Abstract
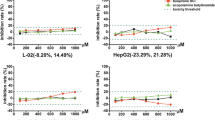
Drug-induced liver injury (DILI) is one of the most common and serious adverse drug reactions and a major cause of drug development failure and withdrawal. Although different molecular mechanisms are implicated in DILI, enhanced ROS levels have been described as a major mechanism. Human-derived cell models are increasingly used in preclinical safety assessment because they provide quick and relatively inexpensive information in early stages of drug development. We have analyzed and compared the phenotype and functionality of two liver cell models (Upcyte human hepatocytes and HepaRG cells) to demonstrate their suitability for long-term hepatotoxicity assessments and mechanistic studies. The transcriptomic and functional analysis revealed the maintenance of phase I and phase II enzymes, and antioxidant enzymes along time in culture, although the differences found between both test systems underlie the differential sensitivity to hepatotoxins. The evaluation of several mechanisms of cell toxicity, including oxidative stress, by high-content screening, demonstrated that, by combining the stable phenotype of liver cells and repeated-dose exposure regimes to 12 test compounds at clinically relevant concentrations, both Upcyte hepatocytes and HepaRG offer suitable properties to be used in routine screening assays for toxicological assessments during drug preclinical testing.











Similar content being viewed by others
Availability of data and material
All data and generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- C max :
-
Therapeutic peak plasmatic concentration
- CYP:
-
Cytochrome P450
- DILI:
-
Drug-induced liver injury
- Fluo-4:
-
Fluo-4 acetoxymethyl ester
- GPX:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- GSR:
-
Glutathione reductase
- GST:
-
Glutathione S-reductase
- HCS:
-
High-content screening
- HLC:
-
Hepatocyte-like cells
- MEC:
-
Minimal effective concentration
- MMP:
-
Mitochondrial membrane potential
- PBGD:
-
Porphobilinogen deaminase
- PHH:
-
Primary human hepatocytes
- PI:
-
Propidium iodide
- TMRM:
-
Tetramethyl rhodamine methyl ester
- UGT:
-
UDP-glucuronosyltransferase
- UHH:
-
Upcyte human hepatocytes
References
Abboud G, Kaplowitz N (2007) Drug-induced liver injury. Drug Saf 30(4):277–294
Antherieu S, Rogue A, Fromenty B, Guillouzo A, Robin MA (2011) Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in heparg cells. Hepatology 53(6):1895–1905. https://doi.org/10.1002/hep.24290
Atienzar FA, Novik EI, Gerets HH et al (2014) Predictivity of dog co-culture model, primary human hepatocytes and hepg2 cells for the detection of hepatotoxic drugs in humans. Toxicol Appl Pharmacol 275(1):44–61. https://doi.org/10.1016/j.taap.2013.11.022
Bell CC, Lauschke VM, Vorrink SU et al (2017) Transcriptional, functional, and mechanistic comparisons of stem cell-derived hepatocytes, heparg cells, and three-dimensional human hepatocyte spheroids as predictive in vitro systems for drug-induced liver injury. Drug Metab Dispos 45(4):419–429. https://doi.org/10.1124/dmd.116.074369
Bell CC, Dankers ACA, Lauschke VM et al (2018) Comparison of hepatic 2D sandwich cultures and 3D spheroids for long-term toxicity applications: a multicenter study. Toxicol Sci 162(2):655–666. https://doi.org/10.1093/toxsci/kfx289
Broeders JJ, Parmentier C, Truisi GL et al (2015) Biokinetics of chlorpromazine in primary rat and human hepatocytes and human heparg cells after repeated exposure. Toxicol In Vitro 30(1 Pt A):52–61. https://doi.org/10.1016/j.tiv.2014.08.012
Bucher S, Jalili P, Le Guillou D et al (2017) Bisphenol a induces steatosis in HepaRG cells using a model of perinatal exposure. Environ Toxicol 32(3):1024–1036. https://doi.org/10.1002/tox.22301
Chan R, Benet LZ (2017) Evaluation of DILI predictive hypotheses in early drug development. Chem Res Toxicol 30(4):1017–1029. https://doi.org/10.1021/acs.chemrestox.7b00025
Chen M, Vijay V, Shi Q, Liu Z, Fang H, Tong W (2011) FDA-approved drug labeling for the study of drug-induced liver injury. Drug Discov Today 16(15–16):697–703. https://doi.org/10.1016/j.drudis.2011.05.007
Donato M, Tolosa L (2021) High-Content screening for the detection of drug-induced oxidative stress in liver cells. Antioxidants (Basel). https://doi.org/10.3390/antiox10010106
Donato MT, Lahoz A, Castell JV, Gomez-Lechon MJ (2008) Cell lines: a tool for in vitro drug metabolism studies. Curr Drug Metab 9(1):1–11
Donato MT, Montero S, Castell JV, Gomez-Lechon MJ, Lahoz A (2010) Validated assay for studying activity profiles of human liver UGTs after drug exposure: inhibition and induction studies. Anal Bioanal Chem 396(6):2251–2263. https://doi.org/10.1007/s00216-009-3441-1
Donato MT, Tolosa L, Jimenez N, Castell JV, Gomez-Lechon MJ (2012) High-content imaging technology for the evaluation of drug-induced steatosis using a multiparametric cell-based assay. J Biomol Screen 17(3):394–400. https://doi.org/10.1177/1087057111427586
Donato MT, Tolosa L, Gomez-Lechon MJ (2015) Culture and functional characterization of human hepatoma hepg2 cells. Methods Mol Biol 1250:77–93. https://doi.org/10.1007/978-1-4939-2074-7_5
Dragovic S, Vermeulen NP, Gerets HH et al (2016) Evidence-based selection of training compounds for use in the mechanism-based integrated prediction of drug-induced liver injury in man. Arch Toxicol 90(12):2979–3003. https://doi.org/10.1007/s00204-016-1845-1
Elsherbiny ME, El-Kadi AO, Brocks DR (2008) The metabolism of amiodarone by various CYP isoenzymes of human and rat, and the inhibitory influence of ketoconazole. J Pharm Pharmaceutical Sci 11(1):147–159. https://doi.org/10.18433/j3sg66
Fabre G, Julian B, Saint-Aubert B, Joyeux H, Berger Y (1993) Evidence for CYP3A-mediated N-deethylation of amiodarone in human liver microsomal fractions. Drug Metab Dispos 21(6):978–985
Funk C, Roth A (2017) Current limitations and future opportunities for prediction of DILI from in vitro. Arch Toxicol 91(1):131–142. https://doi.org/10.1007/s00204-016-1874-9
Garcia-Cortes M, Robles-Diaz M, Stephens C, Ortega-Alonso A, Lucena MI, Andrade RJ (2020) Drug induced liver injury: an update. Arch Toxicol 94(10):3381–3407. https://doi.org/10.1007/s00204-020-02885-1
Garside H, Marcoe KF, Chesnut-Speelman J et al (2014) Evaluation of the use of imaging parameters for the detection of compound-induced hepatotoxicity in 384-well cultures of HepG2 cells and cryopreserved primary human hepatocytes. Toxicol In Vitro 28(2):171–181. https://doi.org/10.1016/j.tiv.2013.10.015
Germano D, Uteng M, Pognan F, Chibout SD, Wolf A (2014) Determination of liver specific toxicities in rat hepatocytes by high content imaging during 2-week multiple treatment. Toxicol In Vitro. https://doi.org/10.1016/j.tiv.2014.05.009
Gomez-Lechon MJ, Donato MT, Castell JV, Jover R (2004) Human hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr Drug Metab 5(5):443–462
Gomez-Lechon MJ, Tolosa L, Donato MT (2016) Metabolic activation and drug-induced liver injury: in vitro approaches for the safety risk assessment of new drugs. J Appl Toxicol 36(6):752–768. https://doi.org/10.1002/jat.3277
Gustafsson F, Foster AJ, Sarda S, Bridgland-Taylor MH, Kenna JG (2014) A correlation between the in vitro drug toxicity of drugs to cell lines that express human P450s and their propensity to cause liver injury in humans. Toxicol Sci 137(1):189–211
Haegler P, Joerin L, Krahenbuhl S, Bouitbir J (2017) Hepatocellular toxicity of imidazole and triazole antimycotic agents. Toxicol Sci 157(1):183–195. https://doi.org/10.1093/toxsci/kfx029
Hewitt M, Enoch SJ, Madden JC, Przybylak KR, Cronin MT (2013) Hepatotoxicity: a scheme for generating chemical categories for read-across, structural alerts and insights into mechanism(s) of action. Crit Rev Toxicol 43(7):537–558. https://doi.org/10.3109/10408444.2013.811215
Jackson JP, Li L, Chamberlain ED, Wang H, Ferguson SS (2016) Contextualizing hepatocyte functionality of cryopreserved heparg cell cultures. Drug Metab Dispos 44(9):1463–1479. https://doi.org/10.1124/dmd.116.069831
Kanebratt KP, Andersson TB (2008) HepaRG cells as an in vitro model for evaluation of cytochrome P450 induction in humans. Drug Metab Dispos 36(1):137–145. https://doi.org/10.1124/dmd.107.017418
Kiang TK, Ho PC, Anari MR, Tong V, Abbott FS, Chang TK (2006) Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol Sci 94(2):261–271. https://doi.org/10.1093/toxsci/kfl096
Kramer NI, Di Consiglio E, Blaauboer BJ, Testai E (2015) Biokinetics in repeated-dosing in vitro drug toxicity studies. Toxicol In Vitro 30(1 Pt A):217–24. https://doi.org/10.1016/j.tiv.2015.09.005
Kyriakidis I, Tragiannidis A, Munchen S, Groll AH (2017) Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 16(2):149–165. https://doi.org/10.1080/14740338.2017.1270264
Lahoz A, Donato MT, Picazo L, Gomez-Lechon MJ, Castell JV (2007) Determination of major human cytochrome P450s activities in 96-well plates using liquid chromatography tandem mass spectrometry. Toxicol In Vitro 21(7):1247–1252. https://doi.org/10.1016/j.tiv.2007.03.022
Lee WM (2003) Drug-induced hepatotoxicity. N Engl J Med 349(5):474–485
Leite SB, Roosens T, El Taghdouini A et al (2016) Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 78:1–10. https://doi.org/10.1016/j.biomaterials.2015.11.026
Lu J, Miyakawa K, Roth RA, Ganey PE (2013) Tumor necrosis factor-alpha potentiates the cytotoxicity of amiodarone in Hepa1c1c7 cells: roles of caspase activation and oxidative stress. Toxicol Sci 131(1):164–178. https://doi.org/10.1093/toxsci/kfs289
Moya M, Benet M, Guzman C et al (2012) Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS ONE 7(1):e30014. https://doi.org/10.1371/journal.pone.0030014
Mueller D, Kramer L, Hoffmann E, Klein S, Noor F (2014) 3D organotypic HepaRG cultures as in vitro model for acute and repeated dose toxicity studies. Toxicol In Vitro 28(1):104–112. https://doi.org/10.1016/j.tiv.2013.06.024
O’Brien PJ, Irwin W, Diaz D et al (2006) High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol 80(9):580–604. https://doi.org/10.1007/s00204-006-0091-3
Park JW, Kim KA, Park JY (2019) Effects of ketoconazole, a CYP4F2 inhibitor, and CYP4F2*3 genetic polymorphism on pharmacokinetics of vitamin K1. J Clin Pharmacol 59(11):1453–1461. https://doi.org/10.1002/jcph.1444
Pomponio G, Savary CC, Parmentier C et al (2015) In vitro kinetics of amiodarone and its major metabolite in two human liver cell models after acute and repeated treatments. Toxicol In Vitro 30(1 Pt A):36–51. https://doi.org/10.1016/j.tiv.2014.12.012
Pradip A, Steel D, Jacobsson S et al (2016) High Content analysis of human pluripotent stem cell derived hepatocytes reveals drug induced steatosis and phospholipidosis. Stem Cells Int 2016:2475631. https://doi.org/10.1155/2016/2475631
Saito J, Okamura A, Takeuchi K, Hanioka K, Okada A, Ohata T (2016) High content analysis assay for prediction of human hepatotoxicity in HepaRG and HepG2 cells. Toxicol in Vitro 33:63–70. https://doi.org/10.1016/j.tiv.2016.02.019
Saruwatari J, Ishitsu T, Nakagawa K (2010) Update on the genetic polymorphisms of drug-metabolizing enzymes in antiepileptic drug therapy. Pharmaceuticals (basel) 3(8):2709–2732. https://doi.org/10.3390/ph3082709
Serras AS, Rodrigues JS, Cipriano M, Rodrigues AV, Oliveira NG, Miranda JP (2021) A critical perspective on 3d liver models for drug metabolism and toxicology studies. Front Cell Dev Biol 9:626805. https://doi.org/10.3389/fcell.2021.626805
Sharanek A, Burban A, Humbert L et al (2015) Cellular accumulation and toxic effects of bile acids in cyclosporine a-treated heparg hepatocytes. Toxicol Sci 147(2):573–587. https://doi.org/10.1093/toxsci/kfv155
Silva MF, Aires CC, Luis PB et al (2008) Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. J Inherit Metab Dis 31(2):205–216. https://doi.org/10.1007/s10545-008-0841-x
Sison-Young RL, Mitsa D, Jenkins RE et al (2015) Comparative proteomic characterization of 4 human liver-derived single cell culture models reveals significant variation in the capacity for drug disposition, bioactivation, and detoxication. Toxicol Sci 147(2):412–424. https://doi.org/10.1093/toxsci/kfv136
Sison-Young RL, Lauschke VM, Johann E et al (2017) A multicenter assessment of single-cell models aligned to standard measures of cell health for prediction of acute hepatotoxicity. Arch Toxicol 91(3):1385–1400. https://doi.org/10.1007/s00204-016-1745-4
Teng S, Barcellini-Couget S, Beaudouin R et al (2015) BK/TD models for analyzing in vitro impedance data on cytotoxicity. Toxicol Lett 235(2):96–106. https://doi.org/10.1016/j.toxlet.2015.03.011
Thompson RA, Isin EM, Li Y et al (2012) In vitro approach to assess the potential for risk of idiosyncratic adverse reactions caused by candidate drugs. Chem Res Toxicol 25(8):1616–1632. https://doi.org/10.1021/tx300091x
Tolosa L, Pinto S, Donato MT et al (2012) Development of a multiparametric cell-based protocol to screen and classify the hepatotoxicity potential of drugs. Toxicol Sci 127(1):187–198. https://doi.org/10.1093/toxsci/kfs083
Tolosa L, Carmona A, Castell JV, Gomez-Lechon MJ, Donato MT (2015) High-content screening of drug-induced mitochondrial impairment in hepatic cells: effects of statins. Arch Toxicol 89(10):1847–1860. https://doi.org/10.1007/s00204-014-1334-3
Tolosa L, Gomez-Lechon MJ, Lopez S et al (2016) Human upcyte hepatocytes: characterization of the hepatic phenotype and evaluation for acute and long-term hepatotoxicity routine testing. Toxicol Sci 152(1):214–229. https://doi.org/10.1093/toxsci/kfw078
Tolosa L, Jimenez N, Pelecha M, Castell JV, Gomez-Lechon MJ, Donato MT (2019) Long-term and mechanistic evaluation of drug-induced liver injury in upcyte human hepatocytes. Arch Toxicol 93(2):519–532. https://doi.org/10.1007/s00204-018-2349-y
Vorrink SU, Zhou Y, Ingelman-Sundberg M, Lauschke VM (2018) Prediction of drug-induced hepatotoxicity using long-term stable primary hepatic 3D spheroid cultures in chemically defined conditions. Toxicol Sci 163(2):655–665. https://doi.org/10.1093/toxsci/kfy058
Waizenegger J, Braeuning A, Templin M, Lampen A, Hessel-Pras S (2018) Structure-dependent induction of apoptosis by hepatotoxic pyrrolizidine alkaloids in the human hepatoma cell line HepaRG: single versus repeated exposure. Food Chem Toxicol 114:215–226. https://doi.org/10.1016/j.fct.2018.02.036
Wewering F, Jouy F, Wissenbach DK et al (2017) Characterization of chemical-induced sterile inflammation in vitro: application of the model compound ketoconazole in a human hepatic co-culture system. Arch Toxicol 91(2):799–810. https://doi.org/10.1007/s00204-016-1686-y
Acknowledgements
The scientific support from the Cytomics Unit from the Instituto de Investigación Sanitaria La Fe and the technical advice of Astrid Nörenberg is acknowledged.
Funding
This work has been supported by the Institute of Health Carlos III (ISCIII, Plan Estatal de I + D + i 2013–2016) and cofinanced by the European Regional Development Fund "A way to achieve Europe" (FEDER) through grants PI18/00993 and CP16/00097, by the Spanish Ministry of Science and Innovation Ministry-Spanish Research Agency through the Project PID2019-106000RB-C22/AEI/1 0.13039/501100011033, and by the Generalitat Valenciana (PROMETEO/2019/060). M.P. was supported by ACIF/2018/0226 (Generalitat Valenciana) and L.T. by ISCIII through grant CP16/00097.
Author information
Authors and Affiliations
Contributions
Conceptualization, MTD, LT: methodology, NJ, MP: writing-original draft preparation, MTD, LT: supervision, MTD, LT: writing-review and editing, MTD, LT: funding acquisition, MTD, LT All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Health Research Institute La Fe (IIS La Fe) (2016/0226, date of approval 06/06/2017). Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Donato, M.T., Jiménez, N., Pelechá, M. et al. Oxidative-stress and long-term hepatotoxicity: comparative study in Upcyte human hepatocytes and hepaRG cells. Arch Toxicol 96, 1021–1037 (2022). https://doi.org/10.1007/s00204-022-03236-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03236-y