study provided the depth of understanding of the tick-borne virus infectivity and transmission under natural conditions. Our findings showed that the regulation of p38 MAPK activation signaling can be used to develop therapeutics to control or prevent tick-borne diseases.
Data availability
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SFTSV:
-
Severe fever with thrombocytopenia syndrome virus
- ZIKV:
-
Zika virus
- TBEV:
-
Tick-borne encephalitis virus
- CCHF:
-
Crimean–Congo hemorrhagic fever virus
- WHO:
-
World Health Organization
- HCV:
-
Hepatitis C virus
- IAV:
-
Influenza A virus
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- RP-HPLC:
-
Reverse-phase high-performance liquid chromatography
- MALDI–TOF:
-
Matrix-assisted laser desorption lionization–time of flight mass spectrometer
- LPS:
-
Lipopolysaccharide
- MyD88:
-
Myeloid differentiation factor 88
- IRAK1/4:
-
Interleukin-1 receptor-associated kinase-1/4
References
Bogovič P, Kastrin A, Lotrič-Furlan S et al (2022) Clinical and laboratory characteristics and outcome of illness caused by tick-borne encephalitis virus without central nervous system involvement. Emerg Infect Dis 28(2):291–301. https://doi.org/10.3201/eid2802.211661
Bouhaddou M, Memon D, Meyer B et al (2020) The global phosphorylation landscape of SARS-CoV-2 infection. Cell 182(3):685–712. https://doi.org/10.1016/j.cell.2020.06.034
Boulanger N, Wikel S (2021) Induced transient immune tolerance in ticks and vertebrate host: a keystone of tick-borne diseases? Front Immunol 12:625993. https://doi.org/10.3389/fimmu.2021.625993
Cabezas-Cruz A, Espinosa P, Alberdi P, de la Fuente J (2019) Tick-pathogen interactions: the metabolic perspective. Trends Parasitol 35(4):316–328. https://doi.org/10.1016/j.pt.2019.01.006
Chander Y, Kumar R, Khandelwal N et al (2021) Role of p38 mitogen-activated protein kinase signalling in virus replication and potential for developing broad spectrum antiviral drugs. Rev Med Virol 31(5):1–16. https://doi.org/10.1002/rmv.2217
Cheng Y, Sun F, Li S et al (2020a) Inhibitory activity of a scorpion defensin BmKDfsin3 against hepatitis C virus. Antibiotics (basel, Switzerland) 9(1):33. https://doi.org/10.3390/antibiotics9010033
Cheng Y, Sun F, Wang L et al (2020b) Virus-induced p38 MAPK activation facilitates viral infection. Theranostics 10(26):12223–12240. https://doi.org/10.7150/thno.50992
Fang M, Tang X, Zhang J et al (2022) An inhibitor of leukotriene-a(4) hydrolase from bat salivary glands facilitates virus infection. Proc Natl Acad Sci U S A 119(10):e2110647119. https://doi.org/10.1073/pnas.2110647119
Hawman DW, Meade-White K, Leventhal S et al (2021) Immunocompetent mouse model for crimean-congo hemorrhagic fever virus. Elife 10:e63906. https://doi.org/10.7554/eLife.63906
Hermance ME, Thangamani S (2015) Tick saliva enhances powassan virus transmission to the host, influencing its dissemination and the course of disease. J Virol 89(15):7852–7860. https://doi.org/10.1128/jvi.01056-15
Jin L, Guo X, Shen C et al (2018) Salivary factor LTRIN from aedes aegypti facilitates the transmission of zika virus by interfering with the lymphotoxin-β receptor. Nat Immunol 19(4):342–353. https://doi.org/10.1038/s41590-018-0063-9
Jones LD, Hodgson E, Nuttall PA (1989) Enhancement of virus transmission by tick salivary glands. J Gen Virol 70(Pt 7):1895–1898. https://doi.org/10.1099/0022-1317-70-7-1895
Jones LD, Hodgson E, Nuttall PA (1990) Characterization of tick salivary gland factor(s) that enhance thogoto virus transmission. In: Calisher C (ed) Hemorrhagic fever with renal syndrome, tick- and mosquito-borne viruses. Springer, Vienna, pp 227–234
Kazimírová M, Thangamani S, Bartíková P et al (2017) Tick-borne viruses and biological processes at the tick-host-virus interface. Front Cell Infect Microbiol 7:339. https://doi.org/10.3389/fcimb.2017.00339
Li CL, Yang R, Sun Y, Feng Y, Song YB (2021) N58A exerts analgesic effect on trigeminal neuralgia by regulating the MAPK pathway and tetrodotoxin-resistant sodium channel. Toxins 13(5):357. https://doi.org/10.3390/toxins13050357
Liu Q, He B, Huang SY, Wei F, Zhu XQ (2014) Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 14(8):763–772. https://doi.org/10.1016/s1473-3099(14)70718-2
Liu BY, Yu XJ, Zhou CM (2021) SAFA initiates innate immunity against cytoplasmic RNA virus SFTSV infection. PLoS Pathog 17(11):e1010070. https://doi.org/10.1371/journal.ppat.1010070
Maqbool M, Sajid MS, Saqib M et al (2022) Potential mechanisms of transmission of tick-borne viruses at the virus-tick interface. Front Microbiol 13:846884. https://doi.org/10.3389/fmicb.2022.846884
Marchant D, Singhera GK, Utokaparch S et al (2010) Toll-like receptor 4-mediated activation of p38 mitogen-activated protein kinase is a determinant of respiratory virus entry and tropism. J Virol 84(21):11359–11373. https://doi.org/10.1128/jvi.00804-10
Nuttall PA, Labuda M (2004) Tick-host interactions: saliva-activated transmission. Parasitology 129(Suppl):S177–S189. https://doi.org/10.1017/s0031182004005633
Shen L, Yang Q, He Y, Zou X, Cao Z (2019) BmK NT1-induced neurotoxicity is mediated by PKC/CaMKII-dependent ERK1/2 and p38 activation in primary cultured cerebellar granule cells. Toxicology 421:22–29. https://doi.org/10.1016/j.tox.2019.03.012
Strobl J, Muendler V, Müller S et al (2022) Tick feeding modulates the human skin immune landscape to facilitate tick-borne pathogen transmission. J Clin Investig 132(21):e161188. https://doi.org/10.1172/jci161188
Tsuji N, Battsetseg B, Boldbaatar D et al (2007) Babesial vector tick defensin against babesia sp. parasites. Infect Immun 75(7):3633–3640. https://doi.org/10.1128/iai.00256-07
Wang XR, Cull B (2022) Apoptosis and autophagy: current understanding in tick-pathogen interactions. Front Cell Infect Microbiol 12:784430. https://doi.org/10.3389/fcimb.2022.784430
Wang XG, Zhu DD, Li N et al (2020) Scorpion venom heat-resistant peptide is neuroprotective against cerebral ischemia-reperfusion injury in association with the NMDA-MAPK pathway. Neurosci Bull 36(3):243–253. https://doi.org/10.1007/s12264-019-00425-1
Wang L, Xia Z, Tang W et al (2022) p38 activation and viral infection. Expert Rev Mol Med 24:e4. https://doi.org/10.1017/erm.2021.29
Xie J, Li H, Zhang X et al (2023) Akkermansia muciniphila protects mice against an emerging tick-borne viral pathogen. Nat Microbiol 8(1):91–106. https://doi.org/10.1038/s41564-022-01279-6
Yu XJ, Liang MF, Zhang SY et al (2011) Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364(16):1523–1532. https://doi.org/10.1056/NEJMoa1010095
Zhang X, Liu Y, Zhao L et al (2013) An emerging hemorrhagic fever in China caused by a novel bunyavirus SFTSV. Sci China Life Sci 56(8):697–700. https://doi.org/10.1007/s11427-013-4518-9
Acknowledgements
We thank Professor Xuejie Yu (Wuhan University, China) for gifting the virus strain of SFTSV and Professor Jiahuai Han (Xiamen University, China) for providing the cDNA plasmid of p38α. This research was funded by the joint research fund of the National Science Fund of China—Science and Technology Development Fund of Macau SAR [No. 32161160303 for NSFC and 0010/2021/AFJ for FDCT], National Science Fund of China [No. 32070525], Innovative Research Groups of Hubei Natural Science Foundation [No. 2020CFA015], Fundamental Research Fund for the Central Universities of China [No. 2042021kf0219] and Open Research Fund Program of the State Key Laboratory of Virology of China [No. 2022KF004].
Author information
Authors and Affiliations
Contributions
LW: conceptualization, methodology, software, writing—original draft preparation. FS: investigation, methodology, software. JH: visualization. YZ and YW: analysis, software. WZ and HFK: methodology, data curation, reviewing and editing. ZC: conceptualization, supervision, reviewing and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Sun, F., Hu, J. et al. The tick saliva peptide HIDfsin2 promotes the tick-borne virus SFTSV replication in vitro by enhancing p38 signal pathway. Arch Toxicol 97, 1783–1794 (2023). https://doi.org/10.1007/s00204-023-03515-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03515-2