Abstract
This review summarises the current state of knowledge regarding the physiology and control of production of thyroid hormones, the effects of chemicals in perturbing their synthesis and release that result in thyroid cancer. It does not consider the potential neurodevelopmental consequences of low thyroid hormones. There are a number of known molecular initiating events (MIEs) that affect thyroid hormone synthesis in mammals and many chemicals are able to activate multiple MIEs simultaneously. AOP analysis of chemical-induced thyroid cancer in rodents has defined the key events that predispose to the development of rodent cancer and many of these will operate in humans under appropriate conditions, if they were exposed to high enough concentrations of the affecting chemicals. There are conditions however that, at the very least, would indicate significant quantitative differences in the sensitivity of humans to these effects, with rodents being considerably more sensitive to thyroid effects by virtue of differences in the biology, transport and control of thyroid hormones in these species as opposed to humans where turnover is appreciably lower and where serum transport of T4/T3 is different to that operating in rodents. There is heated debate around claimed qualitative differences between the rodent and human thyroid physiology, and significant reservations, both scientific and regulatory, still exist in terms of the potential neurodevelopmental consequences of low thyroid hormone levels at critical windows of time. In contrast, the situation for the chemical induction of thyroid cancer, through effects on thyroid hormone production and release, is less ambiguous with both theoretical, and actual data, showing clear dose-related thresholds for the key events predisposing to chemically induced thyroid cancer in rodents. In addition, qualitative differences in transport, and quantitative differences in half life, catabolism and turnover of thyroid hormones, exist that would not operate under normal situations in humans.






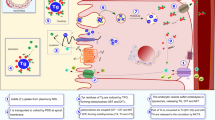
Adapted from Visser et al. (2016)

Redrawn from Fig. 8 from Yu et al. (2002)




Data taken from York et al. (2003)
Similar content being viewed by others
Abbreviations
- T4:
-
Thyroxine
- T3:
-
Tri-iodothyronine
- DIT:
-
Di-iodothyronine
- MIT:
-
Mono-iodothyronine
- TSH:
-
Thyrotropin or thyroid stimulating hormone
- UDPGT:
-
Uridine diphosphate glucuronyltransferase
- SULT:
-
Sulfotransferase
- NIS:
-
Sodium-iodide symporter
- TPO:
-
Thyroperoxidase
- AOP:
-
Adverse outcome pathway
- KE:
-
Key events
- MIE:
-
Molecular initiating event
- MoA:
-
Mode of action
- Ph II:
-
Phase 2 metabolism
- TTR:
-
Transthyretin
- TBG:
-
Thyroxine binding globulin
- NIS:
-
Sodium iodide symporter
- rT3:
-
Reverse T3
- T3S:
-
Sulphated T3
- TRE:
-
Thyroid response elements
- PPAR:
-
Peroxisome proliferator activated receptor
- RXR:
-
Retinoid X receptor
- CAR:
-
Constitutive androstane receptor
- IGF:
-
Insulin like growth factor
- PCB:
-
Polychlorinated biphenyl
- PBB:
-
Polybrominated biphenyl
- TCDD:
-
Tetrachlorodibenzo-p-dioxin
- PTU:
-
6-Propylthiouracil
- CSF:
-
Cerebrospinal fluid
- MCT:
-
Monocarboxylate transporter
- OATP:
-
Organic anion transporter protein
- NOAEL:
-
No observable adverse effect level
References
Abe T, Kakyo M, Sakagami H et al (1998) Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with OATP2. J Biol Chem 273:22395–22401
Ahmed RG (2015) Hypothyroidism and brain developmental players. Thyroid Res 8:2. https://doi.org/10.1186/s13044-015-0013-7
Akosa BT, Sleight SD, Nachreiner RF et al (1982) Effects of purified polybrominated biphenyl congeners on the thyroid and pituitary glands in rats. J Am Coll Toxicol 1:23–36
Alshehri B, D’Souza DG, Lee JY et al (2015) The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol 27:303–323
Andersen BF (1973) Iodide perchlorate discharge test in lithium-treated patients. Acta Endocrinol 73:35–46
Arriagada AA, Albornoz E, Opazo MC et al (2015) Excess iodide induces an acute inhibition of the sodium/iodide symporter in thyroid male rat cells by increasing reactive oxygen species. Endocrinol. 156:1540–1551
Astwood EB, Sullivan J, Bissell A et al (1943) Action of certain sulfonamides and of thiourea upon the function of the thyroid gland of the rat. Endocrinol 32:210–225
Axelstad M, Boberg J, Nellemann C et al (2011) Exposure to the widely used fungicide mancozeb causes thyroid hormone disruption in rat dams but no behavioural effects in the offspring. Toxicol Sci 120:439–446
Bailey DE (1990b) KERB Herbicide (Technical, No Clay): 24-month dietary chronic toxicity/oncogenicity study in rats—24-month oncogenicity phase. Unpublished report of Rohm and Haas Company, a wholly-owned subsidiary of The Dow Chemical Company, Midland, Michigan (cited in Papineni et al 2015).
Bard D, Verger P, Hubert P (1997) Chernobyl, 10 years after: health consequences. Epidemiol Rev 19:187–204
Bartalena L, Robbins J (1993) Thyroid hormone transport proteins. Clin Lab Med 13:583–598
Bartsch R, Brinkmann B, Jahnke G et al (2018) Human relevance of follicular thyroid tumors in rodents caused by nongenotoxic substances. Reg Toxicol Pharmacol 98:199–208
Bianco AC, Kim BW (2006) Deiodinases: implications of the local control of thyroid hormone action. J Clin Investig 116:2571–2579
Bianco AC, Salvatore D, Gereben B et al (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. End Rev 23:38–89
Blount BC, Pirkle JL, Osterloh JD et al (2006) Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect 114:1865–1871
Bogazzi F, Bartalena L, Cosci C et al (2003) Treatment of Type II amiodarone-induced thyrotoxicosis by either iopanoic acid or glucocorticoids: a prospective, randomized study. J Clin Endocrinol Metab 88:1999–2002
Boobis AR, Cohen SM, Dellarco V et al (2006) IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol 36:781–792
Borzelleca JF, Capen CC, Hallagan JB (1987) Lifetime toxicity/carcinogenicity study of FD & C Red No 3 (erythrosine) in rats. Food Chem Toxicol 25:723–733
Bounacer A, Wicker R, Caillou B et al (1997) High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene 15:1263–1273
Brent GA (2008) Clinical practice. Graves’ disease New Engl J Med 358:2594–2605
Brent GA (2012a) Hypothyroidism and thyroiditis. In: Melmed SP, Larsen PR, Kronenberg HM (eds) Williams textbook of endocrinology. Elsevier, Philadelphia
Brent GA (2012b) Mechanisms of thyroid hormone action. J Clin Investig 122:3035–3043
Brucker-Davis F (1998) Effects of environmental synthetic chemicals on thyroid function. Thyroid 8:827–856
Capen C (1996) Toxic responses of the endocrine system. In: Klaassen CD (ed) Casarett and Doull’s toxicology: the basic science of poisons, 5th edn. McGraw-Hill, New York, pp 623–624
Capen CC (1999) Thyroid and parathyroid toxicology. In: Harvey PW, Rush KC, Cockburn A (eds) Endocrine and hormonal toxicolog. Wiley Press, Hoboken, pp 33–66
Capen CC, Martin SL (1989) The effects of xenobiotics on the structure and function of thyroid follicular and C-cells. Toxicol Pathol 17:266–293
Carlson HE, Temple R, Robbins J (1973) Effect of lithium on thyroxine disappearance in man. J Clin Endocrinol Metab 36:1251–1254
Carroll R, Matfin G (2010) Endocrine and metabolic emergencies: thyroid storm. Ther Adv Endocrinol Metab 1:139–145
Cavalieri RR, Pitt-Rivers R (1981) The effects of drugs on the distribution and metabolism of thyroid hormones. Pharmacol Rev 33:55–80
Cayir A, Turan MI, Esin IS (2014) An examination of the effects of phenobarbital on thyroid function tests in childhood epilepsy. Hong Kong J Paed 19:71–74
Chang L, Munro SL, Richardson SJ et al (1999) Evolution of thyroid hormone binding by transthyretins in birds and mammals. Eur J Biochem 259:534–542
Chanoine J-P, Braverman LE, Farwell AP et al (1993) The thyroid gland is a major source of circulating T3 in the rat. J Clin Investig 91:2709–2713
Chauhan KR, Kodavanti PR, McKinney JD (2000) Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transport protein. Toxicol Appl Pharmacol 162:10–21
Cheek AO, Kow K, Chen J et al (1999) Potential mechanisms of thyroid disruption in humans: interaction of organochlorine compounds with thyroid receptor, transthyretin, and thyroid-binding globulin. Environ Health Percept 107:273–278
Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocrinol Rev 31:139–170
Choksi NY, Jahnke GD, St. Hilaire C et al (2003) Role of Thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res (Part B) 68:479–491
Clements FW (1954) The relationship of thyrotoxicosis and carcinoma of the thyroid to endemic goitre. Med J Australia 41:894–897
Cohen SM, Ellwein LB (1991) Genetic errors, cell proliferation, and carcinogenesis. Can Res 51:6493–6505
Cohen HN, Beastall GH, Ratcliffe WA et al (1980) Effects on thyroid functions of sulphonamide and trimethoprim combination drugs. Br Med J 281(6241):646–647
Colnot T, Dekant W (2017) Approaches for grouping of pesticides into cumulative assessment groups for risk assessment of pesticide residues in food. Reg Toxicol Pharmacol 83:89–99
Cooper DS, Axelrod L, DeGroot LJ et al (1981) Congenital goitre and development of metastatic follicular carcinoma with evidence for a leak of non-hormonal iodide: clinical, pathological, kinetic, and biochemical studies and a review of the literature. J Clin Endocrinol Metab 52:924–932
Craft ES, DeVito MJ, Crofton KM (2002) Comparative responsiveness of hypothyroxinemia and hepatic enzyme induction in Long-Evans rats versus C57Bl/6J mice exposed to TCDD-like and Phenobarbital-like Polychlorinated Biphenyl Congeners. Toxicol. Sci 68:372–380
Crofton KM (2008) Thyroid disrupting chemicals: mechanisms and mixtures. Int J Androl 31:209–223
Curran PG, DeGroot LJ (1991) The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocrinol Rev 12:135–150
Curtis SW, Terrell ML, Jacobson MH et al (2019) Thyroid hormone levels associate with exposure to polychlorinated biphenyls and polybrominated biphenyls in adults exposed as children. Environ Health 18(75):2020. https://doi.org/10.1186/s12940-019-0509-zaccessed25thMarch
Daminet S, Ferguson DC (2003) Influence of drugs on thyroid function in dogs. J Vet Int Med 17:463–472
Dellarco VL, McGregor D, Berry C et al (2006) Thiazopyr and thyroid disruption: case study within the context of the 2006 IPCS human relevance framework for analysis of a cancer mode of action. Crit Rev Toxicol 36:793–801
Dentice M, Marsili A, Zavacki A-M et al (2013) The deiodinases and the control of intracellular thyroid hormone signalling during cellular differentiation. Biochim Biophys Acta. 1830:3937–3945
Di Cosmo C, Liao XH, Dumitrescu AM et al (2010) Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Investig 120:3377–3388
Doerge DR, Decker CJ (1994) Inhibition of peroxidase-catalyzed reactions by arylamines: mechanism for the anti-thyroid action of sulfamethazine. Chem Res Toxicol 7:164–169
Dohler KD, Wong CC, Von zur Muhlen A (1979) The rat as a model for the study of drug effects on thyroid function: consideration of methodological problems. Pharmacol Therap B 5:305–318
Dumitrescu AM, Liao XH, Best TB et al (2004) A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Gen 74:168–175
ECHA (European Chemicals Agency) 2015. Guidance on the application of the CLP criteria, version 4.1. 2015. https://echa.europa.eu/documents/10162/23036412/clp_en.pdf/58b5dc6d-ac2a-4910-9702-e9e1f5051cc5. Accessed 21 Nov 2020
ECHA/EFSA (2018). Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. Adapted 5 June 2018
Eelkman RSJ, Kaptein E, Visser TJ (1989) Serum triiodothyronine sulfate in man measured by radioimmunoassay. J Clin Endocrinol Metab 69:552–556
EFSA (European Food Safety Authority), Crivellente F, Hart A, Hernandez-Jerez AF, HougaardBennekou S, Pedersen R, Terron A, Wolterink G, Mohimont L (2019) Scientific report on the establishment of cumulative assessment groups of pesticides for their effects on the thyroid. EFSA J 17(9):5801. https://doi.org/10.2903/j.efsa.2019.5801
EFSA-PPR-Panel (2014) (EFSA Panel on Plant Protection Products and their Residues) Scientific opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile (2014 update). EFSA J 11(131):2014
Elman DS (1958) Familial association of nerve deafness with nodular goitre and thyroid carcinoma. N Engl J Med 259:219–223
EMA (2017) Guideline on strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-strategies-identify-mitigate-risks-first-human-early-clinical-trials-investigational_en.pdf. Accessed 21 Nov 2020
Emi Y, Ikushiro S, Kato Y (2007) Thyroxine-metabolizing rat uridine diphosphate glucuronosyltransferase 1A7 is regulated by thyroid hormone receptor. Endocrinol 148:6124–6133
EPA (2001) The Determination of Whether Dithiocarbamate Pesticides Share a Common Mechanism of Toxicity. https://archive.epa.gov/pesticides/reregistration/web/pdf/dithiocarb.pdf. Accessed 21 Nov 2020
Episkopou V, Maeda S, Nishiguchi S et al (1993) Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc Natl Acad Sci USA 90:2375–2379
Evans RM (1988) The steroid and thyroid hormone receptor superfamily. Science 240:889–895
Fattori J, Campos JLO, Doratioto TR et al (2015) RXR Agonist modulates TR: corepressor dissociation upon 9-cis retinoic acid treatment. Mol Endocrinol 29:258–273
Feldt-Rasmussen U, and Rasmussen AK (2007) Thyroid hormone transport and actions. In: Krassas GE, Rivkees SA, Kiess W (eds) Diseases of the thyroid in childhood and adolescence. Ped Adolesc Med, Basel, Karger, vol 11, pp 80–103
Findlay KAB, Kaptein E, Vissser TJ et al (2000) Characterization of the uridine diphosphate-glucuronosyltransferase-catalyzing thyroid hormone glucuronidation in man. J Clin Endocrinol Metab 85:2879–2883
Flink IL, Bailey TJ, Gustafson TA et al (1986) Complete amino acid sequence of human thyroxine-binding globulin deduced from cloned DNA: close homology to the serine antiproteases. Proc Natl Acad Sci USA 83:7708–7712
Fonseca TL, Correa-Medina M, Campos MP et al (2013) Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Investig 123:1492–1500
Foster JR (1997) The role of cell proliferation in chemically induced carcinogenesis. J Comp Pathol 116:113–144
Fox CS, Pencina MJ, D’Agostino RB et al (2008) Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Int Med 168:587–592
Franceschi S, Preston-Martin S, Dal Maso L et al (1999) A pooled analysis of case–control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control 10:583–595
Friesema EC, Grueters A, Biebermann H et al (2004) Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364(9443):1435–1437
Frumess RD, Larsen PR (1975) Correlation of serum triiodothyronine (T3) and thyroxine (T4) with biologic effects of thyroid hormone replacement in propylthiouracil-treated rats. Metabolism 24:547–554
Gauger KJ, Kato Y, Haraguchi K et al (2004) Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ Health Percept 112:516–523
Gerber H, Studer H, Conti A et al (1981) Reaccumulation of thyroglobulin and colloid in rat and mouse thyroid follicles during intense thyrotropin stimulation A clue to the pathogenesis of colloid goiters. J Clin Investig 68:1338–1347
Gereben B, Zavacki AM, Ribich S et al (2008) Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocrin Rev 29:898–938
Gereben B, McAninch EA, Ribeiro MO et al (2015) Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol 11:642–652
Ghamari-Langroudi M, Vella KR, Srisai D et al (2010) Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Mol Endocrinol 24:2366–2381
Graham SL, Davis KJ, Hansen WH, Graham CH (1975) Effects of prolonged ethylene thiourea ingestion on the thyroid of the rat. Food Cosmet Toxicol 13:493–499
Greer MA, Goodman G, Pleus RC et al (2002) Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Perspect 110:927–937
Haines C, Chatham LR, Vardy A et al (2019) Comparison of the hepatic and thyroid gland effects of sodium phenobarbital and pregnenolone-16α-carbonitrile in wild-type and constitutive androstane receptor (CAR)/pregnane X receptor (PXR) knockout rats. Xenobiotica 49:227–238
Hallgren S, Darnerud PO (2002) Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats—testing interactions and mechanisms for thyroid hormone effects. Toxicol 177:227–243
Hard GC (1998) Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environ Health Perspect 106:427–436
Haseman JK, Huff JE, Rao GN et al (1985) Neo-plasms observed in untreated and corn oil gavage control groups of F344/N rats and (C57BL/6N x C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst 75:975–984
Hassan I, El-Masri H, Kosian PA, Ford J, Degitz SJ, Gilbert ME (2017) Neurodevelopment and thyroid hormone synthesis inhibition in the rat: quantitative understanding within the adverse outcome pathway framework. Toxicol Sci 160:57–73
He Y, Yang J, Huang S et al (2019) Protective effect of mulberry crude extract against nonylphenol-induced thyroid disruption by inhibiting the activity of deiodinase in rats. Gen Comp Endocrinol 270:90–95
Helmreich DL, Tylee D (2011) Thyroid hormone regulation by stress and behavioural differences in adult male rats. Horm Behav 60:284–291
Hershman JM (1974) Clinical application of thyrotropin-releasing hormone. N Engl J Med 290:886–890
Heuer H, Visser TJ (2009) Minireview: pathophysiological importance of thyroid hormone transporters. Endocrinology 150:1078–1083
Hiasa Y, Kitahori Y, Kitamura M et al (1991) Relationships between serum thyroid stimulating hormone levels and the development of thyroid tumors in rats treated with N-bis-(2-hydroxypropyl)nitrosamine. Carcinogenesis 12:873–877
Hill RN, Erdreich LS, Paynter OE et al (1989) Thyroid follicular cell carcinogenesis. Fund Appl Toxicol 12:629–697
Hill RN, Crisp TM, Hurley PM et al (1998) Risk assessment of thyroid follicular cell tumors. Environ Health Percept 106:447–457
Hingorani M, Spitzweg C, Vassauz G et al (2010) The biology of the sodium iodide symporter andits potential for targeted gene delivery. Curr Cancer Drug Targets 10:242–267
Holm LE, Wiklund KE, Lundell GE et al (1988) Thyroid cancer after diagnostic doses of iodine-131: a retrospective cohort study. J Natl Cancer Inst 80:1132–1138
Hood A, Klaassen CD (2000a) Effects of microsomal enzyme inducers on outer ring deiodinase activity towards thyroid hormones in various rat tissues. Toxicol Appl Pharmacol 163:240–248
Hood A, Klaassen CD (2000b) Differential effects of microsomal enzyme inducers on in vitro thyroxine (T(4)) and triiodothyronine (T(3)) glucuronidation. Toxicol Sci 55:78–84
Hornung MW, Korte JJ, Olker JH et al (2019) Screening the toxcast phase 1 chemical library for inhibition of deiodinase type 1 activity. Toxicol Sci 162:570–581
Hsu JH, Zavacki AM, Harney JW, Brent GA (1995) Retinoid-X receptor (RXR) differentially augments thyroid hormone response in cell lines as a function of the response element and endogenous RXR content. Endocrinology 136:421–430
Hurley PM, Hill RN, Whiting RJ (1998) Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ Health Percept 106:437–445
IARC (2017) Species differences in thyroid, kidney and urinary bladder carcinogenesis. In: Capen CC, Dybing E, Rice JM, Wilbourn JD (eds). IARC Scientific Publications No. 147. 1999. https://monographs.iarc.fr/ENG/Publications/pub147/IARCpub147.pdf Accessed 30 Oct 2017
Imai Y, Toyoda N, Maeda A et al (2001) Type 2 iodothyronine deiodinase expression is upregulated by the protein kinase A dependent pathway and is downregulated by the protein kinase C-dependent pathway in cultured human thyroid cells. Thyroid 11:899–907
Ishigaki S, Abramovitz M, Listowsky I (1989) Glutathione-S-transferases are major cytosolic thyroid hormone binding proteins. Arch Biochem Biophys 273:265–272
Ishihara A, Sawatsubashi S, Yamauchi K (2003) Endocrine disrupting chemicals: interference of thyroid hormone binding to transthyretins and to thyroid hormone receptors. Mol Cell Endocrinol 199:105–117
Iwen KA, Schroder E, Brabant G (2013) Thyroid hormone and the metabolic syndrome. Eur Thyroid J 2:83–92
Jahnke GD, Choksi NY, Moore JA et al (2004) Thyroid toxicants: assessing reproductive health effects. Environ Health Percept 112:363–368
Jansen J, Friesema EC, Milici C et al (2005) Thyroid hormone transporters in health and disease. Thyroid 15:757–768
Johnson S, McKillop D, Miller J, Smith IK (1993) The effects on rat thyroid function of an hepatic microsomal enzyme inducer. Hum Exp Toxicol 13:493–499
Judson R, Paul-Friedman K, Houck K, Mansouri K, Browne P, Kleinstreuer N (2019) New approach methods for testing chemicals for endocrine disruption potential. Curr Opin Toxicol 9:40–47
Kabat EA, Moore DH, Landow H (1942) An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J Clin Investig 21:571–577
Kackar R, Srivastava MK, Raizada RB (1997) Studies on rat thyroid after oral administration of mancozeb: morphological and biochemical evaluations. J AppI Toxicol 17:369–375
Kaneko JJ (1989) Thyroid function. In: Kaneko JJ (ed) Clinical biochemistry of domestic animals. Academic Press, Inc., New York, pp 634–635
Karapanou O, Papadimitriou A (2011) Thyroid hormone transporters in the human. Hormones 10:270–279
Kato H, Fukuda T, Parkison C et al (1989) Cytosolic thyroid hormone-binding protein is a monomer of pyruvate kinase. Proc Natl Acad Sci USA 86:7861–7865
Kelly G (2000) Peripheral metabolism of thyroid hormones: a review. Altern Med Rev 5:306–333
Kersseboom S, Visser TJ (2011) MCT8: from gene to disease and therapeutic approach. Ann Endocrinol 72:77–81
Kester MHA, Van Dijk CH, Tibboel D et al (1999) Sulfation of thyroid hormone by estrogen sulfotransferase. J Clin Endocrinol Metab 84:2577–2580
Kester MH, Kaptein E, Van Dijk CH et al (2002) Characterization of iodothyronine sulfatase activities in human and rat liver and placenta. Endocrinology 143:814–819
Kieffer JD, Mover H, Federico P et al (1976) Pituitary–thyroid axis in neonatal and adult rats: comparison of the sexes. Endocrinology 98:295–304
Kitahara CM, Sosa JA (2016) The changing incidence of thyroid cancer. Nat Rev Endocrinol 12:646–653
Klaassen CD, Hood AM (2001) Effects of microsomal enzyme inducers on thyroid follicular cell proliferation and thyroid hormone metabolism. Toxicol Pathol 29:34–40
Klammer H, Schlecht C, Wuttke W et al (2007) Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamo-pituitary-thyroid function in rats. Toxicology 238:192–199
Kliewer SA, Umesono K, Mangelsdorf DJ et al (1992) Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone, and vitamin D3 signalling. Nature 355:446–449
Knowles JM, Garrett GS, Gorstein J et al (2017) Household coverage with adequately iodized salt varies greatly between countries and by residence type and socioeconomic status within countries: results from 10 national coverage surveys. J Nutr 147:1004S-1014S
Knudsen N, Laurberg P, Rasmussen LB et al (2005) Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 90:4019–4024
Kohrle J, Fang SL, Yang Y et al (1989) Rapid effects of the flavonoid EMD 21388 on serum thyroid hormone binding and thyrotropin regulation in the rat. Endocrinology 125:532–537
Kondo T, Ikenaka Y, Nakayama SMM et al (2017) Uridine diphosphate-glucuronosyltransferase (UGT)2B subfamily interspecies differences in carnivores. Toxicol Sci 158:90–100
Kotwal A, Priya R, Qadeer I (2007) Goitre and other iodine deficiency disorders: a systematic review of epidemiological studies to deconstruct the complex web. Arch Med Res 38:1–14
Kwon D, Chung H-K, Shin W-S et al (2018) Toxicological evaluation of dithiocarbamate fungicide mancozeb on the endocrine functions in male rats. Mol Cell Toxicol 14:105–112
Lans MC, Klasson-Wehler E, Willemsen M et al (1993) Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-pdioxins and -dibenzofurans with human transthyretin. Chem-Biol Int 88:7–21
Larsen PR, Zavacki AM (2012) The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur Thyroid J 1:232–242
Leung AM, Pearce EN, Braverman LE (2010) Perchlorate, iodine and the thyroid. Best practice and research. Clin Endocrinol Metab 24:133–141
Lewandowski TA, Seeley MR, Beck BD (2003) Interspecies differences in susceptibility to perturbation of thyroid homeostasis: a case study with perchlorate. Reg Toxicol Pharmacol 39:348–362
Li M, Eastman CJ (2012) The changing epidemiology of iodine deficiency. Nat Rev Endocrinol 8:434–440
Li Y, Kumazawa T, Ishiguro T et al (2011) Hypothyroidism caused by phenobarbital affects patterns of estrous cyclicity in rats. Congenit Anomalies 51:55–61
Liesenkötter KP, Göpel W, Bogner U et al (1995) Earliest prevention of endemic goitre by iodine supplementation during pregnancy. Eur J Endocrinol 134:443–448
Liu YY, Brent GA (2010) Thyroid hormone crosstalk with nuclear receptor signalling in metabolic regulation. Trends Endocrinol Metab 21:166–173
Liu L, Klaassen CD (1996) Regulation of hepatic sulfotransferases by steroidal chemicals in rats. Drug Metab Dispos 24:854–858
Liu J, Liu Y, Barter RA, Klaassen CD (1995) Alteration of thyroid homeostasis by UDP-glucuronosyltransferase inducers in rats: a dose-response study. J Pharmacol Exp Ther 273:977–985
Liu YY, Heymann RS, Moatamed F et al (2007) A mutant thyroid hormone receptor alpha antagonizes peroxisome proliferator-activated receptor alpha signaling in vivo and impairs fatty acid oxidation. Endocrinology 148:1206–1217
Logan A, Black EG, Gonzalez AM et al (1992) Basic fibroblast growth factor: an autocrine mitogen of rat thyroid follicular cells? Endocrinology 130:2363–2372
Longnecker MP, Wolff MS, Gladen BC et al (2003) Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Percept 111:65–70
Lu C, Cheng S-Y (2010) Thyroid hormone receptors regulate adipogenesis and carcinogenesis via crosstalk signalling with peroxisome proliferator-activated receptors. J Mol Endocrinol 44:143–154
Lueprasitsakul W, Alex S, Fang SL et al (1990) Flavonoid administration immediately displaces thyroxine (T4) from serum transthyretin, increases serum free T4, and decreases serum thyrotropin in the rat. Endocrinology 126:2890–2895
Maciel RMB, Moses AC, Villone G et al (1988) Demonstration of the production and physiological role of insulin-like growth factor 11 in rat thyroid follicular cells in culture. J Clin Invest 82:1546–1553
Mackenzie C, Mackenzie JB (1943) Effect of sulfonamides and thioureas on the thyroid gland and basal metabolism. Endocrinology 32:185–209
Mackenzie JB, Mackenzie CG, McCollum EV (1941) The effect of sulfanyl guanidine on the thyroid in the rat. Science 94:518–519
Maglich JM, Watson J, McMillen PJ et al (2004) The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem 279:19832–19838
Malendowicz LK (1977) Sex dimorphism in the thyroid gland. I. Morphometric studies on the thyroid gland of intact adult male and female rats. Endokrinologie. 69(3):326–328
Malendowicz LK, Majchrzak M (1981) Sex dimorphism in the thyroid gland. III. Morphometric studies on the rat thyroid gland in the course of postnatal ontogenesis. Endokrinologie 77(3):297–302
Malik R, Hodgson H (2002) The relationship between the thyroid gland and the liver. Q J Med 95:559–569
Marinovich M, Guizzetti M, Ghilardi F et al (1997) Thyroid peroxidase as toxicity target for dithiocarbamates. Arch Toxicol 71:508–512
Mazonakis M, Tzedakis A, Damilakis J et al (2007) Thyroid dose from common head and neck CT examinations in children: is there an excess risk for thyroid cancer induction? Eur Radiol 17:1352–1357
McClain RM (1989) The significance of hepatic microsomal enzyme induction and altered thyroid function in rats: implications for thyroid gland neoplasia. Toxicol Pathol 17:294–306
McClain RM (1995) Mechanistic considerations for the relevance of animal data on thyroid neoplasia to human risk assessment. Mut Res/Fund Mol Mech Mutagen 333:131–142
McAninch EA, Bianco AC (2016) The history and future of treatment of hypothyroidism. Ann Int Med 164:50–56
McClain RM, Posch RC, Bosakowski T et al (1988) Studies on the mode of action for thyroid gland tumor promotion in rats by phenobarbital. Toxicol Appl Pharmacol 94:254–265
McClain RM, Levin AA, Posch R et al (1989) The effect of phenobarbital on the metabolism and excretion of thyroxine in rats. Toxicol Appl Pharmacol 99:216–228
McGirr EM, Clement WE, Currie AR et al (1959) Impaired dehalogenase activity as cause of goitre with malignant changes. Scott Med J 4:232–241
McNabb FMA, Darras VM (2014) Anatomy, embryology, and histology of thyroid glands. In: Scanes C (ed) Sturkie’s Avian physiology, 6th edn. Academic Press, New York, pp 535–547
McTiernan AM, Weiss NS, Daling JR (1984) Incidence of thyroid cancer in women in relation to previous exposure to radiation therapy and history of thyroid disease. J Natl Cancer Inst 73:575–581
Meek ME, Palermo CM, Bachman AN et al (2014a) Mode of action human relevance (species concordance) framework: evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol 34:595–606
Meek ME, Boobis A, Cote I et al (2014b) New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol 34:1–18
Meerts IA, van Zanden JJ, Luijks EA et al (2000) Potent competitive interactions of some brominated flame retardants and related compounds wuth human transthyretin in vitro. Toxicol Sci 56:96–104
Mendel CM, Cavalieri RR, Weisiger RA (1988) Uptake of thyroxine by the perfused rat liver: implications for the free hormone hypothesis. Am J Physiol 255:E110–E119
Merrill EA, Clewell RA, Gearhart JM et al (2003) PBPK predictions of perchlorate distribution and its effect on thyroid uptake of radioiodide in the male rat. Toxicol Sci 73:256–269
Miller MD, Crofton KM, Rice DC et al (2009) Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Percept 117:1033–1041
Minelli R, Gardini E, Bianconi L et al (1992) Subclinical hypothyroidism, overt thyrotoxicosis and subclinical hypothyroidism: the subsequent phases of thyroid function in a patient chronically treated with amiodarone. J Endocrinol Investig 15:853–855
Monk JA, Sims NA, Dziegielewska KM et al (2013) Delayed development of specific thyroid hormone-regulated events in transthyretin null mice. Am J Physiol Endocrinol Metab 304:E23–E31
Moriarty GC, Tobin RB (1976) An immunocytochemical study of TSH storage in rat thyroidectomy cells with and without D or L thyroxine treatment. J Histochem Cytochem 24:1140–1149
Morse DC, Groen D, Veerman M et al (1993) Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in foetal and neonatal rats. Toxicol Appl Pharmacol 122:27–33
Motomura K, Brent GA (1998) Mechanisms of thyroid hormone action. Implications for the clinical manifestation of thyrotoxicosis. Endocrinol Metab Clin N Am 27:1–23
Mullur R, Liu Y-Y, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382
Murakami M, Araki O, Hosoi Y et al (2001) Expression and regulation of type II iodothyronine deiodinase in human thyroid gland. Endocrinology 142:2961–2967
Nagao H, Imazu T, Hayashi H et al (2011) Influence of thyroidectomy on thyroxine metabolism andturnover rate in rats. J Endocrinol 210:117–123
Narayana SK, Woods DR, Boos CJ (2011) Management of amiodarone-related thyroid problems. Ther Adv Endocrinol Metab 2:115–126
Nishikawa S (1983) Effects of sulfonamide on the pituitary-thyroid gland. 1. Morphological changes of thyroid gland and variation in plasma thyroxine and triiodothyronine. J Toxicol Pathol 8:47–59
Nishimura M, Naito S (2008) Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmaco 23:22–44
Norford DC, Meuten DJ, Cullen JM et al (1993) Pituitary and thyroid gland lesions induced by 2-mercaptobenzimidazole (2-MBI) inhalation in male fischer-344 rats. Toxicol Pathol 21:456–464
Noyes PD, Friedman KP, Browne P et al (2019) Evaluating chemicals for thyroid disruption: opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ Health Perspect. https://doi.org/10.1289/EHP5297
NTP (1995) National Toxicology Program Technical Report Series No. 388. NTP technical report on the perinatal toxicology and carcinogenesis studies of ethylene thiourea (CAS No. 96-45-7) in F344/n rats and B6C3F1 mice (feed studies). https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr388.pdf. Accessed 1 Apr 2020
NTP (2006) NTP technical report on the toxicology and carcinogenesis studies of 2,2’,4,4’,5,5’- Hexachlorobiphenyl (PCB 153) (CAS NO. 35065-27-1) in Female Harlan Sprague-Dawley Rats (Gavage Studies). May 2006. NTP TR529. NIH Publication No. 06-4465, National Institutes of Health Public Health Service, U.S. Department of Health and Human Services
Oetting A, Yen PM (2007) New insights into thyroid hormone action. Best practice & research. Clin Endocrinol Metabol 21:193–208
Olker JH, Korte JJ, Denny JS, Hartig PC, Cardon MC, Knutsen CN, Kent PM, Christensen JP, Degitz SJ (2019) Hornung MW screening the toxcast phase 1, phase 2, and e1k chemical libraries for inhibitors of iodothyronine deiodinases. Tox Sci 168(2):430–442
O'Neil WM, Marshall WD (1984) Goitrogenic effects of ethylenethiourea on rat thyroid. Pestic Biochem Physiol 21:92–101
Oppenheimer JH, Bernstein G, Surks MI (1968) Increased thyroxine turnover and thyroidal function after stimulation of hepatocellular binding of thyroxine by phenobarbital. J Clin Invest 47:1399–1406
Oppenheimer JH, Schwartz HL, Lane JT et al (1991) Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Investig 87:125–132
Palha JA, Episkopoull V, Maeda S et al (1994) Thyroid hormone metabolism in a transthyretin-null mouse strain. J Biol Chem 269:33135–33139
Palha JA, Fernandes R, de Escobar GM et al (2000) Transthyretin regulates thyroid hormone levels in the choroid plexus, but not in the brain parenchyma: study in a transthyretin null mouse model. Endocrinology 141:3267–3272
Palha J, Nissanov J, Fernandes R et al (2002) Thyroid hormone distribution in the mouse brain: the role of transthyretin. Neuroscience 113:837–847
Papineni S, Marty SM, Rasoulpour RJ et al (2015) Mode of action and human relevance of pronamide-induced rat thyroid tumors. Reg Toxicol Pharmacol 71:541–551
Pardridge WM, Mietus LJ (1979) Influx of thyroid hormones into rat liver in vivo: differential availability of thyroxine and triiodothyronine bound by plasma proteins. J Clin Invest 66:367–374
Parker SL, Tong T, Bolden S et al (1997) Cancer statistics. CA Cancer J Clin 47:5–27
Parmentier M, Libert F, Maenhaut C et al (1989) Molecular cloning of the thyrotropin receptor. Science 246(4937):1620–1622
Paul K (2014) Using adverse outcome pathway analysis to identify gaps in high-throughput screening for thyroid disruption. Bayer CropScience. https://ntp.niehs.nih.gov/iccvam/meetings/aop-wksp-2014/presentations/paul-508.pdf. Accessed 29 Oct 2017
Peeters RP, Kester MH, Wouters PJ et al (2005) Increased thyroxine sulfate levels in critically ill patients as a result of a decreased hepatic type I deiodinase activity. J Clin Endocrinol Metabol 90:6460–6465
Pendergrast WJ, Milmore BK, Marcus SC (1961) Thyroid cancer and thyrotoxicosis in the United States: their relation to endemic goitre. J Chronic Dis 13:22–38
Peterson PA, Rask L, Ostberg L et al (1973) Studies on the transport and cellular distribution of vitamin A in normal and vitamin A-deficient rats with special reference to the vitamin A-binding plasma protein. J Biol Chem 248:4009–4022
Piersma AH, Verhoef A, te Biesebeek JD, Pieters MN, Slob W (2000) Developmental toxicity of butyl benzyl phthalate in the rat using a multiple dose study design. Reprod Toxicol 14:417–425
Power DM, Elias NP, Richardson SJ et al (2000) Evolution of the thyroid hormone-binding protein, transthyretin. Gen Comp Endocrinol 119:241–255
Price RJ, Burch R, Chatham LR et al (2020) An assay for screening xenobiotics for inhibition of rat thyroid gland peroxidase activity. Xenobiotica 50:318–322
Qatanani M, Zhang J, Moore DM (2005) Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology 146:995–1002
Radominska-Pandya A, Ouzzine M, Fournel-Gigleux S et al (2005) Structure of UDP-glucuronosyltransferases in membranes. Methods Enzymol 400:116–147
Ramhøj L, Hass U, Gilbert ME et al (2020) Evaluating thyroid hormone disruption: investigations of long-term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (PFHxS). Sci Rep 10:2672. https://doi.org/10.1038/s41598-020-59354-z
Refetoff S (2000) Thyroid hormone serum transport proteins. [Updated 2015 Jun 7]. In: Feingold KR, Anawalt B, Boyce A et al (eds) Endotext [Internet]. South Dartmouth: MDText.com, Inc. https://www.ncbi.nlm.nih.gov/books/NBK285566/
Ren SG, Malozowski S, Simoni C et al (1988) Dose-response relationship between thyroid hormone and growth velocity in cynomolgus monkeys. J Clin Endocrinol Metab 66:1010–1013
Renehan AG, Tyson M, Egger M et al (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371:569–578
Riccabona G (1986) Thyroid cancer and other surgical problems in the areas of endemic goitre. In: Dunn JT, Pretell EA, Daza CH, Viteri FE (eds) Towards the eradication of endemic goitre, cretenism, and iodine deficiency. Pan American Health Organization, Washington DC, p 68
Riccabona G (1987) Correlation of thyroid cancer with endemic goitre. In: Riccabona G (ed) Thyroid cancer, its epidemiology, clinical features and treatment. Springer, Berlin, pp 6–14
Richardson SJ (2002) The evolution of transthyretin synthesis in vertebrate liver, in primitive eukaryotes and in bacteria. Clin Chem Lab Med 40:1191–1199
Richardson SJ (2007) Cell and molecular biology of transthyretin and thyroid hormones. Int Rev Cytol 258:137–193
Richardson SJ, Wettenhall REH, Schreiber G (1996) Evolution of transthyretin gene expression in the liver of Didelphis virginiana and other American marsupials. Endocrinology 137:3507–3512
Richardson VM, Ferguson SS, Sey YM et al (2014) In vitro metabolism of thyroxine by rat and human hepatocytes. Xenobiotica 44:391–403
Rinaldi S, Lise M, Clavel-Chapelon F et al (2012) Body size and risk of differentiated thyroid carcinomas: findings from the EPIC study. Int J Cancer 131:E1004–E1014
Robbins J (2000) Thyroid hormone transport proteins and the physiology of hormone binding. In: Braverman LE, Utiger RD (eds) Werner and Ingbar’s, the thyroid, 8th edn. Lippincott Williams & Wilkins, Philadelphia, pp 105–120
Robbins J (2002) Transthyretin from discovery to now. Clin Chem Lab Med 40:1183–1190
Robbins J, Edelhoch H (1986) Peripheral hormone metabolism Hormone transport in blood. In: lngbar SH, Bravserman LE (eds) Werner's the thyroid, 5edn. Lippincott Press, Philadelphia, pp 116-127
Rosene ML, Wittmann G, Arrojo e Drigo R et al (2010) Inhibition of the type 2 iodothyronine deiodinase underlies the elevated plasma TSH associated with amiodarone treatment. Endocrinology 151:5961–5970
Rouaze-Romet M, Savu L, Vranckx R et al (1992) Re-expression of thyroxine-binding globulin in post-weaning rats during protein or energy malnutrition. Acta Endocrinol (Cph) 127:441–448
Rouquié D, Tinwell H, Blanck O et al (2014) Thyroid tumor formation in the male mouse induced by fluopyram is mediated by activation of hepatic CAR/PXR nuclear receptors. Reg Toxicol Pharmacol 70:673–680
Rousset B, Dupuy C, Miot F et al (2000) Chapter 2 thyroid hormone synthesis and secretion. [Updated 2015 Sep 2]. In: De Groot LJ, Chrousos G, Dungan K (eds) Endotext [Internet]. MDText.com, Inc., South Dartmouth. https://www.ncbi.nlm.nih.gov/books/NBK285550/
Ruf J (2006) Structural and functional aspects of thyroid peroxidase. Arch Biochem Biophys 445:269–277
Russfield AB (1967) Pathology of the endocrine glands, ovary and testis of rats and mice. In: Cotchin E, Roe FJ (eds) Pathology of laboratory rats and mice. Blackwell Scientific Publications, Oxford, pp 391–403
Sarne D (2000) Effects of the environment, chemicals and drugs on thyroid function. [Updated 2016 Sep 27]. In: De Groot LJ, Chrousos G, Dungan K et al (eds) Endotext [Internet]. MDText.com, Inc., South Dartmouth. https://www.ncbi.nlm.nih.gov/books/NBK285560/
Scanlon MF, Toft AD (2000) Regulation of thyrotropin secretion. In: Braverman LE, Utiger RD (eds) Werner and Ingbar’s, the thyroid, 8th edn. Lippincott Williams & Wilkins, Philadelphia, pp 234–253
Schlenker EH, Hora M, Liu Y et al (2008) Effects of thyroidectomy, T4, and DITPA replacement on brain blood vessel density in adult rats. Am J Physiol Regul Integr Comp Physiol 294:R1504-1509
Schroder-van der Elst JP, Van der Heide D, Rokos H et al (1998) Synthetic flavonoids cross the placenta in the rat and are found in foetal brain. Am J Physiol 274:E253–E256
Schussler GC (2000) The thyroxine-binding proteins. Thyroid 10:141–149
Schuur AG, Legger FF, van Meeteren ME et al (1998) In vitro inhibition of thyroid hormone sulfation by hydroxylated metabolites of halogenated aromatic hydrocarbons. Chem Res Toxicol 11:1075–1081
Schwartz CE, Stevenson RE (2007) The MCT8 thyroid hormone transporter and Allan–Herndon–Dudley syndrome. Best practice and research. Clin Endocrinol Metabol 21:307–321
Segni M (2000) Disorders of the thyroid gland in infancy, childhood and adolescence. [Updated 2017 Mar 18]. In: Feingold KR, Anawalt B, Boyce A et al (eds) Endotext [Internet]. MDText.com, Inc.: South Dartmouth. https://www.ncbi.nlm.nih.gov/books/NBK279032/
Seibert FB, Nelson JW (1942) Electrophoretic study of the blood protein response in tuberculosis. J Biol Chem 143:29–38
Seoane LM, Carro E, Tovar S et al (2000) Regulation of in vivo TSH secretion by leptin. Reg Peptides 92:25–29
Sharlin DS, Tighe D, Gilbert ME et al (2008) The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology 149:2527–2536
Shopsin B, Shenkman L, Blum M et al (1973) Iodine and lithium-induced hypothyroidis. Documentation of synergism. Am J Med 55:695–699
Siegel RD, Lee SL (1998) Toxic nodular goitre. Toxic adenoma and toxic multinodular goitre. Endocrinol Metabol Clin N Am 27:151–168
Slob W, Moerbeek M, Rauniomaa E, Piersma AH (2005) A statistical evaluation of toxicity study designs for the estimation of the benchmark dose in continuous endpoints. Toxicol Sci 84(1):167–185 (Epub 2004 Oct 13)
Smith PF, Grossman SJ, Gerson RJ et al (1991) Studies on the mechanism of simvastatin-induced thyroid hypertrophy and follicular cell adenoma in the rat. Toxicol Pathol 19:197–205
Sorenson RL, and Belje TC (2014) Atlas of human histology. In: A Guide to microscopic structure of cells, tissues and organs, 3rd edn. University of Minnesota Bookstore, Minneapolis, pp 367
Sousa J, de Escobar GM, Oliveira P et al (2005) Transthyretin is not necessary for thyroid hormone metabolism in conditions of increased hormone demand. J Endocrinol 187:257–266
Surks MI, Sievert R (1995) Drugs and thyroid function. N Engl J Med 333:1688–1694
Sutherland RL, Brandon MR (1976) The thyroxine-binding properties of rat and rabbit serum proteins. Endocrinol 98:91–98
Suttie A (2017) Boorman’s pathology of the rat. In: Reference and atlas, 2nd edn. Academic Press
Swarm RI, Roberts GKS, Levy AC et al (1973) Observations on the thyroid glands in rats following administration of sulphomethoxazole and trimethoprim. Toxicol Appl Pharmacol 24:351–363
Takayama S, Aihara K, Onodera T et al (1986) Anti-thyroid effects of propylthiouracil ans sulphamonomethoxine in rats and monkeys. Toxicol Appl Pharmacol 82:191–199
Tani Y, Mori Y, Miura Y et al (1994) Molecular cloning of the rat thyroxine-binding globulin gene and analysis of its promoter activity. Endocrinology 135:2731–2736
Tani Y, Maronpot RR, Foley JF et al (2004) Follicular epithelial cell hypertrophy induced by chronic oral administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Harlan Sprague–Dawley rats. Toxicol Pathol 32:41–49
Tanjasiri P, Kozbur X, Florsheim WH (1976) Somatostatin in the physiologic feedback control of thyrotropin secretion. Life Sci 19:657–660
Teubner W, Meinl W, Florian S et al (2007) Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem J 404:207–215
Thomas GA, Williams ED (1991) Evidence for and possible mechanisms of non-genotoxic carcinogenesis in the rodent thyroid. Mut Res 248:357–370
Turyk ME, Anderson HA, Persky VW (2007) Relationships of thyroid hormones with polychlorinated Biphenyls, dioxins, furans, and DDE in adults. Environ Health Percept 115:1197–1203
U.S. EPA (2016) Series 890 - endocrine disruptor screening program test guidelines. Series 890 - Endocrine Disruptor Screening Program Test Guidelines | Test Guidelines for Pesticides and Toxic Substances | US EPA. Accessed 10 Dec 2020
U.S. EPA. (1998) Assessment of thyroid follicular cell tumors. Washington, DC: EPA Document No. DC EPA/630/R-97/002. https://www.epa.gov/sites/production/files/2014-11/documents/thyroid.pdf. Accessed 21 Nov 2020
Van Der Haar F (2007) Goitre and other iodine deficiency disorders: a systematic review of epidemiological studies to deconstruct the complex web. Arch Med Res 38:586–587
Vansell NR, Klaassen CD (2002) Increase in rat liver UDP glucuronosyltransferase mRNA by microsomal enzyme inducers that enhance thyroid hormone glucuronidation. Drug Metab Dispos 30:240–246
Verburg FA, Reiners C (2010) The association between multinodular goitre and thyroid cancer. Minerva Endocrinol 35:187–192
Vinken M, Kramer N, Allen TEH et al (2020) The use of adverse outcome pathways in the safety evaluation of food additives. Arch Toxicol 94:959–9966
Visser TJ (1998) In: Cooke BA, King RJB, Van der Molen HJ (eds) Hormones and their actions, part I. Elsevier Science Publishers, Amsterdam, pp 81–103
Visser TJ (1994) Role of sulfation in thyroid hormone metabolism. Chem Biol Int 92:293–303
Visser TJ (1996) Pathways of thyroid hormone metabolism. Acta Med Austriaca 23:10–16
Visser TJ, Rutgers M, de Herder WW et al (1988) Hepatic metabolism, biliary clearance and enterohepatic circulation of thyroid hormone. Acta Med Austriaca 15(Suppl 1):37–39
Visser TJ, Kaptein E, van Raaij JA et al (1993a) Multiple UDP-glucuronyltransferases for the glucuronidation of thyroid hormone with preference for 3,3’,5’-triiodothyronine (reverse T3). FEBS Lett 315:65–68
Visser TJ, Kaptein E, van Toor H et al (1993b) Glucuronidation of thyroid hormone in rat liver: effects of in vivo treatment with microsomal enzyme inducers and in vitro assay conditions. Endocrinology 133:2177–2186
Visser WE, Friesema EC, Visser TJ (2011) Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol 25:1–14
Visser WE, Visser TJ, Peeters RP (2016) Metabolism of thyroid hormone. http://www.thyroidmanager.org/wp-content/uploads/chapters/metabolism-of-thyroid-hormone.pdf. Accessed 21 Oct 2017
Vranckx R, Rouaze M, Savu L et al (1990a) The hepatic biosynthesis of rat thyroxine binding globulin (TBG): demonstration, ontogenesis, and up-regulation in experimental hypothyroidism. Biochem Biophys Res Commun 167:317–322
Vranckx R, Savu L, Maya M et al (1990b) Characterization of a major development-regulated serum thyroxine-binding globulin in the euthyroid mouse. Biochem J 271:373–379
Wagner MS, Morimoto R, Dora JM et al (2003) Hypothyroidism induces type 2 iodothyronine deiodinase expression in mouse heart and testis. J Mol Endocrinol 31:541–550
Wang LQ, James MO (2006) Inhibition of sulfotransferases by xenobiotics. Curr Drug Metab 7:83–104
Wang LQ, Falany CN, James MO (2004) Triclosan as a substrate and inhibitor of 3’-phosphoadenosine 5’-phosphosulphate-sulphotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos 32:1162–1169
Watson ID (1980) Effects on thyroid functions of sulphonamide and trimethoprim combination drugs. Br. Med J 281:646–647
WHO (2009) Principles and methods for the risk assessment of chemicals in food. ISBN: 9789241572408. https://www.who.int/publications/i/item/principles-and-methods-for-the-risk-assessment-of-chemicals-in-food Accessed 21 Nov 2020
Williams ED (1995) Mechanisms and pathogenesis of thyroid cancer in animals and man. Mut Res 333:123–129
Wu KM, Farrelly JG (2006) Preclinical development of new drugs that enhance thyroid hormone metabolism and clearance: inadequacy of using rats as an animal model for predicting human risks in an IND and NDA. Am J Ther 132:141–144
Wu SY, Green WL, Huang WS et al (2005) Alternate pathways of thyroid hormone metabolism. Thyroid 15:943–958
Yen PM (2003) Molecular basis of resistance to thyroid hormone. Trends Endocrinol Metab 14:327–333
York RG, Funk KA, Girard MF et al (2003) Oral (drinking water) developmental toxicity study of ammonium perchlorate in Sprague–Dawley rats. Int J Toxicol 22:453–464
Yu KO, Narayanan L, Mattie DR et al (2002) The pharmacokinetics of perchlorate and its effect on the hypothalamus-pituitary-thyroid axis in the male rat. Toxicol Appl Pharmacol 182:148–159
Żerek-Meleń G, Lewiński A, Pawlikowski M et al (1987) Influence of somatostatin and epidermal growth factor (EGF) on the proliferation of follicular cells in the organ-cultured rat thyroid. Res Exp Med 187:415–421
Zhang Y, Guo GL, Han X et al (2008) Do polybrominated diphenyl ethers (PBDEs) increase the risk of thyroid cancer? Biosci Hypotheses 1:195–199
Zimmermann MB (2009) Iodine deficiency. Endo Rev 30:376–408
Zimmermann MB, Galetti V (2015) Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 8:8. https://doi.org/10.1186/s13044-015-0020-8
Acknowledgements
The author acknowledges the financial support of the European Crop Protection Agency in financially supporting the preparation of this manuscript.
Funding
The preparation of this manuscript was funded by the European Crop Protection Association.
Author information
Authors and Affiliations
Contributions
All three authors contributed equally to the preparation and review of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Foster, J.R., Tinwell, H. & Melching-Kollmuss, S. A review of species differences in the control of, and response to, chemical-induced thyroid hormone perturbations leading to thyroid cancer. Arch Toxicol 95, 807–836 (2021). https://doi.org/10.1007/s00204-020-02961-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02961-6
Keywords
- Thyroid
- Rodent
- Human
- Species differences
- chemical-induced
- Comparative biology
- Carcinogenesis
- Thyroid hormones
- Thyrotropin
- Hepatic
- Clearance
- Thyroxine
- Triiodothyronine
- Thyroid peroxidase
- Sodium iodide symporter
- Deiodinase
- Inhibition
- Liver
- Glucuronidation
- Sulphation
- Xenobiotics
- Thyroxine binding protein
- Transthyretin
- Albumin