Abstract
Bromate, classified as a EU CLP 1B carcinogen, is a typical by-product of the disinfection of drinking and swimming pool water. The aim of this study was (a) to provide data on the occurrence of bromate in pool water, (b) to re-evaluate the carcinogenic MOA of bromate in the light of existing data, (c) to assess the possible exposure to bromate via swimming pool water and (d) to inform the derivation of cancer risk-related bromate concentrations in swimming pool water. Measurements from monitoring analysis of 229 samples showed bromate concentrations in seawater pools up to 34 mg/L. A comprehensive non-systematic literature search was done and the quality of the studies on genotoxicity and carcinogenicity was assessed by Klimisch criteria (Klimisch et al., Regul Toxicol Pharmacol 25:1–5, 1997) and SciRAP tool (Beronius et al., J Appl Toxicol, 38:1460–1470, 2018) respectively. Benchmark dose (BMD) modeling was performed using the modeling average mode in BMDS 3.1 and PROAST 66.40, 67 and 69 (human cancer BMDL10; EFSA 2017). For exposure assessment, data from a wide range of sources were evaluated for their reliability. Different target groups (infants/toddlers, children and adults) and exposure scenarios (recreational, sport-active swimmers, top athletes) were considered for oral, inhalation and dermal exposure. Exposure was calculated according to the frequency of swimming events and duration in water. For illustration, cancer risk-related bromate concentrations in pool water were calculated for different target groups, taking into account their exposure using the hBMDL10 and a cancer risk of 1 in 100,000. Convincing evidence was obtained from a multitude of studies that bromate induces oxidative DNA damage and acts as a clastogen in vitro and in vivo. Since statistical modeling of the available genotoxicity data is compatible with both linear as well as non-linear dose–response relationships, bromate should be conservatively considered to be a non-threshold carcinogen. BMD modeling with model averaging for renal cancer studies (Kurokawa et al., J Natl. Cancer Inst, 1983 and 1986a; DeAngelo et al., Toxicol Pathol 26:587–594, 1998) resulted in a median hBMDL10 of 0.65 mg bromate/kg body weight (bw) per day. Evaluation of different age and activity groups revealed that top athletes had the highest exposure, followed by sport-active children, sport-active adults, infants and toddlers, children and adults. The predominant route of exposure was oral (73–98%) by swallowing water, followed by the dermal route (2–27%), while the inhalation route was insignificant (< 0.5%). Accepting the same risk level for all population groups resulted in different guidance values due to the large variation in exposure. For example, for an additional risk of 1 in 100,000, the bromate concentrations would range between 0.011 for top athletes, 0.015 for sport-active children and 2.1 mg/L for adults. In conclusion, the present study shows that health risks due to bromate exposure by swimming pool water cannot be excluded and that large differences in risk exist depending on the individual swimming habits and water concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bromate anion is a disinfection by-product (DBP) in drinking and swimming pool water that occurs as a result of ozonation or chlorination of bromide-containing water or using ozone-bromide treatment for disinfection. Since the (European) Directive 2006/7/EC on the management of bathing water quality does not apply to swimming pools, requirements for the quality of swimming pool water are defined within the framework of the legislation of EU member states. In Germany, the legal basis for monitoring the quality of swimming and bathing pool water is the Infection Protection Act (IfSG 2000, para. 37). Swimming pool water disinfectants are product-type 2 usages as laid down in ‘Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products’. Insofar, the DBP bromate is also covered by Regulation (EU) No 528/2012 (ECHA 2017a). Biocidal products require an authorization before they can be placed on the market, and the active substances contained in that biocidal product must be previously approved. The comprehensive requirements for human health risk assessment in the context of the approval and authorization of biocides are described in ECHA (2017b) and ECHA (2018). ECHA’s first tier approach for the human toxicological risk assessment for DBPs from oxidative acting biocidal products in PT2 consists of simply comparing measured DBP concentration of selected DBPs to existing limits for swimming- and/or drinking-water for these DBPs. In principle, the use of drinking-water limits should be viewed as first tier approach which can be refined if needed with a more specific swimming-water limit (ECHA 2017a).
Various thresholds for bromate in pool water (Anses 2012; DIN 2012; RIVM 2014; UBA 2014; EDI 2016; ECHA 2017a) have been proposed, ranging from 8.7 to 2000 µg/L. Often, drinking water is used as fill-up water for swimming pools. For risk evaluation, it is important to know the maximum background concentration of bromate in the fill-up water. Drinking water guidance values for bromate were set at 10 µg/L (World Health Organization 2017; Health Canada 2018; US Environmental Protection Agency, Office of Water 2018; EU 2020). The U.S. EPA’s MCLG (Maximum Contaminant Level Goal, a non-enforceable health benchmark goal) has set the level goal of bromate at zero. The Directive (EU) 2020/2184 indicates that, where possible without compromising disinfection, EU member states should strive for lower bromate values in the future. The drinking water values are of particular interest when cancer estimates have been taken into account. If sea water is utilized as fill-up water, its natural bromide content determines the background concentration before disinfection. In natural sea water, bromate may be present below 1 µg/L (Lim and Shin 2012). The current bathing water guidance values differ by several orders of magnitude, depending partly on the evaluation of the carcinogenic mode of action (MOA), as well as on the exposure assessment. According to CLP regulation EU/1272/2008, potassium bromate is classified as carcinogen in category 1B (presumed to have carcinogenic potential for humans, classification is largely based on animal evidence). Bromate’s mutagenicity has not been classified in the CLP regulation.
Current discussions on the approval of the ozone-bromine method for the disinfection of bathing water, the in comparison to other countries relatively high bromate limit value of 2 mg/L bathing water in the recently implemented baths hygiene regulation of Schleswig–Holstein, Germany, and recurrent findings of even higher bromate concentrations in German seawater pools were the starting-point for the present study.
The aim of this study was (a) to provide data on the occurrence of bromate in pool water, (b) to re-evaluate the carcinogenic MOA of bromate in the light of existing data, (c) to assess the possible exposure to bromate via swimming pool water and (d) to inform the derivation of cancer risk-related bromate concentrations in swimming pool water. For the development of these risk-based guidance values, all relevant exposure routes, different target groups and exposure scenarios were considered.
Physical–chemical properties, formation, occurrence of bromate and guidance values in swimming pool water
As the dissolved bromate anion is the chemical entity that leads to the relevant health effects, different salts, e.g. sodium vs. potassium bromate, are not evaluated separately.
Physical–chemical data
Bromate is an anion that is associated with a cation, forming salts with characteristic physico-chemical properties (molecular weight bromate = 127.9 g/mol, e.g. Potassium Bromate = 167.01 g/mol). As strong electrolytes with a water solubility of more than 10 g/L at 20 °C, bromate salts dissociate in aqueous media. Therefore, the mode of toxic action of the bromate ion is expected to be independent of the counter-ion (ECHA 2010, 2019). The Henry coefficient has not been determined for potassium bromate or sodium bromate; however, an estimate (based on data for sodium oxide and an assumption of 99% dissociation) indicates a Henry-coefficient of 2.53 × 10–13 Pa × m3/mol (S1.1). This low vapor pressure indicates that exposure to bromate by inhalation may occur only in the presence of bromate-loaded aerosols. Further physical data (S1.2) and analytical methods (S1.3) are summarized in the supplemental materials.
Formation of bromate by water disinfection in the presence of bromide ions
Water may contain a natural background of bromide ions in the range of 10 – 40,000 µg/L (Table 1). In pool water, bromate may be formed during disinfection if bromide ions are present.
During disinfection processes chlorine, hypochlorites and ozone may oxidize bromide (Br–) to bromine (Br2) and further to bromate (Huang et al. 2008; Liu et al. 2012; Shi et al. 2013; Fang et al. 2014). Bromide ions (Br–) react rapidly to hypobromite (OBr–) in the presence of ozone (1) and may subsequently and unintentionally disproportionate to bromate (BrO3−) at elevated pH-values, or may also be oxidized to bromate by ozone (2).Footnote 1
The yield of bromate generated during ozonation of water is dependent on several factors such as pH, total dissolved organic carbon (TOC), ammonia, bromide and temperature. An empirical relation between the yield of bromate and the aforementioned parameters has been published (Haag and Hoigne 1983; Siddiqui and Amy 1993; Song et al. 1996). A detailed description of the reactions is available in Supplemental Information S2 and S3. The influence of several parameters on bromate formation may explain why bromate/bromide ratios differ widely between samples taken from waters treated with the same disinfection method (Table 1). For the ozone-bromide treatment of pool waters, 20–40 mg/L bromide is added (Brugger 2014; Hansen et al. 2016) to achieve an ozone-bromide system with acceptable biocidal activity.
Increased bromate concentrations in pool water also can result from an impurity of the disinfection solution. For example, sodium bromate can increase over time in sodium hypochlorite solution (Javel water) used for disinfection, if the chloride solutions for the synthesis of hypochlorites contain bromide ions, these hypochlorite disinfectants may reach bromate concentrations of 2.5–38 mg/kg, and peak concentrations of 77 mg/kg (Binetti and Attias 2007). A further source of bromate is the electrolytic generation of chlorine in pool water, if bromide containing sea water is used instead of pure sodium chloride brine (World Health Organization 2017).
Occurrence of bromate in marine and fresh pool water
Surveillance data for bromate in pool water (not published) reveal the impact of different water sources on the bromate concentrations (Table 2).
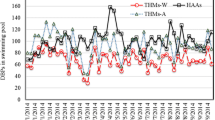
A total of 229 samples taken between 2017 and May 2020 from 31 fresh water facilities and 197 samples from 11 seawater facilities in northern Germany, partially including several pools, were analyzed. Fresh water pools had low concentrations of bromate, with 89% of pools containing less than 100 µg/L and a maximum of 1200 µg/L. Higher concentrations were detected in seawater pools; with 51% of the analyzed samples containing higher than 2000 µg/L. It should be considered that these concentrations were obtained from pools with disinfected seawater and that concentrations in native seawater are much lower. Bromate was not detected in untreated seawater using detection limits of 60 µg/L and 2 µg/L (Chen et al. 2006; Zakaria et al. 2011). Background concentrations of 0.1–0.6 µg/L bromate in five sea water samples from the south sea at Korea were reported (Lim and Shin 2012). Although high concentrations of bromate were detected in some seawater pools, there are also other seawater pools with bromate concentrations below the lower limit of quantitation (LLOQ) of 200 µg/Lover the entire period examined. Moreover, individual seawater pools with disinfection may exhibit a large variation over time (Table 3); rapid increases in bromate concentration within two months have been found (Table 4).
An overview of bromate concentrations in pool and drinking water reported in the literature is given in Table 1. Bromate concentrations in drinking water ranged from < 2 to 770 µg/L, whereas levels reported for pool water ranged from < 2 to 2000 µg/L. In Germany, drinking water analysis does not include bromate on a regular basis. When bromate was analyzed, the drinking water limit for bromate was exceeded in less than 1% of the samples between 2014 and 2016 (Bundesministerium für Gesundheit and Umweltbundesamt 2018).
Pool water standards and guidance values for bromate
Pool water standards are set to ensure a certain level of antimicrobial activity for the protection of swimmers against infections. If ozonation is used for pool water disinfection, bromide ions may have been added to achieve the legally required disinfection capacity defined by sanitation standards (ozone-bromide treatment). Existing sanitation standards for pool water are given in Table 5.
An overview of guideline values for bromate in pool water and drinking water, focusing the toxicological data on which their derivation is based, is given in Table 6. Carcinogenicity has been assessed as the critical effect and key endpoint of concern, whereby linear extrapolation using a non-threshold approach has been used in most cases.
Toxicokinetics
The kinetics of bromate has been studied in rats following oral administration. The only data on dermal absorption are from unpublished studies in guinea pigs, summarized by the Cosmetic Ingredient Expert Review Panel (Anderson 1994).
Absorption
Oral uptake
Following administration of bromate (0.625–100 mg/kg bw) by gavage to rats, the maximum plasma concentration was reached within 15 min (Fujii et al. 1984), indicating fast absorption. Comparison of AUCs following intravenous and oral administration, the latter by gavage, enabled a calculation of the bioavailability. When doses between 0.077 and 15.3 mg/kg bw (only oral administration) were investigated, a linear relationship between AUC and dose was observed between 0.077 and 1.9 mg/kg bw for the intravenous and oral routes; however, at higher doses, the AUC increases exceeded dose proportionality, suggesting saturation of clearance processes (Bull et al. 2012). The percentage of oral absorption was calculated to vary between 19.5 and 24.6%.
Investigations using real and synthetic gastric juice indicate some pre-absorptive breakdown in the stomach (Keith et al. 2006; Cotruvo et al. 2010) which may reduce the amount of absorbable bromate. However, as the rate of breakdown was very slow, it was assumed to be irrelevant under the typical physiological stomach retention time.
Human data on absorption are scarce. In a case report, acute bromate poisoning of a 2-year-old male (13 kg) was described. He ingested 1 to 2 oz (29.5–59 mL) of a permanent wave neutralizer containing 10–12 g/100 mL bromate (dose: between 3 and 7 g; 230 and 540 mg/kg bw). The peak concentration (approximately 160 µg/mL serum) was reached 12 h after ingestion. The total amount of bromide recovered from dialysate and the urine over 6–48 h after ingestion was 1,850 mg (140 mg/kg bw). The boy was admitted to the pediatric intensive care unit, where a urinary catheter was inserted after a decrease of urinary output. He was released from hospital a few days later. Follow-up examinations revealed normal hearing, renal function and urinalysis findings (Lichtenberg et al. 1989).
Inhalation uptake
Data on the uptake of bromate by the inhalation route are not available.
Dermal uptake
Primary data for the dermal absorption of bromate are not available. An unpublished ex vivo dermal absorption study with guinea pig skin is summarized by the Cosmetic Ingredient Expert Review Panel (Anderson 1994). A Kp value (Kp = permeability coefficient) of 4.29 × 10–6 cm/min can be calculated from the reported data to approximate the dermal uptake of bromate for swimmers (S4). This value is in a typical range for anions; Kp values were 5.5 × 10–5 cm/min for bromide, 1.1 × 10–4 cm/min for phosphate in rabbits in vivo, 7.3 × 10–7 cm/min for bromide in male volunteers (Tregear 1966), and 3.8 × 10–5 cm/min for bromide on porcine skin in vitro (Paweloszek et al. 2016).
No studies could be identified for long-term exposure of the whole body in water. However, Gattu and Maibach (2010, 2011) reviewed studies in which the influence of physical or chemical stress and skin disease on dermal absorption of bromate was investigated. They concluded that even in the worst case, the increase in dermal absorption was modest and did not exceed a factor of ten. Likewise, Felter et al. (2017) investigated the influence of diaper rash on skin absorption and reported a 1.45-fold increase compared to healthy skin for substances with low absorption of less than 10% of the applied dose. Considering that human skin is generally less permeable to chemicals than guinea pig skin due to fewer hair follicles, it was assumed that the higher permeability of macerated or injured skin is covered by the use of a permeability coefficient derived from a guinea pig skin absorption model. Therefore, no further uncertainty factor was used for the assessment of dermal bromate exposure.
Distribution
Fujii et al. (1984) reported that in rats after oral dosing with potassium bromate, no bromate was found in any organ or blood 24 h after dosing, although it was found in large amounts in urine. However, bromide concentrations were increased in all organs, particularly in kidney, and in urine. This is consistent with reduction of bromate to bromide in body tissues by glutathione and other thiols (Tanaka et al. 1984). When the bromate dose was increased above 5 mg/kg bw by gavage, the concentration of 18O-labeled bromate in the tissue of the kidneys increased strongly. This may be explained by saturation of the renal elimination of bromate and metabolites at such high concentrations (Delker et al. 2006).
Fisher and Bull (2006) hypothesized that the organ-specificity of bromate-induced adverse effects is the consequence of effective transport of bromate by the sodium iodide symporter (NIS) into the cells of the respective organs. NIS is an energy-dependent transporter which shuttles iodide from the blood stream into cells. In addition, bromate and its stable metabolite bromide are substrates of NIS (Eskandari et al. 1997). However, except for the thyroid, there is only limited evidence for the presence of an active NIS in target tissues.
Metabolism
Bromate is a strong oxidizer, which is effectively reduced to bromide by thiol compounds such as GSH and cysteine (Cys). Rat liver and kidney homogenate, as well as red blood cells, showed the highest activity for degradation of bromate under physiological conditions (Tanaka et al. 1984). Furthermore, bromide is yielded stoichiometrically by GSH-mediated degradation of bromate, which corresponds well with the fact that in vivo the bromide concentration increases in the kidney, pancreas, stomach, red blood cells and plasma and urine of rats 24 h after oral administration of bromate (Fujii et al. 1984). Further information on metabolism was derived from a newer in vitro study in which bromate was added to fresh rat blood. Initially, bromate at concentrations of less than 320 µM was rapidly reduced to bromide. Thereafter, a lower rate of transformation was observed. This slower rate was also measured in samples with higher initial bromate concentrations (Bull et al. 2012). It was proposed that degradation of bromate in vivo occurs to a high degree in plasma. In addition, gastric transformation may take place when bromate is administered orally. In gastric juice, bromate has a half-life of about 20–30 min (Keith et al. 2006).
Elimination and volume of distribution
The average terminal half-life of orally administered bromate was 37 min, according to Bull et al. (2012). Using AUC data and the data from Bull et al. (2012) after intravenous administration, the clearance of bromate value was calculated to be 0.76 ± 0.34 L/kg/h (mean ± SD) and the resulting volume of distribution (according to volume of distribution = clearance/(0.693/half-life)) was calculated to be 47.3 L for a 70 kg person, indicating that bromate is distributed into the body water. From a study with continuous oral administration via drinking water, the daily renal excretion of bromate was 9.1 ± 1.6% (mean ± SD) of the dose and the sum of bromate and bromide excretion was 75.2 ± 8.7% (mean ± SD) of the dose (Bull et al. 2012).
PBPK models
Health Canada employed the results of a PBPK model in its risk assessment (Health Canada 2018). However, besides a preliminary structure of the model for bromate and its metabolite bromide (Fisher and Bull 2006), no further publication was identified in the literature. In addition, the risk assessment document did not contain further details of the model. As explained in a second document (Environment Canada and Health Canada 2010), the model results included some uncertainties, as concentrations in human biological fluids were unavailable for model validation, and differences between rats and humans in bromate kinetics may exist.
In an attempt to estimate human equivalent concentrations in drinking water, Campbell et al. (2019) developed a PBPK model using published experimental results from rat studies (Bull et al. 2012) to calibrate the model. The authors constructed a rat model that was extrapolated to human using species-specific physiological parameters and interspecies scaling factors (flows: body weight3/4, first order rate constants: body weight−1/4). The human model was used to simulate the concentrations of potassium bromate in drinking water in humans, assuming a water consumption of 2 L/d, which would lead to an equivalent internal dose, defined as average steady state plasma concentration, compared to the internal dose in three different carcinogenicity studies in rats (Kurokawa et al. 1986a, b; DeAngelo et al. 1998). Using this procedure, human equivalent drinking water concentrations (HEC) could be established. The HEC factors ranged from 1.92 to 3.47. As the upper limit of this range is close to the standard human adjustment factor of 4, we used this standard value when converting the rat BMDL to a human BMDL.
Health effects
Effects in humans
Systematic studies of the toxicity of bromate in humans are lacking and effects of long-term exposure remain unknown. In the literature, cases of accidental and suicidal intoxications have been published. The target organs of acute toxicity are the kidney and the inner ear. Acute doses which are reported to result in kidney toxicity and ototoxicity are above 100 mg/kg bw, according to a case series in Kurokawa et al. (1990) and Mack (1988). Ototoxicity has been reviewed by Campbell (2006). Further case reports and information are given by Dunsky (1947), Gradus et al. (1984), Kuwahara et al. (1984), and Quick et al. (1975).
Animal studies
The quality of the main animal key studies was evaluated using SciRAP (Science in Risk Assessment and Policy, http://www.scirap.org/), which is a web-based reporting and evaluation tool (Table 9). Several criteria for the evaluation of reliability and relevance of the studies were used, whereby the more precisely the studies were described the higher the scores (given percentage) for reporting and methodological quality (see supplementary SciRAP files) and the lower the total tier (given in Tables 7, 8, 9). The evaluation comprises criteria on the reporting quality, methodological quality und relevance of each study. Each criterion can be marked as fulfilled, partially fulfilled, not fulfilled or not applicable, corresponding to the different color codes in the table. Accordingly, the study by Dodd et al. (2013) is described in the highest detail, whereas Guo et al. (2001) lacked some information.
Acute toxicity studies
Potassium bromate was given to F344 rats, B6C3F mice and Syrian golden hamsters (5/sex/group) as a single intragastric dose, with an observation period of 7 days. In all high dose groups (700–900 mg/kg bw), two thirds of the animals died within 3 h after administration, and the remaining animals died within 48 h. LD50 values were calculated to be 400/495 mg/kg for rats, 280/355 mg/kg for mice and 388/460 mg/kg for hamsters, each for male and female animals, respectively (Kurokawa et al. 1990). In further acute toxicity studies, potassium bromate induced oxidative stress and impaired the antioxidant capabilities in male Wistar rats. The animals were given a single oral dose of 100 mg/kg bw potassium bromate and they were sacrificed 12, 24, 48, 96 and 168 h after treatment. Some changes in blood parameters were observed, indicating the presence of oxidative stress. These effects peaked 48 h after administration, after which recovery took place (Ahmad and Mahmood 2012). In a further study, a single dose of 100 mg/kg bw in male Wistar rats elicited deleterious nephrotoxic effects, including increased reactive oxygen species (ROS) production and induced oxidative stress. After 48 h, the symptoms were reversible (Ahmad et al. 2012). Hassan et al. (2019) treated Swiss albino rats with a single dose of potassium bromate at the sub-lethal dose of 100 mg/kg bw. Extensive toxic effects were reported, such as altered liver function markers, influenced redox status and severe damage of liver tissues characterized by stained granules and vacuoles and a dilated central vein. However, the route of exposure was not stated.
To investigate the occurrence of oxidative stress after potassium bromate exposure, female F344 rats were exposed to 300 mg/kg by a single intragastric injection or to 80 mg/kg by a single intraperitoneal (i.p.) injection. The animals were sacrificed 48 h after administration and 8-oxodeoxyguanosine (8-oxodG) levels in the kidney were measured. The levels were significantly increased when compared to the control groups (Umemura et al. 2004). There were indications of bromate-induced ototoxicity with the cochlea being the primary site of lesion, which might cause irreversible sensorineural hearing loss with unknown incidences. Data from acute exposure studies show corresponding effects at high doses primarily in guinea pigs (Campbell 2006).
The results of these studies indicate that the administration of single sublethal doses of potassium bromate down to 100 mg/kg bw elicited deleterious nephrotoxic and hepatotoxic effects, as well as increased oxidative stress in rats, with some effects being transient. Male mice are the most susceptible sex/species for acute effects, with the lowest LD50 value of 280/355 mg/kg bw for male and female mice, respectively (Kurokawa et al. 1990).
Subacute and subchronic studies
An overview of the subacute, subchronic and chronic animal studies except carcinogenicity studies is given in Table 7.
Factors for converting concentrations of substances in drinking water into a daily dose for rats and mice for subacute, subchronic and chronic study durations were taken from EFSA Scientific Committee (2012).
There is no evidence for central nervous system malformations or brain weight changes in developmental studies (Crofton 2006). However, as in the acute toxicity studies, data from subacute exposure studies showed corresponding effects at high doses in guinea pigs, whereas ototoxicity was the most sensitive effect for which no lowest dose has been established, particularly for long-term low-dose exposure (Campbell 2006).
When exposed to potassium bromate, groups of 10 male and female F344 rats were given potassium bromate for 13 weeks at doses of 150, 300, 600, 1250, 2500, 5000 and 10,000 ppm (13.35/13.95, 26.7/27.9, 53.4/55.8, 111.25/116.25, 222.5/232.5, 445/465, 890/930 mg/kg bw/d for males and females, respectively) via drinking water. All animals given ≥ 2500 ppm died within 7 weeks, body weight gain was decreased in male rats given 600 and 1250 ppm, and significant changes in body weight and clinical chemistry were observed in rats of both sexes at 600 ppm, as well as extensive regenerative changes in the kidneys (Kurokawa et al. 1990). However, the primary literature is not available and the description of the study lacks detailed specifications. A NOAEL was not explicitly given but can be determined to be 300 ppm (26.7/27.9 mg/kg bw/d) from the data provided in the study (Kurokawa et al. 1990).
To further investigate renal effects, male F344 rats were orally exposed via drinking water to 600 ppm (53.4/55.8 mg/kg bw/d for males and females, respectively) potassium bromate for 12 weeks. Four weeks after the initial treatment, eosinophilic bodies were found in renal tubules. However, these deposits were transient and normalized after treatment (Kurokawa et al. 1990). In the following studies, precise information on dosages was not provided (Kurokawa et al. 1990); therefore, no information on doses per kg bodyweight can be given. Rats, dogs and monkeys were fed with bread made from flour treated with potassium bromate. Rats were exposed to flour with 14 and 100 ppm over a period of 10 months and three generations. Histology revealed no pathological alterations, no alterations in reproductive performance and no accumulation of bromide in the tissues. Rats treated with flour containing potassium bromate at levels of ~ 75 ppm for four weeks showed no abnormalities and reproductive performance was comparable with controls. Animals treated with flour containing potassium bromate at 200 ppm for 10 weeks also showed no adverse effects. Three dogs fed with bread made from flour containing up to 200 ppm for 16 days or 76 ppm for 12 weeks and showed no adverse effects.
In addition, three monkeys exposed for 8 weeks to a diet containing 84% bread made from flour containing 75 ppm potassium bromate showed no adverse effects (Ford et al. 1959 as cited in Joint FAO/WHO Expert Committee on Food Additives 1964; Kurokawa et al. 1990).
Male Swiss mice orally exposed to 2 g/L (348 mg/kg bw/d) potassium bromate via drinking water for 2 weeks exhibited oxidative stress and protein oxidative damage in the liver. Elevated plasma transaminase levels, such as AST and ALT, also indicated liver damage, which was accompanied by histological changes e.g. hepatic steatosis, leucocyte infiltration, hepatocyte vacuolization and necrosis (Ben Saad et al. 2016). Swiss Webster mice exposed to up to 100 and 200 mg/kg bw/d for 42 days via gavage showed hematological changes, impaired renal and hepatic histology such as congested central veins and vacuoles and decreased antioxidant capacities (Altoom et al. 2018). Neurotoxic symptoms were described in a study by Ajarem et al. (2016), in which male albino mice were treated via gavage for 42 days with 100 and 200 mg/kg bw/d potassium bromate. All treated animals showed neurobehavioral changes, as well as decreased neurotransmitter levels. They also had reduced brain levels of GSH, accompanied by extensive damage according to the histological sections of the medulla and cerebral cortex of the brains.
Male F344 rats were exposed to potassium bromate via drinking water at 0, 5, 20, 100, 200 or 400 mg/L (0, 0.4, 1.6, 8.1, 16.5 or 34.9 mg/kg bw/d) for 2 or 13 weeks. Increased kidney weights were observed in the highest dose group after exposure for 2 and 13 weeks. In the renal tubules, hyaline droplets were observed at 200 and 400 mg/L after 2 weeks and at 400 mg/L after 13 weeks. For a treatment period of 13 weeks, the NOAEL was determined to be 100 mg/L (8.1 mg/kg bw/d (Dodd et al. 2013)).
To evaluate the immunotoxic potential in female B6C3F1 mice, sodium bromate was administered in the drinking water at doses of 80, 200, 400, 600 and 800 mg/L (15.28, 38.2, 76.4, 114.6, 152.8 mg/kg bw/d) for 28 days. Minimal toxicological and immunotoxic effects were observed. All treated animals revealed significantly increased absolute and relative spleen weights. Some hematological parameters such as MCH or MCHC were slightly decreased in the highest dose group. A dose-related increase in reticulocytes was observed. No further parameters were affected. The number of T cells, B cells, NK cells and macrophages were not altered in any dose group. The suppressive effect of macrophages on the proliferation of B16F10 tumor cells was decreased after exposure to sodium bromate (Guo et al. 2001).
Male and female F344 rats were exposed to 0, 15, 30, 60, 125, 250 or 500 mg/L (0, 1.88/1.82, 3.75/3.63, 7.5/7.26, 15.63/15.13, 31.25/30.25, 62.5/60.5 mg/kg bw/d, respectively) potassium bromate in the drinking water for 4 weeks. In both sexes, the 8-oxodG formation was significantly increased at 250 mg/L and above. Accumulation of α2-macroglobulin in the kidneys of male rats was significantly increased at 125 mg/L and above. These results suggest that DNA oxidation may occur independently of lipid peroxidation and higher levels than 250 mg/L of potassium bromate in the drinking water might exert carcinogenic effects by oxidative stress. The NOAEL was set to 60 mg/L (7.5/7.26 mg/kg bw/d) (Umemura et al. 2004).
In conclusion, potassium bromate was shown to be toxic in repeated dose studies in rats and mice; main targets were kidney, liver, brain and clinical chemistry parameters. The lowest LOAEL of all subchronic and subacute studies was reported in the study of Umemura et al. (2004), with a value of 15.6/15.13 mg/kg bw/d and a corresponding NOAEL of 7.5/7.26 mg/kg bw/d for males and female rats, respectively. At the LOAEL, the kidney was affected, evident as an accumulation of α2u-globulin. These results were supported by the study of Dodd et al. (2013), in which the kidneys of treated animals were affected, with a LOAEL of 16.5 mg/kg bw/d and a NOAEL of 8.1 mg/kg bw/d. However, according to Umemura et al. (2004) and Dodd et al. (2013), male rats were more susceptible to bromate exposure than female rats and rats represented the more sensitive species compared to mice. Therefore, the studies showing the most sensitive results were performed in male rats. Both studies were well performed and, according to the SCiRAP evaluation, the study of Dodd et al. (2013), showed no deficits at all.
Chronic and long-term studies
Mice fed flour containing 15 ppm (1.95/2.51 mg/kg bw/d for males and females) potassium bromate showed no adverse effects over eight generations. Likewise, rats fed for 2 years with flour containing 627 ppm (28.22/36.2 mg/kg bw/d) potassium bromate also showed no significant abnormalities when compared to the controls. However, the description of the study lacks many details and specifications. For example, the sex of the mice is not stated and the dosing regimen and scope of investigation is unclear. Therefore, this study has limited relevance for use in an evaluation (DIN 19643-1:2012-11 2012; Ford et al. 1959 as cited in Joint FAO/WHO Expert Committee on Food Additives (1964)).
In another study, male Wistar rats were exposed to 0.4 g/L (30 mg/kg bw/d) potassium bromate via drinking water for up to 15 months. Body weight gain was markedly inhibited in the treated animals. After 7–11 weeks, histological examination of the kidneys revealed karyopyknotic foci (necrotic changes) in tubules of the inner medulla. At the end of the exposure period, hematology revealed increased blood urea nitrogen and structural abnormalities of the cortical tubules. The LOAEL was set to 30 mg/kg bw/d but no NOAEL was determined (Nakano et al. 1989; as cited in US EPA 2001)).
In a further study, male B6C3F mice were treated with 0, 0.08, 0.4 or 0.8 g/L (0, 8.2, 41.2, 82.4 mg/kg bw/d) for up to 100 weeks and male F344 rats were treated with 0, 0.02, 0.1, 0.2 or 0.4 g/L (0, 1, 5.2, 10.4, 20.8 mg/kg bw/d) via drinking water. In mice, there were no significant differences in survival rate, body weight gain, feed consumption or organ weight observed in any dose group. The only effect observed was significantly decreased water consumption in the top dose group. By contrast, rats exhibited an increased mortality rate at doses of 0.2 and 0.4 g/L. The final mean body weight was significantly decreased; kidney and thyroid weights were significantly increased in the top dose group. Treated rats developed foci of mineralization of the renal papilla, as well as eosinophilic droplets in the proximal tubule epithelium. A dose-dependent increase in urothelial hyperplasia was observed in the renal pelvis at 0.1 g/L and above. Therefore, the NOAEL for non-neoplastic observations in male rats was set at 0.02 g/L (1 mg/kg bw/d) (DeAngelo et al. 1998).
Taken together, long-term studies up to 100 weeks were performed in mice and rats. Since it has been reported that males are more susceptible to bromate-induced effects, only male animals were used in these experiments. A LOAEL was determined in male rats to be 5.2 mg/kg bw/d, with a corresponding NOAEL of 1 mg/kg bw/d (DeAngelo et al. 1998). Adverse effects included the urogenital system, with urothelial hyperplasia observed at the LOAEL, while the testes and kidneys were affected at higher dosages. According to the evaluation by SciRAP, this study was of a high quality. In the study of Narkano et al. (1989), as cited in US EPA (2001)), the urogenital system was also affected, with necrotic changes of the kidneys and structural abnormalities of the cortical tubules in male rats. However, only a single dose of 30 mg bromate/kg bw/d was reported for this study, which is higher than in the study of DeAngelo et al. In the latter study, male mice exhibited no effects up to the highest dose of 82.4 mg/kg bw/d. Therefore, rats clearly seem to be the more susceptible species for chronic effects.
In summary, animal studies for non-cancer effects indicate the urogenital system, especially the kidneys, to be the major target of adverse effects after acute, subchronic and chronic exposure of bromate. Rats appeared to be the more susceptible species, with a NOAEL of 1 mg/kg bw/d via drinking water in the study of (DeAngelo et al. 1998), although there are conflicting results for acute toxicity with LD50 values in mice being reported to be lower than in rats. According to literature data, males are more susceptible than females; therefore, most of the studies were performed in males.
Carcinogenicity studies
The carcinogenicity of bromate was evaluated in various animal studies, mostly in standard 2-year studies with rats. An overview of the following studies is given in Table 8.
Groups of 53 male and 53 female F344 rats were treated with potassium bromate via drinking water at 0, 250 or 500 ppm (0, 12.5, 27.7 mg/kg bw/d in males, 0, 12.5, 25.5 mg/kg bw/d in females) for 110 weeks. At the top dose, significant signs of general toxicity and increased mortality were observed. At 250 and 500 ppm, the incidence of renal cell tumors was significantly increased in both sexes. Mesotheliomas of the peritoneum were increased in male rats in a dose-dependent manner and were significant at 500 ppm (Kurokawa et al. 1983). According to the additional publication of this experiment, the top dose was decreased after week 60 to 400 ppm due to marked decreases in body weight gain (Kurokawa et al. 1986b). There was an increased mortality of male rats in the top dose group. In rats of both sexes, there were incidences of renal cell tumors and, in males, incidences of mesotheliomas of the peritoneum were significantly increased when compared to the control group. In one study with B6C3F mice (50 animals/sex/dose), doses of 500 and 1000 ppm (0, 56.5, 119.8 mg/kg bw/d) were administered via drinking water over 78 weeks (Kurokawa et al. 1986b). There was no significant difference in the incidence of tumors compared to the control group. The mice were sacrificed in week 104. In the top dose group, body weight gain was decreased but no increased mortality was observed in any dose group. Treatment of male mice was, however, discontinued due to highly aggressive behavior.
In 1986, the same authors performed a carcinogenicity study of potassium bromate in 148 male F344 rats divided into 7 groups treated with 0, 15, 30, 60, 125, 250 or 500 ppm (0, 0.9, 1.7, 3.3, 7.3, 16 or 43.4 mg/kg bw/d) via drinking water over a period of 104 weeks. In the top dose group, decreased body weight gain and increased mortality was observed. Combined incidences of renal adenocarcinomas and adenomas were significantly and dose-dependently increased at 125 ppm and above. In the highest dose group, the combined incidences of follicular adenocarcinomas, adenomas of the thyroid, and mesotheliomas of the peritoneum were significantly increased. At 60 ppm and above, dysplastic foci of the kidneys were significantly also increased. These lesions were considered to be pre-neoplastic effects, which gradually develop into neoplasms (Kurokawa et al. 1986a).
The carcinogenicity of potassium bromate was also studied in male B6C3F mice and male F344/N rats. Mice were treated with 0, 0.08, 0.4 or 0.8 g/L (0, 8.2, 41.2, 82.4 mg/kg bw/d) for up to 100 weeks and rats were treated with 0, 0.02, 0.1, 0.2 or 0.4 g/L (0, 1, 5.2, 10.4, 20.8 mg/kg bw/d) via drinking water. A dose-dependent increase in the incidence of mesotheliomas of the tunica vaginalis testis was observed, which was not statistically significant in the lowest dose group but was considered biologically significant. Rats developed renal cell tumors at concentrations of 0.1 g/L and above, which were significantly increased only in the top dose group. Thyroid follicular proliferative lesions, including hyperplasia, adenoma and carcinoma, were observed at 0.02 g/L and above but were significantly increased only in the two top dose groups. The same renal cell tumor observed in rats was also significantly increased in all treated male mice without any dose dependency. There were no other treatment-related increases in benign or malignant neoplasms in any of the examined tissues. However, mesotheliomas of the tunica vaginalis are of questionable relevance for humans and the incidence in the lowest dose group was not significant (DeAngelo et al. 1998).
In a re-evaluation of the rat study by DeAngelo et al. (1998), the time course of the carcinogenic effects of potassium bromate were discussed (Wolf et al. 1998). In addition to the final examination after 100 weeks reported by DeAngelo et al., data on 120 male F344 rats examined after 12, 26, 52, and 78 weeks were reported. Six animals from each group were sacrificed and necropsied at each interim time point. After 52 weeks, renal cell tumors were observed in the top dose group only. Mesotheliomas of the tunica vaginalis were observed after 52 weeks of treatment in both of the highest dose groups. After 78 weeks, mesothelioma were also present in other sites in a decreasing manner in spleen, gastrointestinal tract, mesentery, pancreas, urinary bladder, liver, and rarely in kidney. The incidence of thyroid follicular tumors was increased after 26 weeks at 0.1 g/L but not in a dose-dependent manner. After 100 weeks, even in the lowest dose group of 0.02 g/L, a significantly increased incidence of thyroid tumors was observed. All incidences and numbers of animals for the terminal examination were identical between DeAngelo et al. and Wolf et al., the only discrepancy was the reported incidence of thyroid tumors, for which no explanation was given by Wolf et al. (1998).
In another study, 81 male Fisher 344 rats were treated with a single intragastric administration of 0, 300 and 600 mg/kg potassium bromate and then observed for a period of 87 weeks. In the top dose group, 13.6% of the animals developed renal tumors. By contrast, there were no such tumors in the low dose and control group (Oinuma 1974 as cited in Kurokawa et al. (1990)).
Groups of 27 male mice of B6C3F, BDF and CDF strains treated orally with 0 and 750 ppm (0 and 77 mg/kg bw/d) for 88 weeks revealed no statistically significant differences in growth rate or survival time between the treated and control groups. However, significantly increased incidences of adenoma of the small intestine in CDF mice and significantly increased incidences of adenoma of the liver in B6C3F mice occurred when treated with 750 ppm. Nevertheless, there were no significant differences in the occurrence of adenocarcinoma between treated and control groups (Oinuma 1974 as cited in Kurokawa et al. (1990)). This single dose study is not suitable for a proper assessment of carcinogenicity.
Carcinogenicity of potassium bromate was primarily investigated in Fischer F344 rats, with a few studies being performed in mice. The test substance was predominantly administered via drinking water. In these studies, potassium bromate was shown to be a carcinogen in the rat (kidney, thyroid gland, mesothelium, peritoneum and tunica vaginalis testis) and in mice. However, the mouse studies showed an unclear pattern. In one of the three studies, the kidneys of C6C3F mice were affected at doses of 77.8 mg/kg bw/d (DeAngelo et al. 1998). Kurokawa et al. (1986b) could not find any tumors up to 119.8 mg/kg bw/d in B6C3F mice and Oinuma (1974, as cited in Kurokawa et al. (1990)) reported increased incidences of tumors of the intestine (CDF mice) and the liver (B6C3F mice) at the lowest dose of 60 mg/kg bw/d. According to Umemura and Kurokawa (2006), male rats are the most susceptible species for bromate exposure and only kidney tumors were found in both sexes, while the other tumors were restricted to male animals.
Taken together, studies with male rats were considered as most suitable for the derivation of guidance values. The types of tumors are discussed below.
Modes of action
The toxicity of bromate is related to different MOAs. Generally, genotoxic and non-genotoxic mechanisms can be distinguished from each other. For genotoxicity, mutagenic and clastogenic effects are discussed in the following sections. The non-genotoxic MOAs related to bromate include thyroid and sex hormone imbalance, oxidative stress and alterations in apoptosis. Further references for potential cellular MOAs come from toxicogenomic analyses of potassium bromate. Global transcription analyses of human intestinal epithelial cells treated with 0.42 mM potassium bromate (IC10 value) results in 370 differently expressed genes (DEGs with p ≤ 0:01 and fold change (FC) ≥ 1:2). Biological context analyses using pathway enrichment revealed significantly affected gene ontology (GO) terms in 10 functional categories: inflammation and immune response, cell cycle, cellular processes, chemotaxis, signal transduction, DNA damage and oxidative stress (Procházka et al. 2019), which all reflect possible modes of action.
Genotoxicity
Genotoxicity of bromate was studied in several in vitro and in vivo systems. This included a wide range of studies employing genotoxicity core tests such as micronuclei (MN) formation, sister chromatid exchange (SCE) and chromosomal aberrations (CA). Furthermore, mutagenicity studies were performed in bacteria, cellular systems, as well as in rodents. Indicator tests, such as analyses of oxidative DNA lesions (i.e., mostly 8-oxo-dG) and of DNA strand breaks, serve as biomarkers of exposure and can give additional information on the MOA. An overview of parameters and results of selected studies is given in Table S1 and will be discussed in the following paragraph. A limitation is that none of the studies identified in this publication were conducted according to OECD guidelines. Furthermore, most of the studies fall into category 3 according to Klimisch criteria (Klimisch et al. 1997), mostly because of missing information on the purity of the used bromate or the lack of a positive control in core tests. Where such information was available, studies fell into Klimisch categories 2 or 3. However, it is considered likely that most of the studies that did not report purity of the used bromate nevertheless used chemicals of high purity. Considering the wide range of studies, model systems and endpoints analyzed, in their entirety they give strong evidence for a genotoxic action of bromate without a clear No Observed Genotoxic Effect Level (NOGEL) in the low dose level (see Table S1 In vivo and in vitro data on genotoxicity).
Core tests
Clastogenicity and aneuploidy: analysis of micronuclei formation and chromosomal aberrations
The clastogenic and/or aneugenic properties of bromate were studied in a number of in vitro and in vivo models, in part in parallel to those in which primary DNA damage was analyzed, as reviewed below. Thus, in vitro micronucleus tests were reported in AS52 CHO cells (Ballmaier and Epe 2006), primary cultures of human and rat kidney cells (Robbiano et al. 1999), HepG2 cells (Zhang et al. 2011), human peripheral lymphocytes (Kaya and Topaktaş 2007), and human lymphoblastoid cells (Luan et al. 2007; Platel et al. 2009; Seager et al. 2012). Specifically, Robbiano et al. reported significant induction of micronuclei (MN) in primary rat and human kidney cells at a concentration of 0.56 mM bromate (Robbiano et al. 1999), a concentration that was also found to induce MN in human TK6 cells (Luan et al. 2007). With regards to studies in human cells, Kaya and Topaktas incubated human peripheral blood lymphocytes with 400–550 µg/mL potassium bromate for 24 and 48 h. MN were induced in a concentration-dependent manner at both time points analyzed. The authors also found evidence of increased SCE frequencies and chromosomal aberrations (Kaya and Topaktaş 2007). Of note, potassium bromate-induced the same level of chromosomal aberrations as the positive control, MMC. Platel et al. analyzed MN formation in TK6 cells over a concentration range of 50 µM to 5 mM using different treatment schedules for up to 24 h. Although the authors found some evidence of a threshold concentration of potassium bromate under certain treatment conditions in cells incubated for 24 h, the trend of increase in MN started from 50 µM with significantly increased values at 500 µM and higher. Similarly, Zhang et al. (2011) observed a significant increase in MN formation after treating HepG2 cells with concentrations ≥ 120 µM for 24 h. In another study that analyzed MN formation in human lymphoblastoid AHH1 cells in a low-dose range of 100–800 µM potassium bromate, there was a trend increase in MN at concentrations above 300 µM after a 4-h treatment with potassium bromate followed by a 22-h treatment with cytochalasin B (Seager et al. 2012).
In vivo studies on MN induction were performed in murine red blood cells (Awogi et al. 1992; Allen et al. 2000), bone marrow cells (Dongmei et al. 2015) and spermatids (Allen et al. 2000), as well as in rat red blood cells (Sai et al. 1992a), kidney (Robbiano et al. 1999), liver, stomach, colon and bone marrow cells (Okada et al. 2015). Most of these studies detected significant increases in MN formation in the cell types analyzed. The exceptions included a study using mouse bone marrow cells (Dongmei et al. 2015) and another study by Okada et al., which reported increased MN frequencies in rat stomach and bone marrow but not in liver and colon (Okada et al. 2015). A long-term and low-dose study analyzing male mice treated with ≥ 80 ppm (80 mg/l) potassium bromate in drinking water for 8 and 78 weeks found a significant increase in MN frequencies in erythrocytes after 8 weeks but not in spermatids at the lowest dose tested (Allen et al. 2000). The lowest dose of potassium bromate applied in a single treatment study in mice was 18.8 mg/kg bw (i.p. injection) followed by a 24- to 96-h observation period. Significant induction of MN was observed at 37.5 mg/kg bw after 24 h. No significant induction was evident at the lowest tested concentration of 18.8 mg/kg bw (Awogi et al. 1992).
Sai et al. showed that several antioxidants, such as GSH, Cys, and liposome-coated superoxide effectively prevented MN formation induced by potassium bromate in F344 rat peripheral blood cells (Sai et al. 1992a). The protective role of GSH is probably based on its ability to compete with DNA for reaction with free bromine radicals (Platel et al. 2009).
In conclusion, there is very solid evidence that bromate induces clastogenicity and/or aneuploidy in vitro and in/ex vivo in human and animal cells. It should be noted that most of the studies discussed above do not allow a clear discrimination between clastogenic and aneugenic effects. However, considering the fact that bromate induces oxidative DNA lesions and secondary strand breaks (see below), a clastogenic potential of bromate is very likely. Nevertheless, an aneugenic potential cannot completely be excluded, and may be possible through bromate-induced damage of proteins involved in the regulation of the spindle apparatus. Although there are some indications for a threshold dose/concentration for such effects, the available data do not allow deriving a threshold value for the induction of clastogenicity and aneuploidy according to our assessment.
Mutagenicity studies
Potassium bromate has been reported to be mutagenic at a dose of 3 mg potassium bromate per plate in the Salmonella reverse mutation assay (Ames test) (Ishidate et al. 1984), while in another study, no mutagenic activity of potassium bromate was observed at concentrations up to 600 µg per plate (Dongmei et al. 2015).
Several mutagenicity studies have been performed in cellular systems analyzing mutation frequencies in the Hprt, Tk, or Gpt loci in CHO cells (Speit et al. 1999; Ballmaier and Epe 2006), mouse lymphoma L5178Y cells (Harrington-Brock et al. 2003; Priestley et al. 2010), and human lymphoblastoid cells (Luan et al. 2007; Platel et al. 2011; Seager et al. 2012). All of these studies reported significant increases in mutation frequencies induced by potassium bromate, with the most sensitive response observed by Seager et al., who reported visible increases in mutation frequencies at a concentration of 250 µM potassium bromate after a 24-h treatment (POD in Hockey Stick model 180 µM) (Seager et al. 2012).
The in vivo, mutagenic activity of potassium bromate was analyzed in mice (Arai et al. 2002, 2003; Tsuchiya et al. 2018) and rats (Umemura et al. 2006, 2009; Yamaguchi et al. 2008). Significant increases in mutation frequencies were observed after treatment of animals with 500 ppm potassium bromate in drinking water for several weeks (Umemura et al. 2006; Yamaguchi et al. 2008). In both studies, lower concentrations did not result in significant increases in mutation frequencies.
In conclusion, there is convincing evidence from in vitro and in vivo studies using mammalian cells of a mutagenic potential of bromate. Similar to the results from clastogenicity and aneuploidy studies, the data do not allow the determination of a clear threshold value.
Indicator tests
Analysis of 8-oxo-dG
Levels of 8-oxo-dG as a biomarker of exposure were reported in multiple studies. The analyses were performed via (i) HPLC coupled to UV-detection, electrochemical detection or mass spectrometry, (ii) modified comet assays using the 8-oxo-dG-specific glycosylases FPG or OGG1, (iii) or immunochemical detection using antibodies raised against 8-oxo-dG.
Induction of oxidative DNA damage by bromate in cell-free systems, i.e. treatment of pure DNA, was studied by several groups (Ballmaier and Epe 1995, 2006; Parsons and Chipman 2000; Murata et al. 2001; Kawanishi and Murata 2006). The main type of damage induced by bromate was 8-oxo-dG adduct formation. By contrast, single strand breaks, AP sites, and other base modifications were only formed at minor frequencies (Ballmaier and Epe 1995). The presence of free thiols, such as GSH or other molecules with Cys residues, was necessary for direct damage induction (Murata et al. 2001). Experiments using deuterated water (D2O), as well as different scavenging reagents, such as catalase, SOD, desferoxamine, azide, and tert-butanol, excluded the involvement of hydroxyl radicals or singlet oxygen as the main DNA reactive molecules (Ballmaier and Epe 1995). Instead, the hydroxyl radical scavenger, methional, inhibited the formation of 8-oxo-dG efficiently. Methional not only scavenges hydroxyl radicals but also chemical species with weaker reactivity (Murata et al. 2001). Furthermore, enzymatic reaction or the presence of transition metals were not required for the generation of bromate-induced DNA damage (Ballmaier and Epe 2006). Taken together, these studies led to the conclusion that neither molecular bromine nor ROS are the main DNA-reactive molecules under cell-free conditions. Instead, bromine radicals or oxides appear to be the relevant DNA damaging species (Murata et al. 2001; Ballmaier and Epe 2006). Thus, it is possible that GSH/Cys reduces bromate to BrO2, which in turn oxidizes guanine. Similarly, GSH/Cys can reduce BrO2- and BrO- to BrO and Br*, which may also react with DNA (Murata et al. 2001).
Oxidative DNA damage as a primary marker for DNA damage has been analyzed at various concentrations and treatment schedules in a wide spectrum of cellular models, such as in primary rat kidney cells (Sai et al. 1994; Parsons and Chipman 2000), L1210 mouse leukemia cells and LLC-PK1 porcine kidney cells (Ballmaier and Epe 1995), mouse lymphoma L5178Y cells (Priestley et al. 2010), V79 Chinese hamster lung cells (Speit et al. 1999), AS52 Chinese hamster ovary cells (Ballmaier and Epe 2006), human leukemia HL60 cells (Murata et al. 2001), TK6 human lymphoblastoid cells (Platel et al. 2011), and human HepG2 liver-derived cells (Zhang et al. 2011). Specifically, Sai et al. reported an increase in 8-oxo-dG in rat renal proximal tubule cells after treatment with bromate at ≥ 2 mM and suggested that lipid peroxidation may be involved in the generation of oxidized DNA damage (Sai et al. 1994). Ballmaier and Epe compared damage profiles and 8-oxo-dG levels in L1210 mouse leukemia cells and LLC-PK1 porcine kidney cells after treatment with millimolar concentrations of bromate. The authors observed damage profiles similar to those observed in isolated DNA. In LLC-PK1 cells, which as kidney cells are derived from the target organ of bromate carcinogenicity, 8-oxo-dG levels were twice as high as those in L1210 mouse leukemia cells, which are derived from a non-target organ. However, a straightforward interpretation of this finding appears difficult due to different species origin. Remarkably, under conditions that did not influence cell proliferation, Fpg-sensitive base modifications were quite persistent and were repaired only with moderate efficiency (Ballmaier and Epe 1995). With regard to dose–response and time-course analyses of DNA damage induction, Parson and Chipman reported significant increases in 8-oxo-dG after treating rat kidney epithelial cells with 1.5 mM potassium bromate for 24 h. No increase was observed after short-term treatment of 15 min (Parsons and Chipman 2000). By contrast, Murata et al. observed a significant induction of 8-oxo-dG in human leukemia HL60 cells already 4 h after treatment with ≥ 0.5 mM potassium bromate (Murata et al. 2001). Similarly, Priestly et al. reported significant increases of 8-oxo-dG in mouse lymphoma L5178Y cells already 3 h after treatment at a concentration of 0.5 mM potassium bromate (Priestley et al. 2010). In the latter study, significant removal of 8-oxo-dG lesions was observed after 21 h; however, it is not completely clear if this effect was due to repair or dilution of lesions as a consequences of cell proliferation. In a recent study, using a modified FADU assay, significant induction of Fpg-sensitive sites was observed after treatment of human THP1 macrophage-derived cells for 2 h already at concentrations ≥ 50 µM potassium bromate (Mack et al. 2021).
Consistent with results from cell-free studies, depleting GSH/Cys levels in cells using diethylmaleate (DEM) led to a reduction in DNA damage levels (Ballmaier and Epe 1995; Parsons and Chipman 2000), suggesting a role of free thiols in DNA damage formation also in cellular systems. Such an effect was, however, not observed in in vivo studies (Sai et al. 1992b; Chipman et al. 1998), suggesting different mechanisms of DNA damage formation in whole organisms. In general, a multitude of in vivo studies in mice and rats demonstrated the formation of 8-oxo-dG in several organs after potassium bromate treatment. Studies in rats were performed by Kasai et al. 1987; Sai et al. 1991; Umemura et al. 1995; Chipman et al. 1998; Umemura et al. 1998; Cadenas and Barja 1999; Umemura et al. 2004; Umemura et al. 2009; Kolisetty et al. 2013. There was a significant induction of 8-oxo-dG after single dose i.p. injections at doses of 40–100 mg/kg bw (Sai et al. 1991; Chipman et al. 1998; Cadenas and Barja 1999). There was no increase compared to background levels at 20 mg/kg bw (Sai et al. 1991). When administered via drinking water, increased levels of 8-oxo-dG were observed in kidneys of F344 rats treated for 28 days with bromate doses as low as 46 mg/L in (Kolisetty et al. 2013). However, in most studies, bromate concentrations applied by drinking water were higher, i.e., in the range of 250–2000 mg/L (Umemura et al. 1998, 2004, 2009). Time-course studies demonstrated an increase in 8-oxo-dG levels in kidneys within 24 h after i.p. injections of potassium bromate, followed by a gradual decrease (up to 96 h) (Kasai et al. 1987; Sai et al. 1991). When administered in drinking water (500 mg/L), Umemura et al. observed an increase in 8-oxo-dG levels in male rats within 1 week after the onset of treatment, with levels remaining high during the entire period of bromate exposure. By contrast, in females increases in 8-oxo-dG levels occurred only 3 weeks after the first application of bromate (Umemura et al. 1998). Interestingly, GSH levels were reported to be higher in male than in female rats (Igarashi et al. 1983). Chipman et al. observed a significant twofold increase in in 8-oxo-dG in genomic rat kidney DNA after i.p. injection of bromate (100 mg/kg bw) and a 57% increase in DNA isolated from kidney mitochondria (Chipman et al. 1998). Interestingly, increases in 8-oxo-dG levels were usually higher in kidney than in liver tissue, which is a non-target tissue with regard to bromate-induced carcinogenicity (Kasai et al. 1987; Umemura et al. 1995). Probably as a compensatory mechanism to elevated 8-oxo-dG levels, Delker et al. observed an increase in Ogg1 mRNA expression in kidneys of F344 rats that were treated with 400 mg/L potassium bromate for up to 100 weeks (Delker et al. 2006). Of note, increases in oxidative DNA damage could be prevented or reduced by pre-administration of several antioxidants, such as resveratrol, melatonin, PBN, vitamin E (Cadenas and Barja 1999), as well as sodium ascorbic acid (Umemura et al. 2009). In addition to studies in rats, several drinking water studies were performed in wild-type and genetically modified mice (Arai et al. 2002, 2003, 2006). Increased levels of 8-oxo-dG were observed in kidney, liver and intestine of mice. Peak values of ~ 70-times above background were reported in Ogg1 KO mice treated with 2 g/L potassium bromate in drinking water for 13 weeks. In this study, 8-oxo-dG levels remained constant even 4 weeks after exposure to bromate was terminated. Even though mutation frequencies increased after bromate treatment, in particular in the Ogg1 deficient background (see below) (Arai et al. 2002, 2003), the authors did not find precancerous lesions in kidneys or any other organ after 12 weeks of treatment. In another mouse study, Yokoo et al. observed significantly lower levels of 8-oxo-dG formation in the intestines of Nrf2 KO mice (Yokoo et al. 2016). However, such a difference was not observed in murine kidneys, as reported by Tsuchiya et al. (Tsuchiya et al. 2018).
In conclusion, oxidative DNA damage, such as 8-oxo-dG lesions, appears to be a primary type of damage induced by bromate in vitro and in vivo. The available data support the view of a threshold dose response relationship for 8-oxo-dG formation (Spossova et al. 2015). However, at present, it is not entirely clear if this might be caused by limited sensitivity of the analytical methods used. Thus, it cannot be excluded that, in most of the above-mentioned studies, the reported lack of elevated 8-oxo-dG levels in the low-dose range was due to the induction of 8-oxo-dG below the limit of detection.
Analysis of DNA strand breaks
In addition to oxidative DNA damage, DNA strand breaks were analyzed following bromate treatment in a variety of cell culture models, such as primary rat and human kidney cells (Robbiano et al. 1999), V79 Chinese hamster cells (Speit et al. 1999), mouse lymphoma L5178Y cells (Priestley et al. 2010), human TK6 cells (Luan et al. 2007; Platel et al. 2011), isolated human white blood cells (Parsons and Chipman 2000), and human HepG2 cells (Zhang et al. 2011). Analyses were performed either with a standard alkaline comet assay, detecting DNA single and double strand breaks, as well as a neutral comet assay, preferentially detecting DNA double strand breaks. Some studies directly compared DNA strand break levels measured by the alkaline comet assay to those measured by the modified comet assay using Fpg and/or Ogg1 glycosylases to induce DNA strand breaks after excision of 8-oxo-dG (Speit et al. 1999; Priestley et al. 2010; Platel et al. 2011). Independent of the cell type tested, these studies consistently showed much a stronger increase in DNA strand break formation upon application of Fpg/Ogg1. These results are also consistent with a recent study, which demonstrated the absence of directly induced strand breaks by bromate up to a concentration of 200 µM, while Fpg-sensitive sites were detected already at 50 µM in human THP1 cells (Mack et al. 2021). Taken together, these studies again indicate that bromate mostly induces oxidative DNA damage, which can be converted to DNA strand breaks during base excision repair. This hypothesis is also supported by data from Zhang et al. (2011), which reported the absence of significant DNA strand breaks using the standard comet assay after bromate incubation with HepG2 cells for 40 min, while a significant and dose-dependent increase was obtained after incubation for 1 h, suggesting the occurrence of DNA strand breaks as DNA repair intermediates. At least one study reported the induction of DNA double strand breaks, as measured by the neutral comet assay, after 4 h incubation with 1 mM bromate (Luan et al. 2007). Consistent with the in vitro data from cell cultures, Ahmad et al. (2013) reported DNA strand breaks, analyzed using the alkaline comet assay, in intestines of adult male rats treated with a single oral dose of 100 mg/kg bw potassium bromate. Significant induction of DNA strand breaks was observed 12 h after treatment, with peak values reaching sixfold above background after 48 h. Thereafter, a decline was observed until the last measured time point of 168 h post treatment, suggesting progression of DNA repair.
In conclusion, the available data support the hypothesis that DNA strand breaks are formed as a result of bromate exposure in vitro and in vivo, mainly as secondary damage arising during the repair (or failed repair) of oxidative DNA lesions. Here, modeling of existing data suggests a linear dose–response relationship (Spassova et al. 2015).
General conclusions on the genotoxicity of bromate
As reviewed in the previous sections, there is strong evidence to consider bromate as a genotoxic compound. Bromate-induced clastogenic and aneugenic effects in various in vitro and in vivo assays. Moreover, mutagenic activity has also been demonstrated in vitro and in vivo, and a wealth of data proved oxidative DNA damage and DNA strand break formation upon bromate exposure. The situation becomes more complicated concerning questions, such as: (i) what is the exact underlying MOA of the genotoxicity of bromate, (ii) does a potential threshold dose exist and can it be quantitatively determined, and (iii) is the genotoxicity of bromate the sole contributing factor to its carcinogenic potential or do additional mechanisms play a role?
Concerning the MOA of the genotoxicity of bromate, thiol-dependent bromate reaction products appear to be a major source of primary DNA damage (Bull and Cottruvo 2006). Thus, it was shown in vitro that bromate-mediated oxidative DNA damage can be generated via thiol-dependent reaction products (Parsons and Chipman 2000; Murata et al. 2001; Ballmaier and Epe 2006). For example, Parson and Chipman 2000 concluded that extracellular GSH is protective against bromate-induced DNA damage, yet intracellular GSH actively mediates the genotoxicity of bromate. They assumed that GSH radicals might be involved in DNA damaging mechanisms. Thiols can react with bromate to sulfur-radicals which than can add on carbon–carbon double bonds. Moreover, an alternative and not mutually exclusive MOA for the observed genotoxicity was proposed by Kolisetty et al. (2013) who obtained evidence that bromate influenced apoptosis in the renal tubules in both male and female F344 rats. They hypothesized that suppression of apoptosis may lead to an induction of DNA damage.
Concerning the question if a potential threshold dose for the genotoxicity of bromate may exist, authors of some studies indeed proposed a thresholded dose–response relationship. However, in most cases, solid dose–response data, in particular in the low dose range, are not available. Furthermore, a series of statistical and modeling analyses of selected key genotoxicity studies was published and concluded that the dose–response relationships of bromate were also consistent with low-dose linear models of genotoxicity—at least for endpoints downstream of primary oxidative DNA lesions, i.e., DNA strand breaks, formation of MN and mutation frequencies (Spassova et al. 2013, 2015; Spassova 2019). The authors concluded that the data analyzed do not provide convincing evidence for the presence of a threshold for bromate genotoxicity. However, it should be noted that this conclusion may have been due to data limitations arising from the experimental studies. For example, the highest experimental concentrations not leading to detectable 8-oxo-dG induction originate from Umemura et al. (2006), who did not find a significant effect at 60 and 125 mg/L potassium bromate in drinking water in Gpt delta rats after 13 weeks of exposure. By contrast, the lowest exposure concentration to induce 8-oxo-dG adducts was reported by Kolisetty et al. (2013) who found a significant effect on 8-oxo-dG formation at a concentration of 46 mg/L bromate in drinking water in a 28-day study in F344 rats in both sexes. Interestingly, this roughly corresponds to the BMDL10 values derived for renal cancer in the same rat strain. Yet, in addition to genotoxicity, other mechanisms may also contribute to the carcinogenicity of bromate. In mice, the lowest BMDL10 reported for genotoxicity was 2.4 mg/L (i.e., for MN formation in mouse erythrocytes) (Health Canada 2018). This suggests that mice are more sensitive to genotoxicity induced by bromate than rats, while the carcinogenic potential of bromate in mice appears to be lower, which indicates that, in addition to genotoxicity, other parameters may contribute to the carcinogenic effects in rats (as discussed below).
Taken together, a threshold-like MOA for the genotoxicity of bromate may be possible; however, this cannot currently be assumed with reasonable certainty. In addition, the data do not allow a quantitative estimate of a potentially existing threshold dose. Therefore, we consider bromate as a genotoxic substance without a threshold-like dose–response relationship. At present, it is not completely clear to what extent bromate genotoxicity translates to carcinogenicity potential and if a potential threshold for carcinogenic effects exists (as discussed below).
Non-genotoxic effects
As described by Health Canada (2018), other MOAs of the carcinogenicity of potassium bromate may exist. These MOAs include thyroid and sex hormone imbalance, immunosuppression and alterations of apoptosis (Health Canada 2018). Some recent findings of these MOAs will be discussed in the following paragraphs.
Thyroid hormone imbalance
The sodium iodide symporter (NIS) mediates the transport of iodide into thyroid epithelial cells and therefore accounts for one of the first steps in thyroid hormone synthesis. Both bromate and its stable metabolite, bromide, are substrates of NIS (Eskandari et al. 1997) and thus can be taken up by the thyroid. Consequently, damage by reactive intermediates or NIS inhibition followed by TSH stimulation were proposed as MOAs for the disruption of thyroid hormone homeostasis (Fisher and Bull 2006). Indeed, substrates of NIS, such as thiocyanate or nitrate, have been shown to competitively inhibit iodide uptake by NIS into the thyroid and to inhibit thyroid hormone synthesis. For bromate, significantly decreased T3 and T4 levels and significantly increased TSH levels were observed in male rats at a dose of 20 mg/kg bw given twice a week for four weeks (Khan 2017). Other studies, however, reported diverging effects of bromate on thyroid hormone synthesis. Whereas Wolf et al. (1998) reported decreased T3 levels at all doses investigated (0.02–0.4 g/L drinking water), but no effects on T4 concentrations after 12 weeks of treatment in rats, Dodd et al. (2013) reported unchanged T3 and T4 levels and a significant decrease of TSH only at 20 and 100 mg/L, but not at 5, 200 and 400 mg/L in a subchronic rat study.
Another proposed MOA of bromate in thyroids is direct oxidative damage. Rats administered potassium bromate at a dose of 110 mg/kg bw showed significant induction of lipid peroxidation in homogenates of thyroid glands (Karbownik et al. 2005). Lipid peroxidation accompanied by reduced activities of antioxidant enzymes e.g. catalase (CAT) and superoxide dismutase (SOD) as well as phase II metabolizing enzymes e.g. glutathione-S-transferase (GST) and glutathione reductase (GR), in thyroid tissue from rats was also observed in the study by Khan (2017). In vitro investigations using primary human thyroid cells from three donors treated with subtoxic potassium bromate concentrations from 1.25 to 5 mM showed a significant dose-dependent increase in the frequency of DNA single strand breaks and activation of DNA repair synthesis. In the same study, potassium bromate was administered to rats at a single dose corresponding to 1/2 LD50 which induced a statistically significant degree of DNA fragmentation in the thyroid (Mattioli et al. 2006). These data indicate a relevant uptake of bromate into the thyroid gland where the induction of oxidative stress leads to DNA damage.
In a 66 day study in which male rats were administered 10, 50 and 100 mg/L bromide (Velický et al. (1997)), T4 was decreased on day 16 and day 66 and T3 was decreased on day 66. The observed histopathological changes in the thyroid were in line with an activation of the hypothalamic-pituitary-thyroid axis at all dose levels. However, only a non-significant trend of a TSH increase at the high dose on day 66 was observed. Similarly, in male rats treated with 100, 200 and 400 mg/L for 133 days, histopathological changes in the thyroid suggested an activation of the hypothalamic-pituitary-thyroid axis. Further findings were a decrease of intrathyroidal iodide concentration, albeit without dose dependency, and decreased T4 levels. T3 concentrations in this study were unchanged. An increase in TSH was only observed at 100 mg/L, whereas only a mild decrease was observed at the higher doses (Velický et al. 1998).
Overall, there is evidence for a NIS-mediated uptake of bromate and bromide into the thyroid, although investigations on the disruption of thyroid hormone homeostasis or TSH stimulation by bromate led to conflicting results. The relevance of thyroid hormone homeostasis as a carcinogenic MOA in humans is, however, under discussion (see Sect. 6.1, Meek et al. 2003). Bromate and its metabolites were reported to cause DNA-strand breaks, as well as oxidative stress, in the thyroid gland, which may represent a possible MOA with respect to human relevance and a respective threshold.
Sex hormone imbalance
An altered balance of sex hormones can potentially result in promotion of tumors. Male rats treated for 4 weeks with 20 mg/kg bw potassium bromate showed reduced levels of FSH, LH and testosterone accompanied by reduced levels of antioxidant enzymes, e.g., catalase (CAT), peroxidase (POD), superoxide dismutase (SOD) and phase II metabolizing enzymes, e.g., glutathione reductase (GSR), glutathione peroxidase (GSHpx), GST and GSH (Khan et al. 2012). FSH enhances the production of an androgen-binding protein by the Sertoli cells, which plays an important role in the maintenance of spermatogenesis (Grover et al. 2005). A decrease in epididymal sperm density in potassium bromate-treated rats was observed in a reproductive study (Wolf and Kaiser (1996) as cited in (US EPA 2001)). LH stimulates testosterone production from Leydig cells. Leydig cell hyperplasia could also be a consequence of low testosterone. Leydig cell tumors were observed in rats exposed to potassium bromate, but also occurred in the control group (Health Canada 2018). Taken together, circumstantial evidence of a potential contribution of potassium bromate to sex hormone influenced carcinogenesis has been presented but further studies would be required for clarification.
Alterations in apoptosis
Sodium bromate has been suggested to increase apoptosis, which is followed by a compensatory suppression of apoptosis (Health Canada 2018). This alteration in apoptosis regulation is supported by in vitro data from human renal cells incubated with subtoxic concentrations of potassium bromate. This exposure led to a downregulation of TRAF3, NF-kB and IL1 gene expression, which counteract apoptosis and induce cellular dedifferentiation (Obaidi et al. 2018). Apoptosis suppression allows for survival and replication of cells with DNA damage, increasing the likelihood of renal tumor development (Bull and Cottruvo 2006). However, these mechanisms have not yet been confirmed in vivo.
Derivation of the point of departure for risk assessment
The relevant and sensitive endpoints to be taken into account for human risk assessment are carcinogenicity and chronic renal toxicity as points of departure.
Human relevance of the tumors observed in experimental animals
Potassium bromate was tested for carcinogenicity in hamsters, mice, and in rats. The clearest and most potent carcinogenic effect was evident from the studies performed in rats. All of the experiments in rats were carried out with the Fischer F344 strain. Potassium bromate-induced tumors in the kidney, the mesothelium, and the thyroid. Only kidney tumors were found in both sexes, the other tumors were restricted to male animals. One major site for the mesothelial tumors was the tunica vaginalis of the testis (DeAngelo et al. 1998). Tunica vaginalis mesotheliomas were found already after 52 weeks of treatment and were present at other mesothelial sites only after 78 weeks. This suggested that the primary tumor was the one located in the tunica vaginalis of the testis (Wolf et al. 1998; Crosby et al. 2000). In the kidney and thyroid, the specific tumor types induced were renal cell adenoma/carcinoma and thyroid follicular adenoma/adenocarcinoma, respectively.
For the derivation of human cancer risk estimates from bromate exposure, a decision on which of these tumors are appropriate for assessment is required. Thus, the tumor types induced by potassium bromate in the rat are discussed with regard to their relevance for humans.
Rat thyroid follicular tumors may be induced via several MOAs. One of them is considered not to be relevant for humans, namely UDP glucuronyltransferase (UGT) mediated induction in the absence of genotoxicity (IARC 1999; ECHA 2021). Furthermore, humans are generally considered to be less sensitive with respect to the induction of thyroid follicular tumors (IARC 1999). BMDL10 values for thyroid follicular tumors were derived using BMDS 3.1 and PROAST 67 using the data from (DeAngelo et al. 1998; Wolf et al. 1998) and (Kurokawa et al. 1983; Kurokawa et al. 1986a). The derived BMDL10 values ranged between 69.5 and 369 ppm (see supplementary files). The BMDL10 values for kidney cancer derived from the data were generally lower by a factor of up to sixfold (Table 10). Thus, if the thyroid follicular tumors would be used for risk assessment this would result in lower cancer risk estimates.
Tunica vaginalis mesotheliomas in rat testis were reviewed and evaluated for their relevance to humans (e.g. (Haber et al. 2009; Maronpot et al. 2009, 2016). This tumor type is found nearly exclusively in F344 rats. Mesothelial tumors are generally rare in female F344 rats. In other rat strains, tunica vaginalis mesotheliomas only appear occasionally and mainly after intraperitoneal application. Tunica vaginalis mesotheliomas are very rare in humans and were estimated to appear at a frequency of 2 × 10–7 (Haber et al. 2009). About a third of the cases were reported to be associated with asbestos exposure. With respect to this type of tumor induced by acrylamide, Haber et al. (2009) concluded that for risk assessment, a non-linear dose–response may be adequate due to an assumed non-genotoxic MOA. For tunica vaginalis mesotheliomas in rat testis, this would lead to very low risk estimates. Consequently, it is recommended not to use tunica vaginalis mesotheliomas in rat testis for extrapolation to derive human cancer risks. Considering Maronpot et al. (2016), tunica vaginalis mesotheliomas are most likely secondary to Leydig cell tumors, a common spontaneous tumor in Fischer rats. The key event might be hormone imbalance associated with Leydig cell tumors. Alternatively, mechanical pressure from Leydig cell tumors might implicate the generation of tunica vaginalis mesotheliomas. However, since in the respective rat study (DeAngelo et al 1998). Leydig cell tumors or pre-stages were not described, the mode of action of the observed tunica vaginalis mesotheliomas is unclear. Direct interaction via oxidative stress could be another mode of action plausible for bromate. Thus, human relevance cannot be entirely excluded. Since tunica vaginalis mesotheliomas in testis is very rare in humans and rats may be more sensitive with respect to tunica vaginalis mesothelioma development, this effect is not used as a basis for the BMDL derivation.
Renal cell adenoma and carcinoma induced in male rats caused by protein droplet accumulation containing α2u-globulin in tubule cells are known to be species-specific in the male rat and should not be used for extrapolation to humans (IARC 1999; ECHA 2021). However, in the case of bromate, the issue is more complex. On the one hand, several studies are available showing that there is clear evidence for α2-macroglobulin protein droplet accumulation in tubule cells in potassium bromate-treated male but not in female rats (for review, see (Health Canada 2018)). This would support lack of human relevance of the renal tumors found in male rats. On the other hand, positive genotoxicity (as evident for bromate) is one reason to assign a male renal cell tumor as human-relevant. Renal cell tumors were also induced in female rats (Kurokawa et al. 1983) indicating that another MOA was involved. The tumor incidences were at mostly only slightly higher in male than in female rats in the study performed by Kurokawa et al. (1983). Thus, the MOA of renal tumor induction via the α2-macroglobulin protein droplet accumulation pathway does not seem to be applicable to bromate. It is considered adequate to use the renal cell adenoma and carcinoma to derive human cancer risk estimates for bromate. This approach was also used for risk evaluation in other studies (Anses 2012; RIVM 2014; ECHA 2017a).
Threshold limit derivation based on cancer endpoints
The available data on renal carcinogenicity (Kurokawa et al. 1983; Kurokawa et al. 1986a; DeAngelo et al. 1998; Wolf et al. 1998) were analyzed with BMDS 3.1, PROAST 66.40 (all but Wolf et al. 1998) and PROAST 69 (Wolf et al. 1998) to derive carcinogenicity potency estimates (Table 10). According to the recommendations in the respective guidances, model averaging was performed (EFSA Scientific Committee 2017; US EPA 2020; RIVM 2021). Both BMDS and PROAST are established approaches to model dose–response curves, details on the differences can be found in e.g. Haber et al. (2018) or WHO (2020). Both approaches were used in the standard and recommended settings mirroring the requirements of the respective guidances. BMDL10 values were used as recommended by the modeling results. The details of each single modeling can be found in the supplementary data. The BMDL10 values of BMDS and PROAST were generally found lying numerically quite close together with one exception for the female animals. The latter may be due to the underlying data in the tumor incidence dose-reponse curve. The hBMDL10 was derived by using an allometric factor of 4 and assuming a daily water consumption of 50 mL/kg rat (ECHA 2012, EFSA 2012). The median hBMDL10 (lower 95% confidence limit on the benchmark dose for a 10% human cancer response) derived from the models was 0.65 mg bromate/kg bw/d. This median BMDL10 value is identical to the results of the model with the lowest AIC value (i.e. Kurokawa et al. 1986a). One value of 0.02 mg Bromate/kg bw/d was excluded as an obvious outlier compared to the other model results.
Threshold limit derivation based on non-cancer endpoints
Two subchronic studies (Kurokawa et al. 1990; Dodd et al. 2013) and several chronic studies (Kurokawa et al. 1986a, b; DeAngelo et al. 1998) have demonstrated non-cancer effects in the kidney following oral exposure to bromate. Only the study by DeAngelo et al. (1998) adequately described the dose–response relationship of the non-cancer effects for the derivation of BMDL, NOAEL, or LOAEL values for non-cancer effects. Moreover, this data set proved to be the most sensitive, with a NOAEL of 0.02 g potassium bromate/L (1 mg/kg bw/d).
Assuming a daily water consumption of 50 mL/kg bw for rats (ECHA 2012; EFSA Scientific Committee 2012) and using a default assessment factor of 100, a health-based guidance value to protect from deterministic effects by bromate would be 7.7 µg bromate/kg bw/d, respectively, including the correction from potassium bromate to bromate.
Comparison of the cancer and non-cancer threshold limit derivation
By linear extrapolation, the health-based guidance value to protect from urothelial hyperplasia by bromate with 7.7 µg bromate/kg bw/d is associated with a renal cancer risk of 0.12% (12/10,000). As this would lead to an unacceptable high tumor risk, the mean hBMDL10 of 0.65 mg bromate/kg bw/d for carcinogenicity is taken forward for exposure risk evaluation.
Exposure assessment
To assess the exposure of swimmers to bromate, three routes of exposure were considered, namely oral, inhalation and dermal. Furthermore, different target groups (infants and toddlers, children and adults), exposure scenarios, including recreational and sport-active swimmers and top athletes, were considered in the calculation of pool water uptake for each exposure route. The default parameters for bodyweight and the specific swimming parameters for the duration per day and the frequency per year were taken from Anses (2012). While Anses (2012) assumed a frequency of 48 swimming sessions per year for children, RIVM (2006) assumed a frequency of 104 times per year. In the present risk assessment, the group of children participating in competitive sports, further referred to as ‘sport-active children’ was addressed separately, assuming a frequency of 238 times per year. For another group of children, it seemed reasonable to assume a long-term average frequency of not more than one pool visit per week. It is acknowledged that these values are somewhat arbitrary and may need to be adjusted to other countries with different climate and habits. Schets et al. (2011) reported lower swimming frequencies than other sources. This is probably due to the fact that the population answering the questionnaire included an unknown number of responders of non-swimmers, thus reducing the calculated average frequency. Their data were therefore not further considered. Footnotes of table S2 describe in detail, why other reported values were not considered for our risk assessment.
For exposure assessment exposure to bromate was averaged over a year. As the risk assessment focuses on a chronic adverse effect of carcinogenicity and an additional life-long extra risk for kidney cancer, this was considered to be justified.
As our risk assessment was not intended to be made in the context of the biocide product regulation, the exposure values used in this paper differ in part from the default values taken by ECHA (2007). Nevertheless, for a better possibility to compare both approaches, an additional exposure assessment for the oral exposure based on the default values and boundary conditions given in the framework of the biocide product regulation was added in the supplementum (table S3).
Oral exposure route
Ingestion rates were taken from Dufour et al. (2017) and were derived for sport-active groups from Briggle et al. (1981) and Allen et al. (1982). The daily pool water uptake per kg bw and day was calculated for the different target groups based on the data for bodyweight, swimming duration per day and swimming frequency per year given by Anses (2012) (Table 11).
A larger experimental human-biomonitoring study for cyanuric acid determined the ingestion of swimming pool water by 549 children and adult recreational swimmers (Dufour et al. 2017). It is important to note that swimmers were directed to perform normal swimming activities for approximately 1 h for this study and calculation of the ingestion rate was based on the self-reported data of the minutes spent in the water. Thus, this study does not give any information on the average swimming duration in swimming pools per day, and the ingestion rates were suggested by the authors not to be taken too strictly. Values calculated for children were 23.9 mL/h and for adults 12.4 mL/h (geometric means, Dufour et al. 2017).
For sport-active swimmers, an ingestion rate of about 322 mL/h was reported (Dufour et al. 2017), based on a small study with five competitive swimmers (Briggle et al. 1981; Allen et al. 1982). As the uptake for sport-active children was not investigated (Dufour et al. 2017), we used the data given by Briggle et al. (1981) and Allen et al. (1982). These studies differ in the methods: concentration of cyanuric acid in the swimming pool of 29.9 µg/mL (C(p)) and a swimming period of 2 h were applied in one study (Briggle et al. 1981) and a determination of the amount of cyanuric acid excreted via urine within 24 h for five long-distance swimmers between 9 and 17 years was the basis of the other study (Allen et al. 1982). The mean cyanuric acid excretion was 9.8 mg (A(u)). No information on the cyanuric acid concentration in the pool water and the swimming duration were given in this study (Allen et al. 1982). From the available information, the ingested water volume can be calculated as follows:
V(p) is the ingested water volume from pool water, V(u) is the volume of urine (24 h), C(p) is the concentration of cyanuric acid in pool water, C(u) is the concentration of cyanuric acid in urine (24 h) and A(u) is the amount of cyanuric acid in urine (24 h).
This resulted in an estimated total ingested volume of 328 mL for sport-active swimmers. Considering a 98% recovery rate of cyanuric acid (Allen et al. 1982) the resulting value is 322 mL (Dufour et al. 2017). As this volume refers to the swimming period of 2 h (personal communication, Briggle et al. (1981)), it is divided by two to obtain the ingestion rate per hour, leading to a rate of 161 mL/h (arithmetic mean), the same amount that was calculated previously (Dufour et al. 2006). As ingestion rates were given as geometric means (Dufour et al. 2017), we calculated a geometric mean of 127 mL/h for the sport-active children, based on the same data (single values for each swimmer) (Allen et al. 1982). This average ingestion rate was also used for adults. Nevertheless, it should be considered that, on average, adults might swallow less water than children aged between 9 and 17 years. In conclusion, based on the bodyweight, the range of average daily oral pool water uptake is between 23 µL/kg bw/d for the group of recreational swimming adults and 5915 µL/kg bw/d for adult top athletes.
An overview of other existing values which were checked for their relevance for the further calculation of the average oral water ingestion per day and the reasons for not being further considered for our assessment of the oral exposure are given in Table S2.
To make our data comparable to exposure assessment approaches recommended under the biocide product regulation, in Table S3 water uptake was calculated based on the grouping of swimmers and the corresponding default values for body weight suggested bei ECHA (2007). Indicative exposure values for the ingestion rates based on the same data as above were derived here according to ECHA (2017c): the 75th percentile of the exposure data was used for moderate, the 95th percentile for considerable and the maximum for high data uncertainty. In addition, ConsExpo data for secondary exposure scenario (post-application) was used. Special data for infants and toddlers or sport-active swimmers are not given by ConsExpo. Thus, we used the ConsExpo-data just for the group of the adults. The ConsExpo default value for body weight is 65 kg.
Based on these approaches, the average daily oral pool water uptake would be between 59 µL/kg bw/d for the group of recreational swimming adults and 24,891 µL/kg bw/d for sport-active children of the age 2 to < 6 years and 110 µL/kg bw/d for the group of adults according to ConsExpo as averaged daily exposure. The much higher values are mainly due to the higher exposure values that had to be used due to data uncertainty according to the ECHA (2017c) approach, if the data for the sport-active swimmers of Allen et al. (1982), consisting of only 5 values would be considered with its maximum.
Inhalation exposure route
Bromate is a non-volatile compound (see also the very low estimated Henry coefficient in the supplement S1.1.). Gas-phase inhalation has been shown to contribute up to 5% of the body burden of swimmers for chlorinated acetates, whereas oral uptake contributed 94% (Cardador and Gallego 2011). As chloro-acetates have a much higher Henry coefficient of about 3.50 × 10–7 (m3 × Pa)/mol (Bowden et al. 1998) than bromate (about 2.53 × 10–13 (m3 × Pa)/mol, see S1.2.), it can be assumed that gas-phase inhalation of bromate from pool water is negligible compared to oral uptake.
However, exposure may be possible via aerosol formation. Uptake of disinfection by-products via aerosol formation has been considered of minor importance (ECHA 2017a). In a study on aerosol formation during showering, Zhou et al. (2007) measured an aerosol particle mass concentration of 5000–14,000 µg/m3 inside the shower for hot water (43–44 °C, mass median diameter 6.3–7.5 µm) and 20–100 µg/m3 for cold water (24–25 °C, mass median diameter 2.5–3.1 µm), the latter being much closer to the temperature generally observed in swimming pools. The recommendation of the FINA (Fédération Internationale de Natation) for the temperature of pool water used by competitive swimmers is 25–28 °C (FINA 2020).
Nevertheless, an aerosol concentration in the given range for hot water of 10,000 µg/m3 was used to further calculate the inhalation uptake to be on the safe side.
For hot water, furthermore, an alveolar deposition fraction of about 10% was given by Zhou et al. (2007). Assuming an average aerosol particle mass concentration of 10,000 µg/m3 and an alveolar uptake fraction of 10%, an aerosol particle uptake of 1000 µg per m3 inhaled air (10%), i.e. 1 µL pool water per m3, was derived for further calculations of the inhalation uptake fraction. Exposure scenarios for the inhalation uptake route are summarized in Table 12.
The range of average inhalation daily pool water uptake was between 0.3 nL/kg bw/d for the group of infants and toddlers and 150 nL/kg bw/d for the adult top athletes.
Dermal exposure route
No studies on the dermal uptake of bromate in humans are available. However, the dermal uptake of bromate is expected to be negligible, as bromate occurs in pool water as anion (SCCS 2018). The fraction taken up via skin while swimming can be approximated in the following way: for the dermal route, an exposure of the total body surface has to be assumed, which is 17,500 cm2 for an adult, according to the Scientific Committee on Consumer Safety (SCCS 2018); for children, surface areas according to Phillips et al. (1993) were used. The dermal permeability coefficient Kp of 4.29 × 10–6 cm/min was calculated from data of a dermal absorption study for guinea pig skin (Anderson 1994) (S4). The dermal permeability coefficient, Kp, was derived from the absorption rate through guinea pig skin. Due to the higher density of hair follicles, the transfer through guinea pig skin is expected to be considerably higher than through human skin. Therefore, a possible increased absorption through injured or macerated skin suggested by the studies of Gattu and Maibach (2011) has not been further considered by using an additional extrapolation factor. The average daily dermal ‘pool water exposure’ considered for the calculation of dermal bromate uptake, is calculated as follows:
Dermal permeability coefficient [cm/min) × body surface [cm2] × (minutes of swimming per day [min] × (days of swimming per year [d] / days of a year [d]) / bodyweight [kg].
Data for all exposure groups are given in Table 13.
Amount of pool water considered for bromate exposure via all routes of uptake
The total amount of pool water that was considered for the calculation of maximum acceptable bromate concentrations and the corresponding fractions for each uptake pathway are summarized in Table 14. Swallowing swimming pool water represents the main uptake route (73–98%). The dermal uptake of bromate (2–27%) is less relevant and the uptake of bromate via respiration of aerosol can be neglected in this context as it is far below 1%.
Derivation of cancer risk-related bromate concentrations in swimming pool water for different exposure scenarios and a risk of 1:100,000
Considering a human cancer potency estimate hBMD10 for bromate of 0.65 mg bromate/kg bw/d based on F344 rat renal tumors (see previous chapter, Table 10) and the relevant pool water volume summarized for each uptake pathway (oral, dermal, inhalation), different target groups (infants, children, adults) and exposure scenarios (recreational, sport-active, top athletes) (Table 14) the corresponding bromate concentration in swimming pool water can be calculated for the cancer risk of interest. If, for example, a tolerable maximum additional theoretical lifelong cancer risk of 1:100,000 is addressed, which corresponds to the risk taken as a basis for the derivation of bromate drinking water guidance values by several institutions (World Health Organization 2017; Health Canada 2018; US Environmental Protection Agency, Office of Water 2018; EU 2020), the calculated maximum bromate concentrations that result for pool water would be in the range between 0.011 and 2.096 mg/L bromate (Table 15). It is a wide range mostly because the amount of swallowed water differs widely between the target groups (Table 14).
The data show that among the three target groups of infants (0–1 year), children (2–15 years) and adults referring to the same cancer risk, lower maximum bromate concentrations would be acceptable for children (0.544 mg/L) and infants (0.385 mg/L) than for adults (2.096 mg/L). Between one and two orders of magnitude lower maximum bromate concentrations are derived for sport-active children (0.015 mg/L) as well as for adults (0.044 mg/L). The lowest maximum bromate concentration was calculated for the group of adult top athletes (0.011 mg/L) (Table 15).
In the supplementum (Table S4), cancer risk-related bromate concentrations calculated according to the approaches for exposure assessment (ECHA 2007, 2017c) used under the biocide product regulation for an additional theoretical life-long cancer risk of 1:100,000 and 1,000,000 are given. For a risk of 1:1,000,000 maximum bromate concentrations would be in the range of 0.0004 mg/L for sport-active children (age 6 to < 12 years) and adults (top athletes) and 0.11 mg/L for adults (recreational swimmers).
Discussion
Formation and occurrence of bromate in swimming pool water
National and international standards require the disinfection of pool water to protect swimmers against microbial infections. Due to the disinfection processes, bromate may be produced by accident. As bromide ions may be present in natural freshwater and especially in seawater, any treatment of these waters by chlorine, ozone or hypochlorite is liable to oxidize bromide to bromate. Background levels of bromate in sea water are below 1 µg/L (Chen et al. 2006; Zakaria et al. 2011; Lim and Shin 2012).
Bromate was rarely detected in samples from fresh water pools (Table 2). This is due to the low concentrations of bromide. The high concentration of bromide in sea water may result in the formation of bromate at high levels. However, this is not necessarily the case, since there are several sea water swimming pools with chlorine as the disinfecting agent, where bromate was not detectable, i.e. below 200 µg/L. Considering the relatively high LLOQ, there is no evidence that these samples would comply with a lower threshold value. Other pools exhibit high or even very high concentrations of bromate. In some cases, the source of bromate formation was identified in the treatment process. In these facilities the disinfectant, chlorine, is produced in situ by electrolysis of seawater. Due to the high concentration of bromide in seawater it is impossible to prevent the formation of bromate by electrolysis. This formation would not occur if pure sodium chloride brine would be used as the electrolyte instead of seawater. However, the reason for the high bromate formation rate could not be identified in one seawater pool; one scenario has been monitored where after a complete water exchange concentrations increased from 2.5 to 14.6 mg/L within two months (Table 4).
Several physical and chemical properties influence the final content of bromate in pool water (supplement S3), and it was claimed that by controlling of some of these parameters, the bromate concentration can be kept at or below 100 µg/L when using bromide/ozone treatment (Hoffmann 2015).
Concerning swimming pool water limit values, it has to be considered that especially for seawater the LOQ of 0.1–0.2 mg/L compared to the LOQ of fresh water of ~ 0.02 mg/L is significantly higher than the calculated maximum acceptable bromate concentrations (risk of 1:100,000) for the population group of sport-active swimmers (0.011—0.044 mg/L).
Critical endpoint/critical study
Bromate was shown to induce cancer in F344 rats by two independent research groups (Kurokawa et al. 1990; DeAngelo et al. 1998). There was no clear evidence for carcinogenicity in mice or hamsters (Table 8). In rats, the tumors induced were renal and thyroid follicular adenomas and adenocarcinomas, and mesotheliomas of the tunica vaginalis. The follicular cell tumors of the thyroid and the mesotheliomas were not considered adequate for human cancer risk assessment due to an assumed higher sensitivity of rats compared to humans and for other reasons described above.
Mode of action
Bromate-induced protein droplet accumulation containing α2u-globulin in renal tubule cells of male rats. In general, the α2-macroglobulin induction MOA is considered a reason for renal cell tumor development specifically in male rats. However, bromate caused renal cell tumors in both male and female rats with a similar potency (Kurokawa et al. 1983). Thus, α2-macroglobulin does not seem to contribute significantly to renal cell tumor induction in rats, leaving genotoxicity as the primary MOA in this case. However, alternative MOAs might contribute to bromate-induced carcinogenicity of other tumors. These MOAs include thyroid and sex hormone imbalance, alterations of apoptosis and immunosuppression. Bromate and its metabolites were reported to cause indirect and direct damage to the thyroid gland. Indirect damage includes NIS inhibition followed by TSH stimulation. Direct damage of bromate in thyroids includes oxidative damage followed by DNA-strand breaks. Consequently, there is a relevant uptake of bromate into the thyroid gland leading to cellular damage and disruption of thyroid hormone homeostasis. The induction of DNA strand breaks in combination with compensatory cell proliferation to balance thyroid hormone homeostasis is regarded as a potential MOA for tumors of the thyroid gland. In a similar way the altered balance of sex hormones can potentially result in promotion of tumors. Potassium bromate-treated rats showed clearly reduced levels of FSH, LH and testosterone, accompanied by reduced levels of antioxidant enzymes. These alterations were potential contributors to induction of observed mesotheliomas in the tunica vaginalis of the testis. Further, sodium bromate has been suggested to suppress apoptosis by downregulation of TRAF3, NF-kB and IL1 and to influence macrophage reactivity against tumor cells. Consequently, apoptosis suppression may allow for survival and replication of cells with DNA damage, while additional suppression of the immunological tumor defense allows the survival and progression of mutated cells increasing the likelihood of tumor development.
Cancer potency estimates
The renal cell tumors in rats were used as point of departure for human cancer risk assessment in the present study. The median hBMDL10 value derived was 0.65 mg Bromate/kg bw/d (range 0.16–1.02). BMDS 3.1 and PROAST versions 66.40, 67 and 69 generally provided similar results (Table 10) except for the renal cell tumor in female rats. This was due to the underlying dose–response data. Our modeling results were similar to the hLEDL10 derived by US EPA (2001) and OEHHA (2009), with 0.59 mg bromate/kg bw/d and 0.96 mg bromate/kg bw/d, respectively, and also with the hBMDL10 value of 1 mg bromate/kg bw/d derived by Health Canada (2018), respectively (Table 16).
The derived bromate concentrations in swimming pool water compared to existing regulatory values
Values for bromate in pool water and drinking water proposed by various organizations are given in Table 6. In all cases, as with our approach, carcinogenicity was the key endpoint of concern, and linear extrapolation using a non-threshold approach has mostly been used for the derivation of pool water and drinking water values (supplemental information S5). This implies that bromate is considered to be a genotoxic carcinogen and a substance without effect threshold. By contrast, the German national standard for bromate in pool water (UBA 2014) was derived considering bromate as a carcinogen with an effect threshold resulting in a limit of 2 mg bromate/L that was also set within the DIN 19,643–1:2012–11. Without exception in previous risk assessments as well as in ours, oral uptake was considered the relevant route of exposure to bromate via pool water, while the dermal and inhalation exposure are expected to be negligible when deriving guidance values for bromate in swimming pool water. Our estimated dermal exposure using a permeability coefficient Kp of 4.29E-06 cm/min on the basis of Anderson (1994) (cf. chapter 7.3), with a maximum dermal fraction of 27% of all exposure pathways in case of adults, represents a conservative assumption, since a significantly lower bromate absorption should be assumed for human skin compared to guinea pig skin. Similarly, our estimate of the inhalation exposure to bromate using an average aerosol particle mass concentration of 10,000 µg/m3 for a water temperature of 43–44 °C also represents a conservative value (cf. chapter 7.2).
Naturally, both the dose response assessment of bromate as a genotoxic chemical substance and the exposure assessment have a decisive impact on the result of risk characterization.
Whether an additional carcinogenic lifetime risk of 1:100,000 should be taken as a basis when deriving bromate pool water values, as in most of the existing proposals, is ultimately also a question of risk management in the specific individual case. Although there is no EU legislation setting the 'tolerable' risk level for carcinogens in the society, cancer risk levels of 1:100,000 and 1:1,000,000 could be seen as indicative tolerable risks levels for workers and the general population, respectively (ECHA 2012).
A detailed comparison of the suggested values according to Table 6 and our derived values is challenging partly because the derivation of the specific bromate pool water guidance values is incompletely described. The oral doses based on renal tumors, all attributed to a lifetime cancer risk of 1:100,000, ranging from 0.0143 (Anses 2012) to 0.065 µg/kg bw/d (this study) and are within one order of magnitude. Anses' bromate pool water values and the values we derived (Table 15) are comparable; however, for infants and toddlers, as well as adults (recreational and sport-active swimmers), larger differences with factors of 6.4 (infants/toddlers), 5.6 (adult occasional swimmers), and 4.4 (adult sport-active swimmers), were observed. This can mainly be explained by discrepancies in the assumed ingestion rates (Table 11, Table S2) and the oral body doses (Table 6) but to a lesser extent by differences of the exposure scenarios for the inhalation of pool water aerosol or dermal bromate uptake. Since Anses used the (higher) arithmetic means instead of the geometric means to describe the ingestion rates, these differ from our data by approximately a factor of 2.3 (Dufour et al. 2017). RIVM (2014) has derived bromate pool water guidance values for toddlers, adults, and competitive swimmers, however, without any further differentiation of the individual exposure groups. RIVM (2014) finally set a guidance value of 0.1 mg bromate/L for an extra cancer risk of about 1:100,000 (Table 6) using an oral body dose of 0.05 µg/kg bw/d. The values of RIVM are comparable to those bromate concentrations derived by us, with the exception of the values for adults and sport-active swimmers. Lower values (0.117 mg/L vs. 2.1 mg/L) were derived by RIVM because RIVM based its calculation for adults on parameters for the duration and frequency of swimming taken from data for competitive swimmers as ‘worst case’ for adults in general (Table S2). Additionally, an oral ingestion rate of 50 mL per hour is suggested by RIVM (2016) also used as default value in ConsExpo for the secondary exposure scenario (post-application), which is more than four times higher than the 12 mL per hours derived by Dufour et al. (2017). As it is not completely clear, how RIVM derived the 50 mL per hour and the data of Dufour et al. (2017) is based on human biomonitoring and n = 362 swimmers, it seems reasonable to harmonize the RIVM/ConsExpo default data used under the biocide product regulation with the results of Dufour et al. (2017). Even if indicative exposure values according to ECHA (2007) are derived, the 75th percentile for moderate data uncertainty results still in a lower ingestion rate of 27 mL per hour.
For disinfection by-products like bromate, for which inhalation and dermal exposures are of minor importance, existing drinking water guidance values or standards for bromate are considered to be adequately protective for swimming pool water. The reason is that the ingested amount of water during swimming is lower than 2 L per day over a lifetime, which is the default assumption for the derivation of drinking water limits. Thus, ECHA (2017c) suggested using drinking water limits as a first-tier approach to assess bromate in pool water. Using drinking water limits of bromate as suggested by ECHA to assess chemical substances in pool water would overestimate the health risk. Risk-related bromate drinking water standards associated with the excess cancer risk of 1:100,000 account for 2 µg/L (World Health Organization 2017) or 4 µg/L (Health Canada 2019) (Table 6). A somewhat higher provisional bromate drinking water guideline value and limit value, respectively, have been set by World Health Organization (2017) and EU (2020) with a bromate concentration of 10 µg/L. The World Health Organization (2017) pointed out that their guideline value is provisionally higher because of limitations in available analytical and treatment methods. These health-based bromate drinking water values are lower than recommended maximum bromate concentrations in pool water derived even for the most susceptible exposure group of competitive swimmers, with values of 8.7 µg/L (Anses 2012), 11 µg/L (this study), and 58.5 µg/L (RIVM 2014). If calculations would be based on the indicative exposure values according to ECHA (2007) in biocide product regulation combined with a protective risk level for the general population of 1:1,000,000 even lower tolerable bromate concentrations would result down to 0.3–0.4 µg/L for sport-active children and adult top athletes (Table S4).
Further aspects
Pool water risk assessment covering all disinfection by-products (DIBP) is beyond the scope of this study. A first cursory look revealed that the quantitative differences between bromine-based disinfection versus chlorine-based disinfection almost all concern levels of the bromoform and chloroform. For mixed trihalomethanes, chlorinated pool water shows higher levels than brominated pool water, and the sum of irritating chloro-amines in water and air achieved slightly lower concentrations in case of brominated pool water (Richardson et al. 2010; Hoffmann 2015), perhaps because in the reduction of free chlorine, bromide competes with mono- and dichloramine (Luong et al. 1982). A guidance document addressing DBP was published by the European Chemicals Agency (ECHA 2017a).
It also has to be considered that no extra factor was applied for children, although this is proposed for mutagenic substances, e.g., by Risk Assessment Forum (2005). Furthermore, the derived bromate levels in pool water do not consider possible other sources. Only the allocation to bathing water was considered. There might be other sources such as the drinking water or the use of potassium bromate as an additive in flour (E924) (Johnson 2013), which, although banned in the EU since 1990, is still permitted in other countries, e.g., the USA (Shanmugavel et al. 2020).
Conclusion
There is convincing evidence from a multitude of studies that bromate induces oxidative DNA damage and acts as a clastogen in vitro and in vivo. Since statistical modeling of the available genotoxicity data is compatible with both linear and non-linear dose–response relationships, bromate should be considered as a non-threshold carcinogen. A threshold-like MOA for the genotoxicity of bromate may be possible; however, it can currently not be assumed with reasonable certainty. In addition, the data do not allow the calculation of a quantitative estimate of a potential existing threshold dose. BMD modeling with model averaging for renal cancer studies (Kurokawa et al. 1983; Kurokawa et al. 1986a; DeAngelo et al. 1998) resulted in a median hBMDL10 of 0.65 mg bromate/kg bw /day.
Evaluation in different age and activity groups revealed that top athletes had the highest exposure, followed by sport-active children, sport-active adults, infants and toddlers, children and adults.
The predominant route of exposure was oral (73–98%), by swallowing water, followed by the dermal route (2–27%); whereas the inhalation route was insignificant (< 0.5%).
Accepting the same risk level for all population group results in different guidance values due to the large variation in exposure. For an additional risk of 1:100,000, the bromate concentrations would range between 0.011 for top athletes, 0.015 for sport-active children and 2.1 mg/L for adults and 0.385 to 0.544 mg/L for non-sport active children including toddlers and infants.
Notes
The equations shown are a simplification; in aqueous solutions, the ions Br−, Br3−, OBr− and BrO3− result from/in dis- and com-proportionation reactions of Br2. At low pH, compropotionation is favored, whereas at higher (alkaline) pH values the disproportionation predominates. The reaction may oscillate around the equilibrium, and the bromate-bromide system is a model for oscillating reactions (p.e. Zaikin and Zhabotinsky 1970; Toporek et al. 2015).
References
Ahmad MK, Mahmood R (2012) Oral administration of potassium bromate, a major water disinfection by-product, induces oxidative stress and impairs the antioxidant power of rat blood. Chemosphere 87:750–756. https://doi.org/10.1016/j.chemosphere.2011.12.073 (Epub 2012 Jan 25)
Ahmad MK, Naqshbandi A, Fareed M, Mahmood R (2012) Oral administration of a nephrotoxic dose of potassium bromate, a food additive, alters renal redox and metabolic status and inhibits brush border membrane enzymes in rats. Food Chem 134:980–985. https://doi.org/10.1016/j.foodchem.2012.03.004 (Epub 2012 Mar 7)
Ahmad MK, Zubair H, Mahmood R (2013) DNA damage and DNA-protein cross-linking induced in rat intestine by the water disinfection by-product potassium bromate. Chemosphere 91:1221–1224. https://doi.org/10.1016/j.chemosphere.2013.01.008 (Epub 2013 Feb 4)
Ajarem J, Altoom NG, Allam AA, Maodaa SN, Abdel-Maksoud MA, Chow BK (2016) Oral administration of potassium bromate induces neurobehavioral changes, alters cerebral neurotransmitters level and impairs brain tissue of swiss mice. Behav Brain Funct 12:14. https://doi.org/10.1186/s12993-016-0098-8 (Epub 2016 May 12)
Allen LM, Briggle TV, Pfaffenberger CD (1982) Absorption and excretion of cyanuric acid in long-distance swimmers. Drug Metab Rev 13:499–516. https://doi.org/10.3109/03602538209029992
Allen JW, Collins BW, Lori A, Afshari AJ, George MH, DeAngelo AB, Fuscoe JC (2000) Erythrocyte and spermatid micronucleus analyses in mice chronically exposed to potassium bromate in drinking water. Environ Mol Mutagen 36:250–253. https://doi.org/10.1002/1098-2280(2000)36:3%3c250:aid-em9%3e3.0.co;2-6
Altoom NG, Ajarem J, Allam AA, Maodaa SN, Abdel-Maksoud MA (2018) Deleterious effects of potassium bromate administration on renal and hepatic tissues of Swiss mice. Saudi J Biol Sci 25:278–284. https://doi.org/10.1016/j.sjbs.2017.01.060 (Epub 2017 Jan 31)
Anderson FA (1994) Final report on the safety assessment of sodium bromate and potassium bromate. J Am Coll Toxicol 13:400–414. https://doi.org/10.3109/10915819409140615
Anses (2012) Évaluation des risques sanitaires liés aux piscines. Partie I: piscines réglementées: Avis de l’Afsset Rapport d’expertise collective. https://www.anses.fr/fr/content/avis-et-rapport-de-lanses-relatif-%C3%A0-levaluation-des-risques-sanitaires-li%C3%A9s-aux-piscines-0 (accessed 28.1.2022)
Arai T, Kelly VP, Minowa O, Noda T, Nishimura S (2002) High accumulation of oxidative DNA damage, 8-hydroxyguanine, in Mmh/Ogg1 deficient mice by chronic oxidative stress. Carcinogenesis 23:2005–2010. https://doi.org/10.1093/carcin/23.12.2005
Arai T, Kelly VP, Komoro K, Minowa O, Noda T, Nishimura S (2003) Cell proliferation in liver of Mmh/Ogg1-deficient mice enhances mutation frequency because of the presence of 8-hydroxyguanine in DNA. Cancer Res 63:4287–4292
Arai T, Kelly VP, Minowa O, Noda T, Nishimura S (2006) The study using wild-type and Ogg1 knockout mice exposed to potassium bromate shows no tumor induction despite an extensive accumulation of 8-hydroxyguanine in kidney DNA. Toxicology 221:179–186. https://doi.org/10.1016/j.tox.2006.01.004 (Epub 2006 Feb 21)
Arvai A, Jasim S, Biswas N (2012) Bromate formation in ozone and advanced oxidation processes. Ozone 34:325–333. https://doi.org/10.1080/01919512.2012.713834
Awogi T, Murata K, Uejima M, Kuwahara T, Asanami S, Shimono K, Morita T (1992) Induction of micronucleated reticulocytes by potassium bromate and potassium chromate in CD-1 male mice. Mutat Res 278:181–185. https://doi.org/10.1016/0165-1218(92)90231-n
Ballmaier D, Epe B (1995) Oxidative DNA damage induced by potassium bromate under cell-free conditions and in mammalian cells. Carcinogenesis 16:335–342. https://doi.org/10.1093/carcin/16.2.335
Ballmaier D, Epe B (2006) DNA damage by bromate: mechanism and consequences. Toxicology 221:166–171. https://doi.org/10.1016/j.tox.2006.01.009 (Epub 2006 Feb 21)
Ben Saad H, Driss D, Ben Amara I, Boudawara O, Boudawara T, Ellouz Chaabouni S, Mounir Zeghal K, Hakim A (2016) Altered hepatic mRNA expression of immune response-associated DNA damage in mice liver induced by potassium bromate: Protective role of vanillin. Environ Toxicol 31:1796–1807. https://doi.org/10.1002/tox.22181 (Epub 2015 Aug 21)
Beronius A, Molander L, Zilliacus J, Rudén C, Hanberg A (2018) Testing and refining the Science in Risk Assessment and Policy (SciRAP) web-based platform for evaluating the reliability and relevance of in vivo toxicity studies. J Appl Toxicol 38:1460–1470. https://doi.org/10.1002/jat.3648
Binetti R, Attias L (2007) Sodium hypochlorite: risk assessment report. European Union Risk Assessment Report. https://echa.europa.eu/documents/10162/330fee6d-3220-4db1-add3-3df9bbc2e5e5 (accessed 8.12.2021)
Briggle TV, Allen LM, Duncan RC, Pfaffenberger CD (1981) High performance liquid chromatographic determination of cyanuric acid in human urine and pool water. J Assoc off Anal Chem 64:1222–1226
Bull RJ, Cottruvo JA (2006) Research strategy for developing key information on bromate’s mode of action. Toxicology 221:135–144. https://doi.org/10.1016/j.tox.2005.10.007 (Epub 2005 Nov 17)
Bull RJ, Kolisetty N, Zhang X, Muralidhara S, Quiñones O, Lim KY, Guo Z, Cotruvo JA, Fisher JW, Yang X et al (2012) Absorption and disposition of bromate in F344 rats. Toxicology 300:83–91. https://doi.org/10.1016/j.tox.2012.06.002 (Epub 2012 Jun 12)
Bundesministerium für Gesundheit, Umweltbundesamt (2018) Bericht des Bundesministeriums für Gesundheit und des Umweltbundesamtes an die Verbraucherinnen und Verbraucher über die Qualität von Wasser für den menschlichen Gebrauch (Trinkwasser)* in Deutschland (2014–2016): gemäß § 21 Trinkwasserverordnung
Cadenas S, Barja G (1999) Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBr O3. Free Radic Biol Med 26:1531–1537. https://doi.org/10.1016/s0891-5849(99)00019-2
Campbell KCM (2006) Bromate-induced ototoxicity. Toxicology 221:205–211. https://doi.org/10.1016/j.tox.2005.12.015 (Epub 2006 Feb 7)
Campbell JL, Bull RJ, Clewell HJ (2019) Development of a rat and human PBPK model for bromate and estimation of human equivalent concentrations in drinking water. Int J Environ Health Res. https://doi.org/10.1080/09603123.2019.1702628 (Epub 2019 Dec 18)
Cardador MJ, Gallego M (2011) Haloacetic acids in swimming pools: swimmer and worker exposure. Environ Sci Technol 45:5783–5790. https://doi.org/10.1021/es103959d (Epub 2011 Jun 7)
Chen ZL, Megharaj M, Naidu R (2006) Determination of bromate and bromide in seawater by ion chromatography, with an ammonium salt solution as mobile phase, and inductively coupled plasma mass spectrometry. Chroma 65:115–118. https://doi.org/10.1365/s10337-006-0128-z
Chipman JK, Davies JE, Parsons JL, Nair J, O’Neill G, Fawell JK (1998) DNA oxidation by potassium bromate; a direct mechanism or linked to lipid peroxidation? Toxicology 126:93–102. https://doi.org/10.1016/s0300-483x(97)00174-1
Cotruvo JA, Keith JD, Bull RJ, Pacey GE, Gordon G (2010) Bromate reduction in simulated gastric juice. J Am Water Works Assoc 102:77–86. https://doi.org/10.1002/j.1551-8833.2010.tb11341.x
Crofton KM (2006) Bromate: concern for developmental neurotoxicity? Toxicology 221:212–216. https://doi.org/10.1016/j.tox.2006.01.021 (Epub 2006 Mar 3)
Crosby LM, Morgan KT, Gaskill B, Wolf DC, DeAngelo AB (2000) Origin and distribution of potassium bromate-induced testicular and peritoneal mesotheliomas in rats. Toxicol Pathol 28:253–266. https://doi.org/10.1177/019262330002800205
DeAngelo AB, George MH, Kilburn SR, Moore TM, Wolf DC (1998) Carcinogenicity of potassium bromate administered in the drinking water to male B6C3F1 mice and F344/N rats. Toxicol Pathol 26:587–594. https://doi.org/10.1177/019262339802600501
Delker D, Hatch G, Allen J, Crissman B, George M, Geter D, Kilburn S, Moore T, Nelson G, Roop B et al (2006) Molecular biomarkers of oxidative stress associated with bromate carcinogenicity. Toxicology 221:158–165. https://doi.org/10.1016/j.tox.2005.12.011 (Epub 2006 Jan 27)
DIN 19643–1:2012–11 (2012) Aufbereitung von Schwimm- und Badebeckenwasser_- Teil_1: Allgemeine Anforderungen. Berlin: Beuth Verlag GmbH (19643–1). [updated 2012]. https://doi.org/10.31030/1916007
Dodd DE, Layko DK, Cantwell KE, Willson GA, Thomas RS (2013) Subchronic toxicity evaluation of potassium bromate in Fischer 344 rats. Environ Toxicol Pharmacol 36:1227–1234. https://doi.org/10.1016/j.etap.2013.10.005 (Epub 2013 Oct 19)
Dongmei L, Zhiwei W, Qi Z, Fuyi C, Yujuan S, Xiaodong L (2015) Drinking water toxicity study of the environmental contaminant–Bromate. Regul Toxicol Pharmacol 73:802–810. https://doi.org/10.1016/j.yrtph.2015.10.015 (Epub 2015 Oct 22)
Donzé G, Vonarburg UP, von Wartburg U (2012) Chlorate et bromate dans les piscines, évaluation de la situation et mesures à prendre pour minimiser ces polluants. Eur J Water Qual 43:53–74. https://doi.org/10.1051/wqual/2013009
Dufour AP, Evans O, Behymer TD, Cantú R (2006) Water ingestion during swimming activities in a pool: a pilot study. J Water Health 4:425–430
Dufour AP, Behymer TD, Cantú R, Magnuson M, Wymer LJ (2017) Ingestion of swimming pool water by recreational swimmers. J Water Health 15:429–437. https://doi.org/10.2166/wh.2017.255
Dunsky I (1947) Potassium bromate poisoning. Am J Dis Child 74:730–734. https://doi.org/10.1001/archpedi.1947.02030010748011
ECHA (2007) Default human factor values for use in exposure assessments for biocidal products. Recommendation no. 14 of the BPC Ad hoc Working Group on Human Exposure. 8 p
ECHA (2010) Potassium bromate—registration dossier. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/12144/1/2. (accessed 28.1.2022)
ECHA (2012) Guidance on information requirements and chemical safety assessment: Chapter R.8: Characterisation of dose [concentration]-response for human health [Version 2.1]. https://echa.europa.eu/documents/10162/13632/information_requirements_r8_en.pdf/e153243a-03f0-44c5-8808-88af66223258. (accessed 8.12.2021)
ECHA (2017a) Guidance on the biocidal products regulation. Volume V, Guidance on Disinfection By-Products [Version 1.0] 79 p
ECHA (2017b) Guidance on the biocidal products regulation. Volume III, Human Health—Assessment & Evaluation (Parts B+C). Version 4.0. https://echa.europa.eu/documents/10162/2324906/biocides_guidance_human_health_ra_iii_part_bc_en.pdf/30d53d7d-9723-7db4-357a-ca68739f5094 (accessed 29.1.2022)
ECHA (2017c) Human exposure to biocidal products. Technical notes for guidance (TNsG). 102 p
ECHA (2019) Sodium bromate—registration dossier. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14239/7/7/2. (accessed 28.1.2022)
ECHA (2021) Guidance on labelling and packaging in accordance with Regulation (EC) No 1272/2008: Version 4.2. https://doi.org/10.2823/697587
[EDI] Eidgenössisches Departement des Innern (2016) Verordnung des EDI über Trinkwasser sowie Wasser in öffentlich zugänglichen Bädern und Duschanlagen (TBDV), Annex 7: Höchstkonzentrationen von Schadstoffen und bei der Desinfektion anfallenden Nebenprodukten für Badewasser. (817.022.11). 2016 Dec 16; [updated 2016 Dec 16]. https://www.gesetze.ch/inhm/inhsub817.022.11.htm. (accessed 28.1.2022)
EFSA Scientific Committee (2012) Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFS2. 10. https://doi.org/10.2903/j.efsa.2012.2579.
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert-Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G, Schlatter JR (2017) Update: use of the benchmark dose approach in risk assessment. EFSA J 15(1):e04658. https://doi.org/10.2903/j.efsa.2017.4658
Environment Canada and Health Canada (2010) Screening assessment for the Challenge: Bromic acid, potassium salt (potassium bromate): Chemical Abstracts Service Registry Number 7758–01–2. https://www.ec.gc.ca/ese-ees/47CCC26F-88C5-40E4-8C3D-FE88049DC9C7/batch9_7758-01-2_en.pdf (accessed 8.12.2021)
Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N (1997) Thyroid Na+/I- symporter. Mechanism, stoichiometry, and specificity. J Biol Chem 272:27230–27238. https://doi.org/10.1074/jbc.272.43.27230
[EU] European Union (2020) Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption
Fang L, Zhu Q, Xu JH (2014) The influence of bromate formation by chlorination in drinking water. AMM 675–677:951–954. https://doi.org/10.4028/www.scientific.net/AMM.675-677.951
Felter SP, Carr AN, Zhu T, Kirsch T, Niu G (2017) Safety evaluation for ingredients used in baby care products: consideration of diaper rash. Regul Toxicol Pharmacol 90:214–221. https://doi.org/10.1016/j.yrtph.2017.09.011 (Epub 2017 Sep 12)
FINA (Fédération Internationale de Natation) (2020) FINA facilities rules 2017–2021. https://resources.fina.org/fina/document/2021/01/19/da8798f0-e89f-4e8c-9e21-5452c16a55dd/fina_swimming_pool_certificate_guide_february_2020_1.pdf (accessed 28.1.2022)
Fisher J, Bull RJ (2006) Development of a rat dosimetry model for bromate. Toxicology 221:235–240. https://doi.org/10.1016/j.tox.2006.01.022 (Epub 2006 Feb 24)
Fujii M, Oikawa K, Saito H, Fukuhara C, Onosaka S, Tanaka K (1984) Metabolism of potassium bromate in rats I. In vivo studies. Chemosphere 13:1207–1212. https://doi.org/10.1016/0045-6535(84)90120-6
Gattu S, Maibach HI (2010) Enhanced absorption through damaged skin: an overview of the in vitro human model. Skin Pharmacol Physiol. 23:171–176. https://doi.org/10.1159/000288163 (Epub 2010 Feb 25)
Gattu S, Maibach HI (2011) Modest but increased penetration through damaged skin: an overview of the in vivo human model. Skin Pharmacol Physiol 24:2–9. https://doi.org/10.1159/000314995 (Epub 2010 Jun 30)
Gradus D, Rhoads M, Bergstrom LB, Jordan SC (1984) Acute bromate poisoning associated with renal failure and deafness presenting as hemolytic uremic syndrome. Am J Nephrol 4:188–191. https://doi.org/10.1159/000166804
Grover A, Smith CE, Gregory M, Cyr DG, Sairam MR, Hermo L (2005) Effects of FSH receptor deletion on epididymal tubules and sperm morphology, numbers, and motility. Mol Reprod Dev 72:135–144. https://doi.org/10.1002/mrd.20303
von Gunten U (2003) Ozonation of drinking water: part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res 37:1469–1487. https://doi.org/10.1016/S0043-1354(02)00458-X
von Gunten U, Hoigne J (1994) Bromate formation during ozonization of bromide-containing waters: interaction of ozone and hydroxyl radical reactions. Environ Sci Technol 28:1234–1242. https://doi.org/10.1021/es00056a009
Guo TL, McCay JA, Karrow NA, Brown RD, Musgrove DL, Luebke RW, Germolec DR, White KL (2001) Immunotoxicity of sodium bromate in female B6C3F1 mice: a 28-day drinking water study. Drug Chem Toxicol 24:129–149. https://doi.org/10.1081/DCT-100102606
Haag WR, Hoigne J (1983) Ozonation of bromide-containing waters: kinetics of formation of hypobromous acid and bromate. Environ Sci Technol 17:261–267. https://doi.org/10.1021/es00111a004
Haber LT, Maier A, Kroner OL, Kohrman MJ (2009) Evaluation of human relevance and mode of action for tunica vaginalis mesotheliomas resulting from oral exposure to acrylamide. Regul Toxicol Pharmacol 53:134–149. https://doi.org/10.1016/j.yrtph.2008.12.008 (Epub 2008 Dec 31)
Harrington-Brock K, Collard DD, Chen T (2003) Bromate induces loss of heterozygosity in the thymidine kinase gene of L5178Y/Tk(+/-)-3.7.2C mouse lymphoma cells. Mutat Res 537:21–28. https://doi.org/10.1016/s1383-5718(03)00044-5
Health Canada (2018) Guidelines for canadian drinking water quality: guideline technical document — bromate. www.canada.ca/content/dam/hc-sc/documents/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-bromate/bromate-2018-final-eng.pdf. (accessed 28.1.2022)
Health Canada (2019) Guidelines for Canadian drinking water quality: guideline technical document: manganese. Ottawa, ON: Health Canada = Santé Canada. 107 p. ISBN: 9780660074962. www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/water-eau/sum_guide-res_recom/sum_guide-res_recom-eng.pdf. (accessed 28.1.2022)
Hoffmann M (2015) Ozone-bromine treatment – water treatment in public pools without chlorine: a new standard? Ozone 37:456–466. https://doi.org/10.1080/01919512.2015.1053014
Huang X, Gao N, Deng Y (2008) Bromate ion formation in dark chlorination and ultraviolet/chlorination processes for bromide-containing water. J Environ Sci (china) 20:246–251. https://doi.org/10.1016/s1001-0742(08)60038-8
IARC (1999) Potassium bromate. IARC monographs on the evaluation of carcinogenic risks to humans. 73:481–496. https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono73.pdf (accessed 9.12.2021)
IfSG (Infection Protection Act) (2000) Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (Infektionsschutzgesetz - IfSG)). https://www.gesetze-im-internet.de/ifsg/IfSG.pdf (accessed 18.12.2021)
Igarashi T, Satoh T, Ueno K, Kitagawa H (1983) Sex-related difference in the hepatic glutathione level and related enzyme activities in rat. J Biochem 93:33–36. https://doi.org/10.1093/oxfordjournals.jbchem.a134174
Ishidate M, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M, Matsuoka A (1984) Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol 22:623–636. https://doi.org/10.1016/0278-6915(84)90271-0
Johnson OR (2013) Analysis of potassium bromate and hydrocyanic acid contents of commonly consumed loaves of bread and wheat flour samples In Karu, Nasarawa State, Nigeria. IOSR-JESTFT 6:42–46. https://doi.org/10.9790/2402-0614246
Joint FAO/WHO Expert Committee on Food Additives (1964) Specifications for the identity and purity of food additives and their toxicological evaluation: emulsifiers, stabilizers, bleaching and maturing agents, seventh report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization technical report series. https://apps.who.int/iris/handle/10665/40595
Karbownik M, Stasiak M, Zasada K, Zygmunt A, Lewinski A (2005) Comparison of potential protective effects of melatonin, indole-3-propionic acid, and propylthiouracil against lipid peroxidation caused by potassium bromate in the thyroid gland. J Cell Biochem 95:131–138. https://doi.org/10.1002/jcb.20404
Kasai H, Nishimura S, Kurokawa Y, Hayashi Y (1987) Oral administration of the renal carcinogen, potassium bromate, specifically produces 8-hydroxydeoxyguanosine in rat target organ DNA. Carcinogenesis 8:1959–1961. https://doi.org/10.1093/carcin/8.12.1959
Kawanishi S, Murata M (2006) Mechanism of DNA damage induced by bromate differs from general types of oxidative stress. Toxicology 221:172–178. https://doi.org/10.1016/j.tox.2006.01.002 (Epub 2006 Feb 2)
Kaya FF, Topaktaş M (2007) Genotoxic effects of potassium bromate on human peripheral lymphocytes in vitro. Mutat Res 626:48–52. https://doi.org/10.1016/j.mrgentox.2006.08.006 (Epub 2006 Nov 22)
Keith JD, Pacey GE, Cotruvo JA, Gordon G (2006) Experimental results from the reaction of bromate ion with synthetic and real gastric juices. Toxicology 221:225–228. https://doi.org/10.1016/j.tox.2006.02.003 (Epub 2006 Mar 10)
Khan RA (2017) Effect of Launaea procumbens on thyroid glands lipid peroxidation and hormonal dysfunction: a randomized control trial. Lipids Health Dis 16:168. https://doi.org/10.1186/s12944-017-0557-8 (Epub 2017 Sep 11)
Khan RA, Khan MR, Sahreen S (2012) Protective effects of Sonchus asper against KBr O3 induced lipid peroxidation in rats. Lipids Health Dis 11:164. https://doi.org/10.1186/1476-511X-11-164 (Epub 2012 Nov 27)
Klimisch HJ, Andreae M, Tillmann U (1997) A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol 25:1–5. https://doi.org/10.1006/rtph.1996.1076
Kolisetty N, Bull RJ, Muralidhara S, Costyn LJ, Delker DA, Guo Z, Cotruvo JA, Fisher JW, Cummings BS (2013) Association of brominated proteins and changes in protein expression in the rat kidney with subcarcinogenic to carcinogenic doses of bromate. Toxicol Appl Pharmacol 272:391–398. https://doi.org/10.1016/j.taap.2013.06.018 (Epub 2013 Jun 26)
Kurokawa Y, Hayashi Y, Maekawa A, Takahashi M, Kokubo T, Odashima S (1983) Carcinogenicity of potassium bromate administered orally to F344 rats. J Natl Cancer Inst 71:965–972
Kurokawa Y, Aoki S, Matsushima Y, Takamura N, Imazawa T, Hayashi Y (1986a) Dose-response studies on the carcinogenicity of potassium bromate in F344 rats after long-term oral administration. J Natl Cancer Inst 77:977–982
Kurokawa Y, Takayama S, Konishi Y, Hiasa Y, Asahina S, Takahashi M, Maekawa A, Hayashi Y (1986b) Long-term in vivo carcinogenicity tests of potassium bromate, sodium hypochlorite, and sodium chlorite conducted in Japan. Environ Health Perspect 69:221–235. https://doi.org/10.1289/ehp.8669221
Kurokawa Y, Matsushima Y, Takamura N, Imazawa T, Hayashi Y (1987) Relationship between the duration of treatment and the incidence of renal cell tumors in male F344 rats administered potassium bromate. Jpn J Cancer Res 78:358–364
Kurokawa Y, Maekawa A, Takahashi M, Hayashi Y (1990) Toxicity and carcinogenicity of potassium bromate—a new renal carcinogen. Environ Health Perspect 87:309–335. https://doi.org/10.1289/ehp.9087309
Kuwahara T, Ikehara Y, Kanatsu K, Doi T, Nagai H, Nakayashiki H, Tamura T, Kawai C (1984) 2 cases of potassium bromate poisoning requiring long-term hemodialysis therapy for irreversible tubular damage. Nephron 37:278–280. https://doi.org/10.1159/000183265
Lichtenberg R, Zeller WP, Gatson R, Morrison Hurley R (1989) Bromate poisoning. J Pediatr 114:891–894. https://doi.org/10.1016/s0022-3476(89)80160-x
Lim H-H, Shin H-S (2012) Sensitive and robotic determination of bromate in sea water and drinking deep-sea water by headspace solid-phase micro extraction and gas chromatography-mass spectrometry. Anal Chim Acta 741:32–37. https://doi.org/10.1016/j.aca.2012.06.046 (Epub 2012 Jul 5)
Liu C, von Gunten U, Croué J-P (2012) Enhanced bromate formation during chlorination of bromide-containing waters in the presence of CuO: catalytic disproportionation of hypobromous acid. Environ Sci Technol 46:11054–11061. https://doi.org/10.1021/es3021793 (Epub 2012 Sep 26)
Luan Y, Suzuki T, Palanisamy R, Takashima Y, Sakamoto H, Sakuraba M, Koizumi T, Saito M, Matsufuji H, Yamagata K et al (2007) Potassium bromate treatment predominantly causes large deletions, but not GCTA transversion in human cells. Mutat Res 619:113–123. https://doi.org/10.1016/j.mrfmmm.2007.02.029 (Epub 2007 Mar 4)
Luong TV, Peters CJ, Perry R (1982) Influence of bromide and ammonia upon the formation of trihalomethanes under water-treatment conditions. Environ Sci Technol 16:473–479. https://doi.org/10.1021/es00102a009
Haber LT, Dourson ML, Allen BC, Hertzberg RC, Parker A, Vincent MJ, Maier A, Boobis AR (2018) Benchmark dose (BMD) modeling: current practice, issues, and challenges. Crit Rev Toxicol 48(5):387–415. https://doi.org/10.1080/10408444.2018.1430121
Mack RB (1988) Round up the usual suspects. Potassium bromate poisoning. N C Med J 49:243–245
Mack M, Schweinlin K, Mirsberger N, Zubel T, Bürkle A (2021) Automated screening for oxidative or methylation-induced DNA damage in human cells. Altex 38:63–72. https://doi.org/10.14573/altex.2001221 (Epub 2020 Jul 13)
Maronpot RR, Zeiger E, McConnell EE, Kolenda-Roberts H, Wall H, Friedman MA (2009) Induction of tunica vaginalis mesotheliomas in rats by xenobiotics. Crit Rev Toxicol 39:512–537. https://doi.org/10.1080/10408440902969430
Maronpot RR, Nyska A, Foreman JE, Ramot Y (2016) The legacy of the F344 rat as a cancer bioassay model (a retrospective summary of three common F344 rat neoplasms). Crit Rev Toxicol 46:641–675. https://doi.org/10.1080/10408444.2016.1174669 (Epub 2016 Jun 9)
Mattioli F, Martelli A, Gosmar M, Garbero C, Manfredi V, Varaldo E, Torre GC, Brambilla G (2006) DNA fragmentation and DNA repair synthesis induced in rat and human thyroid cells by chemicals carcinogenic to the rat thyroid. Mutat Res 609:146–153. https://doi.org/10.1016/j.mrgentox.2006.06.028 (Epub 2006 Aug 30)
Meek MEB, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE (2003) A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol 33:591–653. https://doi.org/10.1080/713608373
Michalski R, Mathews B (2007) Occurrence of chlorite, chlorate and bromate in disinfected swimming pool water. [place unknown]: [publisher unknown]
Murata M, Bansho Y, Inoue S, Ito K, Ohnishi S, Midorikawa K, Kawanishi S (2001) Requirement of glutathione and cysteine in guanine-specific oxidation of DNA by carcinogenic potassium bromate. Chem Res Toxicol 14:678–685. https://doi.org/10.1021/tx000209q
Obaidi I, Higgins M, Bahar B, Davis JL, McMorrow T (2018) Identification of the multifaceted chemopreventive activity of curcumin against the carcinogenic potential of the food additive, KBrO3. Curr Pharm Des 24:595–614. https://doi.org/10.2174/1381612824666171226143201
[OEHHA] Office of Environmental Health Hazard Assessment (2009) Public Health Goal for Bromate in Drinking Water
Okada E, Fujiishi Y, Narumi K, Kado S, Wako Y, Kawasako K, Kaneko K, Ohyama W (2015) Evaluation of repeated dose micronucleus assays of the liver and gastrointestinal tract using potassium bromate: a report of the collaborative study by CSGMT/JEMS.MMS. Mutat Res Genet Toxicol Environ Mutagen 780–781:94–99. https://doi.org/10.1016/j.mrgentox.2014.03.002 (Epub 2014 Mar 14)
Parsons JL, Chipman JK (2000) The role of glutathione in DNA damage by potassium bromate in vitro. Mutagenesis 15:311–316. https://doi.org/10.1093/mutage/15.4.311
Paweloszek R, Briançon S, Chevalier Y, Gilon-Delepine N, Pelletier J, Bolzinger M-A (2016) Skin absorption of anions: part two. Skin absorption of halide ions. Pharm Res 33:1576–1586. https://doi.org/10.1007/s11095-016-1898-0 (Epub 2016 Mar 21)
Phillips LJ, Fares RJ, Schweer LG (1993) Distributions of total skin surface area to body weight ratios for use in dermal exposure assessments. J Expo Anal Environ Epidemiol 3:331–338
Platel A, Nesslany F, Gervais V, Marzin D (2009) Study of oxidative DNA damage in TK6 human lymphoblastoid cells by use of the in vitro micronucleus test: determination of no-observed-effect levels. Mutat Res 678:30–37. https://doi.org/10.1016/j.mrgentox.2009.06.006 (Epub 2009 Jun 24)
Platel A, Nesslany F, Gervais V, Claude N, Marzin D (2011) Study of oxidative DNA damage in TK6 human lymphoblastoid cells by use of the thymidine kinase gene-mutation assay and the in vitro modified comet assay: determination of No-Observed-Genotoxic-Effect-Levels. Mutat Res 726:151–159. https://doi.org/10.1016/j.mrgentox.2011.09.003 (Epub 2011 Sep 13)
Priestley CC, Green RM, Fellows MD, Doherty AT, Hodges NJ, O’Donovan MR (2010) Anomalous genotoxic responses induced in mouse lymphoma L5178Y cells by potassium bromate. Toxicology 267:45–53. https://doi.org/10.1016/j.tox.2009.10.012 (Epub 2009 Oct 22)
Procházka E, Melvin SD, Escher BI, Plewa MJ, Leusch FDL (2019) Global transcriptional analysis of nontransformed human intestinal epithelial cells (FHs 74 Int) after exposure to selected drinking water disinfection by-products. Environ Health Perspect 127:117006. https://doi.org/10.1289/EHP4945 (Epub 2019 Nov 22)
[PWTAG] Pool Water Treatment Advisory Group (2019) PWTAG Code of Practice: THE MANAGEMENT AND TREATMENT OF SWIMMING POOL WATER. https://www.pwtag.org/code-of-practice/ (accessed 28.1.2022)
Quick CA, Chole RA, Mauer M (1975) Deafness and renal failure due to potassium bromate poisoning. Arch Otolaryngol 101:494–495. https://doi.org/10.1001/archotol.1975.00780370036013
Richardson SD, DeMarini DM, Kogevinas M, Fernandez P, Marco E, Lourencetti C, Ballesté C, Heederik D, Meliefste K, McKague AB et al (2010) What’s in the pool? A comprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ Health Perspect 118:1523–1530. https://doi.org/10.1289/ehp.1001965
Righi E, Fantuzzi G, Predieri G, Aggazzotti G (2014) Bromate, chlorite, chlorate, haloacetic acids, and trihalomethanes occurrence in indoor swimming pool waters in Italy. Microchem J 113:23–29. https://doi.org/10.1016/j.microc.2013.11.007
Risk Assessment Forum (2005) Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. Washington, DC 20460. 125 p
RIVM (2006) Disinfectant products fact sheet: To assess the risks for the consumer [RIVM rapport 320005003]. https://www.rivm.nl/bibliotheek/rapporten/320005003.pdf (accessed 9.12.2021)
RIVM (2014) Normen en methoden voor kwaliteitsparameters in het te wijzigen Besluit hygiëne en veiligheid badinrichtingen en zwemgelegenheden: RIVM rapport 2014–0121. https://www.rivm.nl/publicaties/normen-en-methoden-voor-kwaliteitsparameters-in-te-wijzigen-besluit-hygiene-en. (accessed 9.12.2021)
RIVM (2021) https://www.rivm.nl/en/proast. (accessed 16.11.2021)
Robbiano L, Carrozzino R, Puglia CP, Corbu C, Brambilla G (1999) Correlation between induction of DNA fragmentation and micronuclei formation in kidney cells from rats and humans and tissue-specific carcinogenic activity. Toxicol Appl Pharmacol 161:153–159. https://doi.org/10.1006/taap.1999.8796
Sai K, Takagi A, Umemura T, Hasegawa R, Kurokawa Y (1991) Relation of 8-hydroxydeoxyguanosine formation in rat kidney to lipid peroxidation, glutathione level and relative organ weight after a single administration of potassium bromate. Jpn J Cancer Res 82:165–169. https://doi.org/10.1111/j.1349-7006.1991.tb01824.x
Sai K, Hayashi M, Takagi A, Hasegawa R, Sofuni T, Kurokawa Y (1992a) Effects of antioxidants on induction of micronuclei in rat peripheral blood reticulocytes by potassium bromate. Mutat Res 269:113–118. https://doi.org/10.1016/0027-5107(92)90166-y
Sai K, Umemura T, Takagi A, Hasegawa R, Kurokawa Y (1992b) The protective role of glutathione, cysteine and vitamin C against oxidative DNA damage induced in rat kidney by potassium bromate. Jpn J Cancer Res 83:45–51. https://doi.org/10.1111/j.1349-7006.1992.tb02350.x
Sai K, Tyson CA, Thomas DW, Dabbs JE, Hasegawa R, Kurokawa Y (1994) Oxidative DNA damage induced by potassium bromate in isolated rat renal proximal tubules and renal nuclei. Cancer Lett 87:1–7. https://doi.org/10.1016/0304-3835(94)90402-2
[SCCS] Scientific Committee on Consumer Safety (2018) SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation: 10th revision. https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_224.pdf (accessed 9.12.2021)
Schets FM, Schijven JF, Roda Husman AM (2011) Exposure assessment for swimmers in bathing waters and swimming pools. Water Res 45:2392–2400. https://doi.org/10.1016/j.watres.2011.01.025 (Epub 2011 Mar 1)
Schleswig-Holstein (2018) Landesverordnung über die Hygiene- und Qualitätsanforderungen in Einrichtungen des Badewesens. https://www.gesetze-rechtsprechung.sh.juris.de/jportal/?quelle=jlink&query=B%C3%A4derHygV+SH&psml=bsshoprod.psml&max=true. (accessed 28.1.2022)
Seager AL, Shah U-K, Mikhail JM, Nelson BC, Marquis BJ, Doak SH, Johnson GE, Griffiths SM, Carmichael PL, Scott SJ et al (2012) Pro-oxidant induced DNA damage in human lymphoblastoid cells: homeostatic mechanisms of genotoxic tolerance. Toxicol Sci 128:387–397. https://doi.org/10.1093/toxsci/kfs152 (Epub 2012 Apr 26)
Shanmugavel V, Komala Santhi K, Kurup AH, Kalakandan S, Anandharaj A, Rawson A (2020) Potassium bromate: effects on bread components, health, environment and method of analysis: a review. Food Chem 311:125964. https://doi.org/10.1016/j.foodchem.2019.125964 (Epub 2019 Dec 9)
Shi H, Qiang Z, Adams C (2013) Formation of haloacetic acids, halonitromethanes, bromate and iodate during chlorination and ozonation of seawater and saltwater of marine aquaria systems. Chemosphere 90:2485–2492. https://doi.org/10.1016/j.chemosphere.2012.09.073 (Epub 2012 Nov 22)
Siddiqui MS, Amy GL (1993) Factors affecting DBP formation during ozone-bromide reactions. J Am Water Works Assoc 85:63–72. https://doi.org/10.1002/j.1551-8833.1993.tb05922.x
Song R, Donohoe C, Minear R, Westerhoff P, Ozekin K, Amy G (1996) Empirical modeling of bromate formation during ozonation of bromide-containing waters. Water Res 30:1161–1168. https://doi.org/10.1016/0043-1354(95)00302-9
Spassova MA (2019) Statistical approach to identify threshold and point of departure in dose-response data. Risk Anal 39:940–956. https://doi.org/10.1111/risa.13191 (Epub 2018 Sep 25)
Spassova MA, Miller DJ, Eastmond DA, Nikolova NS, Vulimiri SV, Caldwell J, Chen C, White PD (2013) Dose-response analysis of bromate-induced DNA damage and mutagenicity is consistent with low-dose linear, nonthreshold processes. Environ Mol Mutagen 54:19–35. https://doi.org/10.1002/em.21737 (Epub 2012 Sep 26)
Spassova MA, Miller DJ, Nikolov AS (2015) Kinetic modeling reveals the roles of reactive oxygen species scavenging and DNA repair processes in shaping the dose-response curve of KBrO3-induced DNA damage. Oxid Med Cell Longev 2015:764375. https://doi.org/10.1155/2015/764375
Speit G, Haupter S, Schütz P, Kreis P (1999) Comparative evaluation of the genotoxic properties of potassium bromate and potassium superoxide in V79 Chinese hamster cells. Mutat Res 439:213–221. https://doi.org/10.1016/s1383-5718(98)00200-9
Tanaka K, Oikawa K, Fukuhara C, Saito H, Onosaka S, Min K-S, Fujii M (1984) Metabolism of potassium bromate in rats II. In vitro studies. Chemosphere 13:1213–1219. https://doi.org/10.1016/0045-6535(84)90121-8
The European Parliament and the council of the European Union (2008) Regulation (EC) No 1272/2008: on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Official Journal of the European Union
Toporek M, Michalowska-Kaczmarczyk AM, Michalkowski T (2015) Symproportionation versus disproportionation in bromine redox systems. Electrochim Acta 171:176–187. https://doi.org/10.1016/j.electacta.2015.05.012
Tregear RT (1966) The permeability of mammalian skin to ions. J Invest Dermatol 46:16–23. https://doi.org/10.1038/jid.1966.4
Tsuchiya T, Kijima A, Ishii Y, Takasu S, Yokoo Y, Nishikawa A, Yanai T, Umemura T (2018) Mechanisms of oxidative stress-induced in vivo mutagenicity by potassium bromate and nitrofurantoin. J Toxicol Pathol 31:179–188. https://doi.org/10.1293/tox.2018-0024 (Epub 2018 Jun 2)
UBA (2014) Hygieneanforderungen an Bäder und deren Überwachung. Bundesgesundheitsbl 57:258–279. https://doi.org/10.1007/s00103-013-1899-7
U.S Department of Health and Human Services, Centers for Disease Control and Prevention (2016) 2016 Model Aqauatic Health Code: Code Language. 2nd Edition. https://www.cdc.gov/mahc/pdf/2016-mahc-code-final.pdf (accessed 9.12.2021)
U.S. Environmental Protection Agency, Office of Water (2018) 2018 Edition of the Drinking Water Standards and Health Advisories Tables (EPA 822-F-18–001). https://www.epa.gov/sites/default/files/2018-03/documents/dwtable2018.pdf (accessed 9.12.2021)
Umemura T, Sai K, Takagi A, Hasegawa R, Kurokawa Y (1995) A possible role for oxidative stress in potassium bromate (KBrO3) carcinogenesis. Carcinogenesis 16:593–597. https://doi.org/10.1093/carcin/16.3.593
Umemura T, Takagi A, Sai K, Hasegawa R, Kurokawa Y (1998) Oxidative DNA damage and cell proliferation in kidneys of male and female rats during 13-weeks exposure to potassium bromate (KBrO3). Arch Toxicol 72:264–269. https://doi.org/10.1007/s002040050500
Umemura T, Kitamura Y, Kanki K, Maruyama S, Okazaki K, Imazawa T, Nishimura T, Hasegawa R, Nishikawa A, Hirose M (2004) Dose-related changes of oxidative stress and cell proliferation in kidneys of male and female F344 rats exposed to potassium bromate. Cancer Sci 95:393–398. https://doi.org/10.1111/j.1349-7006.2004.tb03221.x
Umemura T, Kurokawa Y (2006) Etiology of bromate-induced cancer and possible modes of action-studies in Japan. Toxicology 221:154–157. https://doi.org/10.1016/j.tox.2006.01.007 (Epub 2006 Feb 13)
Umemura T, Kanki K, Kuroiwa Y, Ishii Y, Okano K, Nohmi T, Nishikawa A, Hirose M (2006) In vivo mutagenicity and initiation following oxidative DNA lesion in the kidneys of rats given potassium bromate. Cancer Sci 97:829–835. https://doi.org/10.1111/j.1349-7006.2006.00248.x (Epub 2006 Jun 29)
Umemura T, Tasaki M, Kijima A, Okamura T, Inoue T, Ishii Y, Suzuki Y, Masui N, Nohmi T, Nishikawa A (2009) Possible participation of oxidative stress in causation of cell proliferation and in vivo mutagenicity in kidneys of GPT delta rats treated with potassium bromate. Toxicology 257:46–52. https://doi.org/10.1016/j.tox.2008.12.007 (Epub 2008 Dec 14)
US EPA (2001) Toxicological review of bromate (CAS No. 15541-45-4). In Support of Summary Information on the Integrated Risk Information System (IRIS). https://iris.epa.gov/static/pdfs/1002tr.pdf (accessed 9.12.2021)
US EPA (2020) https://www.epa.gov/sites/production/files/2020-09/documents/bmds_3.2_user_guide.pdf (accessed 16.11.2021)
US EPA (2021) IRIS Assessment Bromate. [place unknown]: [publisher unknown]; https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=1002 (accessed 8.5.2021)
Velický J, Titlbach M, Dušková J, Vobecký M, Štrbák V, Raška I (1997) Potassium bromide and the thyroid gland of the rat: morphology and immunohistochemistry, RIA and INAA analysis. Ann Anatomy Anatomischer Anzeiger 179:421–431. https://doi.org/10.1016/S0940-9602(97)80041-6
Velický J, Titlbach M, Lojda Z, Dušková J, Vobecký M, Štrbák V, Raška I (1998) Long-term action of potassium bromide on the rat thyroid gland. Acta Histochem 100:11–23. https://doi.org/10.1016/S0065-1281(98)80003-2
Wolf DC, Crosby LM, George MH, Kilburn SR, Moore TM, Miller RT, DeAngelo AB (1998) Time- and dose-dependent development of potassium bromate-induced tumors in male Fischer 344 rats. Toxicol Pathol 26:724–729. https://doi.org/10.1177/019262339802600602
World Health Organization (2006) Swimming pools and similar environments. Geneva: [publisher unknown]. 118 p. (Guidelines for safe recreational water environments;/World Health Organization ; Vol. 2). ISBN: 9241546808
World Health Organization (2017) Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. Geneva: [publisher unknown]. ISBN: 9789241549950
WHO (2020) Chapter 5 “Dose-response assessment and derivation of health-based guidance values” of EHC 240 https://www.who.int/docs/default-source/food-safety/publications/chapter5-dose-response.pdf?sfvrsn=32edc2c6_5 (accessed 18.12.2021)
Yamaguchi T, Wei M, Hagihara N, Omori M, Wanibuchi H, Fukushima S (2008) Lack of mutagenic and toxic effects of low dose potassium bromate on kidneys in the Big Blue rat. Mutat Res 652:1–11. https://doi.org/10.1016/j.mrgentox.2007.11.004 (Epub 2007 Nov 26)
Yokoo Y, Kijima A, Ishii Y, Takasu S, Tsuchiya T, Umemura T (2016) Effects of Nrf2 silencing on oxidative stress-associated intestinal carcinogenesis in mice. Cancer Med 5:1228–1238. https://doi.org/10.1002/cam4.672 (Epub 2016 Feb 21)
Zaikin AN, Zhabotinsky AM (1970) Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 225:535–537. https://doi.org/10.1038/225535b0
Zakaria P, Bloomfield C, Shellie RA, Haddad PR, Dicinoski GW (2011) Determination of bromate in sea water using multi-dimensional matrix-elimination ion chromatography. J Chromatogr A 1218:9080–9085. https://doi.org/10.1016/j.chroma.2011.10.029 (Epub 2011 Oct 19)
Zhang Y, Jiang L, Jiang L, Geng C, Li L, Shao J, Zhong L (2011) Possible involvement of oxidative stress in potassium bromate-induced genotoxicity in human HepG2 cells. Chem Biol Interact 189:186–191. https://doi.org/10.1016/j.cbi.2010.12.011 (Epub 2010 Dec 21)
Zhou Y, Benson JM, Irvin C, Irshad H, Cheng Y-S (2007) Particle size distribution and inhalation dose of shower water under selected operating conditions. Inhal Toxicol 19:333–342. https://doi.org/10.1080/08958370601144241
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Röhl, C., Batke, M., Damm, G. et al. New aspects in deriving health-based guidance values for bromate in swimming pool water. Arch Toxicol 96, 1623–1659 (2022). https://doi.org/10.1007/s00204-022-03255-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03255-9