Abstract
Glyphosate is the most widely used herbicide worldwide. It is a broad spectrum herbicide and its agricultural uses increased considerably after the development of glyphosate-resistant genetically modified (GM) varieties. Since glyphosate was introduced in 1974, all regulatory assessments have established that glyphosate has low hazard potential to mammals, however, the International Agency for Research on Cancer (IARC) concluded in March 2015 that it is probably carcinogenic. The IARC conclusion was not confirmed by the EU assessment or the recent joint WHO/FAO evaluation, both using additional evidence. Glyphosate is not the first topic of disagreement between IARC and regulatory evaluations, but has received greater attention. This review presents the scientific basis of the glyphosate health assessment conducted within the European Union (EU) renewal process, and explains the differences in the carcinogenicity assessment with IARC. Use of different data sets, particularly on long-term toxicity/carcinogenicity in rodents, could partially explain the divergent views; but methodological differences in the evaluation of the available evidence have been identified. The EU assessment did not identify a carcinogenicity hazard, revised the toxicological profile proposing new toxicological reference values, and conducted a risk assessment for some representatives uses. Two complementary exposure assessments, human-biomonitoring and food-residues-monitoring, suggests that actual exposure levels are below these reference values and do not represent a public concern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glyphosate is the most widely used herbicide in the world. A broad spectrum herbicide, its uses include weed control in agriculture, vegetation control in non-agricultural areas, and harvesting aid as crop desiccant. Its use in agriculture has increased considerably due to the development of glyphosate-resistant GM crop varieties; the herbicide has also been used to control illegal crops through massive aerial applications (Solomon et al. 2007). The widespread use and public debate regarding these uses have aroused societal concern and a scientific controversy on the toxicity of glyphosate (Faria 2015) beyond the scientific debate (Blaylock 2015).
Glyphosate was considered an advantageous herbicide until its use led to the evolution of glyphosate-resistant weeds (Duke and Powles 2008) and studies suggesting effects of glyphosate-based formulations in humans and wildlife were published. Interest in glyphosate has increased exponentially among scientists, and the subject accounted for 5% of the articles on pesticides included in PubMed during 2015. About 25% of the articles cover the toxicity endpoints in humans and all types of organisms, and the majority is conducted with glyphosate-based formulations, containing other ingredients. Some ingredients may be more toxic than glyphosate for non-plant species (Kim et al. 2013; Mesnage et al. 2013; Nobels et al. 2011), ingredients classified as carcinogenic or mutagenic are not expected to be used and must be indicated in the label, however, the full composition of the formulation is not disclosed by the manufacturers, therefore, it is impossible for researchers to apply mixture toxicity methods and attribute toxicity to specific ingredients.
The risk assessment of a pesticide for human health integrates two aspects. First, the hazard identification clarifies the toxicological profile of the substance, setting the type of health effects it is expected to produce in humans depending on the level of exposure, triggering the hazard classification and setting the toxicological reference values to be used in the risk assessment. Then, for each intended use, the expected level of exposure is calculated and compared with the reference values. While the hazard potential is intrinsic and, therefore, expected to be equivalent in all evaluations, the risk is related to the use of the substance—which is defined as the likelihood and magnitude of adverse effects—and strongly depends on the patterns and conditions of use.
Glyphosate has been the subject of regular assessments by national and international regulatory agencies (JMPR 2006; Williams et al. 2000). All had established that glyphosate has a relatively low toxicity in mammals. However, a recent report from the International Agency for Research on Cancer (IARC) concluded that the herbicide and its formulated products are probably carcinogenic in humans (Guyton et al. 2015a, b; IARC 2015). The aim of IARC’s assessments is to identify carcinogenicity hazards as a first step in carcinogenic risk assessment. IARC assessments do not include recommendations regarding regulatory or legislative decisions; they are scientific evaluations informing regulatory assessments. Consequently, the IARC conclusion triggered a reconsideration of the evidence on carcinogenicity in the EU evaluation, and more recently by the Joint FAO/WHO Meeting on Pesticide Residues. The European Union renewal process (European Food Safety Authority 2015a, b; Germany 2015) was the first comprehensive regulatory assessment of glyphosate conducted after the IARC evaluation. Following a detailed assessment of all available information, the European assessment reached a different conclusion, increasing the scientific and social debate. In 2016 the Joint FAO/WHO Meeting on Pesticide Residues concluded that glyphosate is not carcinogenic in rats but could not exclude the possibility that it is carcinogenic in mice at very high doses, this information was used in the risk assessment concluding that glyphosate is unlikely to pose a carcinogenic risk to humans from exposure through the diet (JMPR 2016). This manuscript explores possible reasons for the different conclusions, with a focus on the EU assessment, as this is the evaluation in which the authors have been involved.
Typically, regulatory assessments come to conclusions similar to those of IARC, but there are exceptions (Pearce et al. 2015). Scientific divergences may result from different sets of evidence, different approaches and methods, or different interpretations when weighing ambiguous results. Divergences are particularly likely when one evaluation includes additional evidence. In this context, it is important to mention that the EU evaluation, which considered studies not available to IARC, also updated the toxicological profile of glyphosate, proposing new toxicological reference values.
IARC monographs cover carcinogenicity hazard identification. When statistical associations between exposure and cancer incidences are observed in epidemiological studies, the assessment of causal relationships may lead to divergent conclusions (Rhomberg 2015a, b). The comparison of both glyphosate assessments is used below to explain the different aims, methods and possible divergences between regulatory and IARC assessments—focusing on the glyphosate carcinogenicity hazard identification as a case study—and, more importantly, their role in the assessment of risks to consumers and public health concerns. The example is particularly useful as both evaluations were conducted within the same period, and as the EU assessment, based on the United Nations Globally Harmonised System (UN-GHS) for classification of chemicals, is also relevant in the broad international context.
Methodology: scientific assessment of carcinogenicity and its use in the regulatory context
Pesticides are heavily regulated chemicals and require pre-marketing authorisation in most jurisdictions. The EU system also includes a renewal process, requiring all pesticides to be regularly re-assessed in the light of new scientific developments and information requirements. The EFSA assessment (European Food Safety Authority 2015b) followed an evaluation carried out by the European Commission in 2002.
The identification of carcinogenic chemicals and carcinogens in food is of high societal and scientific interest (Barlow and Schlatter 2010). The communication of the outcome of the risk assessment is complex and controversial in the case of equivocal results (Downes and Foster 2015). The identification of a mutagenic or genotoxic mechanism plays an important role in risk assessment and requires a critical evaluation of the data as well as expert judgment (Eastmond 2012). The hazard assessment is linked to the classification; the EU uses the hazard assessment system for chemicals developed by the United Nations following the 1992 UN Earth Summit (Pratt 2002). This Globally Harmonised System for classifying chemicals replaces previous national and international approaches, is specifically recommended by FAO to be used for pesticides, and is implemented in the EU Classification, labelling and packaging (CLP) regulation—(EC) No 1272/2008—and other jurisdictions (UNECE http://www.unece.org/trans/danger/publi/ghs/implementation_e.html).
IARC and regulatory assessments are usually complementary. The different roles, methods and information sources of IARC and regulatory assessments, as well as the implications for public health, must be considered in case of divergences and are summarised in Table 1. IARC identifies carcinogenic hazards resulting from occupational, environmental, and lifestyle exposures and agents as a first step of the risk assessment process, and has developed an internationally recognised grouping system that includes defined criteria and methodology (Guyton et al. 2015a, b; Lauby-Secretan et al. 2016; Pearce et al. 2015; Straif et al. 2014). The recently developed approach for assessing mechanistic information, based on the characteristics of IARC group 1 carcinogens, was applied for glyphosate (Smith et al. 2016). Regarding data sources, IARC assessments are primarily based on published evidence, i.e. scientific publications and regulatory assessments; industry-sponsored studies are used when reviewed and reported in regulatory evaluations, becoming a relevant secondary source for regulated agents such as pesticides. Both, scientific publications and mandatory industry-sponsored studies, were primary sources in the EU evaluation.
For pesticides, IARC identifies the “carcinogenic agent” as the active pesticide substance and its commercial formulations; the specific role of the other formulation ingredients in the occurrence of effects is not considered separately from the active ingredients. This is in line with the role of human evidence in IARC assessments. Epidemiological studies of farmers and consumers have very limited information on actual exposure levels (Ntzani et al. 2013), and use the pesticide active substance as descriptor, combining individuals exposed to different formulations without discriminating the different compositions. In the regulatory context, each formulation should be assessed according to its composition, identifying the role of the active substance and of the other ingredients; and the risk management measures are set for the chemical responsible for the effect, either active substance or co-formulant.
The UN-GHS and IARC frameworks use different terminology, but the definitions for sufficient and limited evidence in humans and in animals are similar and can be used to establish equivalences between both schemes, as presented in Table 2.
This approach allows a comparison of the pesticides evaluated by IARC with the current EU classification (Table 3 and supplementary material Annex 1). The EU classification includes scientific assessments conducted by the European Chemicals Bureau of the European Commission—some, but not all, based on EFSA evaluations—and by the Committee for Risk Assessment of the European Chemicals Agency.
A total of 53 pesticides have been assessed under both systems. For about half—29 out of 53—the classifications are equivalent; the EU classification is more severe/conservative for 14 pesticides and less severe/conservative for 11. It should be noted that 8 out of the 11 pesticides with more severe/conservative classification by IARC are those assessed in recent IARC monographs. New substances are evaluated and others re-evaluated regularly, leading to changes in the classification; thus the table represents just a “screen-shot” of two rolling processes. Differences with IARC and between jurisdictions have also been reported for other regulatory assessments (Choi and Lim 2010). Both IARC and regulatory classifications are based on the information available at the time of the evaluation. For pesticides, the identification of possible concerns triggers the generation of additional evidence and a subsequent evaluation; consequently, some differences are not real scientific divergences but the result of expert re-evaluations based on different sources of evidence. This may have played a role in the case of glyphosate, as discussed below.
Discussion
Understanding the divergence: glyphosate carcinogenicity assessment
The carcinogenicity of glyphosate has been reviewed by several national and international agencies (Ibrahim 2015). The outcome of the EU assessment, the differences with the IARC evaluation (IARC 2015), and the authors’ views explaining these differences, are summarised below. Additional details are provided in the supporting information.
Human evidence
IARC (2015) offered the most up-to-date review of human epidemiological studies on glyphosate. Positive evidence regarding an association between exposure to glyphosate and non-Hodgkin lymphoma, observed in some case-control studies but not confirmed by cohort studies, was considered sufficient by IARC to conclude on “limited evidence” in humans. Limited evidence is defined as a positive association observed between exposure to the agent and cancer, for which a causal interpretation is considered to be credible, but chance, bias or confounding could not be ruled out with reasonable confidence. This definition was developed by IARC and introduced in the UN-GHS criteria (United Nations 2003) and EU Regulation (EC) No 1272/2008. EFSA re-assessed the same information; the association with non-Hodgkin lymphoma was discussed during an expert meeting. The statistically significant association was considered limited due to low power, lack of consistency, and the view that greater weight should be given to the cohort study for non-rare tumours. Considering causality, the majority of the experts concluded that the epidemiological evidence was very limited, and insufficient for classification. Although the role of the weight attributed to case–control studies versus cohort studies cannot be fully ruled out, the main reason for the divergent views could be the possibility of bias, chance results and confounding effects, as IARC concluded that the limited evidence in humans was supported by sufficient evidence of carcinogenic potential in animals and strong mechanistic evidence for genotoxicity and oxidative stress. As explained below, the EU evaluation used additional evidence regarding animal carcinogenicity and genotoxicity, and reached different conclusions.
Carcinogenicity in animals
Information sources
There is only one published study on the carcinogenicity of the active substance glyphosate in rats (Chruscielska et al. 2000), which showed no significant increase in tumour incidences in any treated group. Two additional published studies on glyphosate formulations, the first one on initiation-promotion in mice (George et al. 2010) and the second one, a study of rats (Seralini et al. 2014) that was retracted and republished creating some controversies (Fagan et al. 2015), were considered inadequate by IARC and EFSA for carcinogenicity assessment (European Food Safety Authority 2012; IARC 2015). Consequently, industry-sponsored studies, required by several jurisdictions worldwide, have constituted the basis for the assessment of animal carcinogenicity by both IARC and EFSA. As expected for a regulatory assessment, EFSA assessed the original study reports. According to their principles, IARC used unpublished studies based on secondary sources, i.e. the information on the studies as published by JMPR (2004) and US-EPA (1993). The time difference, over a decade, between the IARC monograph and the published regulatory assessments must be considered. Five new studies, not assessed by the JMPR and US-EPA, and therefore, not considered by IARC, were considered valid and included in the EU assessment. The IARC assessment is based on the re-assessment of industry-sponsored studies, two in mice and four studies in rats, plus the negative published study in rats. The EU assessment included five additional valid studies, two in mice and three in rats; one mouse study was excluded due to a likely viral infection in the experimental population and one rat study was considered inadequate due to study deficiencies. Table 4 summarises the studies used in the EU assessment; additional information is provided in Table S-2 as supplementary material, with links to the detailed summaries for each study and its assessment as published in the EFSA background document (Germany 2015). Additional information and raw data have been published as supplementary information in a recent industry-sponsored review of glyphosate carcinogenicity (Greim et al. 2015).
Assessment of the available evidence
In its weight of evidence, the IARC Working Group considered a statistically significant trend for renal tumours in male mice in one study (study A in Tables 4, 5) and for haemangiosarcoma in the other (study B in Tables 4, 5). No statistically significant increase in tumour incidence in females was observed in these studies. In the weight of evidence in rats, the IARC Working Group considered increases in the incidence of adenomas, with no evidence of progression to carcinomas, in pancreatic islet cells in males (studies E and F in Table 4), hepatic cells in males (study E in Table 4) and thyroid C-cell in females (study E in Table 4). No increase in tumour incidence was observed in three studies (studies G, K and M in Table 4). The EU assessment followed the weight of evidence approach required by the UN-GHS criteria (United Nations 2015) and further clarified in the ECHA guidance (European Chemicals Agency 2015). The statistical significance found in trend analysis in some studies was balanced against the lack of statistical significance in pair-wise comparison tests, lack of consistency in multiple animal studies, slightly increased incidences only at dose levels at or above the Maximum Tolerable Dose (MTD), lack of pre-neoplastic lesions and/or whether the studies fell within the relevant historical control range. A specific comparison of tumour incidences in male CD-1 mice from four carcinogenicity studies (no change in tumour incidence was observed in females) is provided in Table 5, and the detailed scientific assessment and weight of evidence for each tumour type is summarised in Table 6.
Comparison of both weight of evidence approaches
As indicated by Portier et al. (Portier et al. 2014), individual scientific studies are rarely, if ever, conclusive. In our view, this is particularly relevant when assessing the carcinogenicity potential in humans using animal studies, and supports the need for a consistency check combining all available studies as mandated in the UN-GHS criteria.
In the absence of conclusive human evidence, and despite some views suggesting the need for re-assessing its relevance (Beyer et al. 2011; Marone et al. 2014; Osimitz et al. 2013), rodent long-term toxicity/carcinogenicity studies are used for predicting carcinogenicity in humans (Doktorova et al. 2012). False positives and false negatives should both be considered, weighing the evidence (Lutter et al. 2015; Rhomberg 2015a, b; Rhomberg et al. 2013) and assessing specifically human relevance; and linked to the MTD concept, the relevance of toxicity-induced carcinogenic effects observed in experimental animals only at very high doses. The UN-GHS, and therefore, the EU CLP approach are based on UN harmonised criteria for weighing the evidence from rodent studies. Regulatory (European Chemicals Agency 2015) and non-regulatory (McGregor et al. 2010) guidance is available for weighing the evidence in line with the UN-GHS criteria. Table 7 summarises the assessment of the different UN-GHS Weight of Evidence elements in the EU assessment, and includes a comparison with the weight provided in the IARC evaluation. It should be noted that the authors of this paper did not participate in the IARC assessment, and therefore, the IARC columns are based on the information extracted from the IARC preamble and monograph, and do not reflect the Working Group discussions except when specifically reported in the monograph. The elements detailed in Tables 5, 6 and 7, and used in the EU evaluation, are not only specific components of the regulatory guidance (European Chemicals Agency 2015), but, as described below, are also fully supported by current scientific knowledge on the assessment of animal studies.
Due to the large number of studies, the assessment of chance results is particularly relevant. Dose–response within the study, consistency among similar studies, consistency or justified differences between sexes, and comparison with historical controls, are considered key elements for identifying chance effects. The Bradford Hill guidelines published in 1965 are still considered a reference for assessing causality (Wakeford 2015), and have been included in the IPCS framework and its respective updates (Boobis et al. 2006, 2008; Meek et al. 2014a; Sonich-Mullin et al. 2001). Although the framework focuses on the relevance of the mode of action, dose–response relationships and consistency among studies are also indicated as key elements. The statistical assessment is the first step for assessing the results of the toxicity tests, and has received significant attention from both, regulatory bodies (e.g. OECD guidelines on testing and assessments of chemicals) and academics (Hothorn 2014); nevertheless, the statistical analysis should be considered part of an overall assessment. This is particularly relevant in cases such as glyphosate, where the statistical analysis is inconsistent or inconclusive, with significant differences in the trend, but not in the pair-wise analysis. Lack of consistency at similar doses in the same species and strain and lack of dose–response relationships can be observed for malignant lymphomas in mice (Tables 5, 6) and adenomas in rat (Table 6). Kobayashi et al. (2010) reviewed the grounds for considering statistically significant changes as incidental, observing similar trends for unpublished and peer-reviewed scientific publications. Lack of dose–response is reported as the main justification for disregarding the results as incidental, followed by lack of physiological/toxicological significance of the effects and the comparison with historical controls. These studies support the concern surrounding conclusions that are based only on statistical significance of increased tumour incidences in a particular study, without considerations of the biological relevance of the finding.
Although the concurrent control group is always the most relevant comparator, the use of historical control data, also in combination with background incidental lesions (McInnes and Scudamore 2014), can be essential in cases of equivocal results to detect both, false positive and false negative situations. In addition to best practices (Greim et al. 2003; Keenan et al. 2009), graphical visualisations (Elmore and Peddada 2009) and statistical approaches (Dinse and Peddada 2011; Peddada et al. 2007) have been developed, although direct comparison with the historical control range in the test laboratory around the time of the study is the approach mostly used in the regulatory context, and preferred in the EU assessment. This approach was considered for malignant lymphomas and haemangiosarcomas in mice when the studies reported the historical range for the test laboratory.
Excessive toxicity, for instance toxicity at doses exceeding the MTD, can cause effects such as cell death (necrosis) with associated regenerative hyperplasia, which in turn can lead to tumour development as a secondary effect, unrelated to the intrinsic potential of the substance itself to cause tumours at lower and less toxic doses (European Chemicals Agency 2015; Knight et al. 2006). Also in the assessment of cell proliferation as mode of action for non-genotoxic carcinogens, systemic toxicity and overt cytotoxicity in the target tissue should be avoided (Wood et al. 2015). It has been suggested that almost all chemicals, including those non-genotoxic and without structural alerts for carcinogenicity, would produce statistically significant trends if tested at or above the MTD in a sufficiently large number of animals (Gaylor 2005). Significant trends for tumour induction were observed in two mouse studies but only at very high doses, well above the proposed top dose for carcinogenicity studies (OECD 2012) of 1000 mg/kg bw per day; clear indications of toxicity were observed at these high doses, such as reduced body weight, histopathological changes in the bladder and liver, and other toxic signs; consequently, the tumour induction trends were considered confounding effects due to excessive toxicity.
Mechanistic assessment
The relevance of the mode of action for humans constitutes the basis of the IPCS framework (Boobis et al. 2006, 2008; Meek et al. 2014a; Sonich-Mullin et al. 2001). Mode of action is defined as a biologically plausible series of key events leading to an effect (Sonich-Mullin et al. 2001) and involves interdependent networks of events with feedback loops. Differences in networks between and within human and animal populations account, in part, for interspecies differences and human variability (Meek et al. 2014a). Current approaches explore the applicability of the Adverse Outcome Pathway approach (Collier et al. 2016; Edwards et al. 2016; Zhou 2015) as a framework for linking the initial molecular interactions with the tumour promotion though plausible key events (Becker et al. 2015; Downes and Foster 2015). As the EU evaluation concluded that the incidences were due to chance and bias and the evidence does not indicate that glyphosate is an animal carcinogen, no further assessment of relevance for humans was required.
IARC, with a different focus, not targeted to individual chemicals but to a broad range of agents, has recently developed a new weight of evidence scheme, by extracting the “key characteristics” from the physical/chemical/biological/behavioural agents classified by IARC in category 1 (Smith et al. 2016). These key characteristics are defined as common properties, not to be considered mechanisms of Adverse Outcome Pathways, although are postulated as a method to synthesize information and develop adverse outcome networks. The ten characteristics are the abilities of an agent to: (1) act as an electrophile either directly or after metabolic activation; (2) be genotoxic; (3) alter DNA repair or cause genomic instability; (4) induce epigenetic alterations; (5) induce oxidative stress; (6) induce chronic inflammation; (7) be immunosuppressive; (8) modulate receptor-mediated effects; (9) cause immortalization; and (10) alter cell proliferation, cell death, or nutrient supply. It should be noted that this new approach has been applied to the recent IARC monographs, including the assessment of glyphosate.
Genotoxicity
The EU evaluation considers in vitro genotoxicity tests and in vivo studies performed in mammals, as those are considered to be more relevant for the assessment of the risk to humans (Yauk et al. 2015). Sixteen in vivo studies in somatic cells and two in vivo studies on germ cells were reported on rodents orally treated with dose levels up to 5000 mg/kg bw, or via intraperitoneal injections. All studies were conducted according to internationally validated guidelines; some non-GLP published studies gave negative results, while two non-GLP studies were positive in mice treated intraperitoneally with dose levels in the range of the intraperitoneal LD50 for mice, one study presenting major flaws. No genotoxic effects on germ cells were detected in rats or mice treated orally at dose levels up to 2000 mg/kg bw. The induction of DNA strand breaks observed in mice treated intraperitoneally with doses close to or in excess of the LD50 has been associated to secondary effects of cytotoxicity (JMPR 2006; Kier 2015). Modes of action associated with secondary cytotoxicity should be excluded from the assessment of the intrinsic genotoxicity potential (Bryce et al. 2014; Kitamoto et al. 2015).
IARC combines information on glyphosate and glyphosate-based formulations, compiling studies on humans, other mammals, other vertebrates, invertebrates, and plants. Regarding in vivo mammalian studies, IARC reports positive effects for 5 out of 11 studies; four negative studies on micronucleus formation and dominant lethal mutation reported by JMPR (2006) are not included in the IARC evaluation. Positive effects are described only for intraperitoneal administrations at doses of 300 mg/kg bw. Although these effects had been previously postulated as secondary to (cyto)toxicity (Heydens et al. 2008; JMPR 2006), the role of (cyto)toxicity is not discussed in the IARC monograph. Positive effects are mostly observed in the liver, an organ that is considered inappropriate for assessing in vivo genotoxic effects after intraperitoneal administration (JMPR 2006).
A recent meta-analysis on micronuclei frequency (Ghisi et al. 2016) has confirmed that positive effects are limited to intraperitoneal administrations, and that the response is much higher for glyphosate-based formulations than for the active substance. Cytotoxicity of the surfactants added to the formulations is presented as a plausible explanation, while the cytotoxicity of glyphosate in intraperitoneal administrations at high doses is not discussed. Significant differences are observed for males but not for females, a general difference is reported in the comparison of mammalian and non-mammalian systems, although similar responses are observed for mice and crocodilians (Ghisi et al. 2016).
Non-genotoxic modes of action
Non-genotoxic modes of action for carcinogenicity are assumed for about 9% of IARC classifications (Hernandez et al. 2009) and include endocrine disruption, tumour promotion, tissue-specific toxicity and inflammation, cytotoxicity and immune suppression, inhibition of gap-junction intercellular communications (GJICs), and other mechanisms (Benigni et al. 2013; Hernandez et al. 2009).
In the EU evaluation, the lack of evidence for carcinogenic potential of glyphosate meant that no further thought regarding the mode of action was considered necessary. IARC assessed the “key characteristics of human carcinogens” (Smith et al. 2016), concluding that there is weak evidence for receptor-mediated effects, cell proliferation or death, and immune effects, and strong evidence of oxidative stress.
Role of surfactants and other co-formulants
The EU assessment focuses on glyphosate, aiming to establish the properties of the active substance to be considered in the assessment of each formulation by individual Member States. IARC has a different approach, addressing both glyphosate and its formulations. The potential role of the co-formulants, which differ among formulations, is not assessed; however, the IARC monograph reports a large number of mechanistic studies with negative results for glyphosate but positive results for glyphosate-based formulations, as well as differences between formulations containing similar concentrations of glyphosate, indicating that other ingredients could lead to the effects observed when testing formulations (Coalova et al. 2014; Cox and Surgan 2006). Similar results are observed for other pesticides and particularly for herbicides (Cavas 2011); this is not surprising, as the mode of action leading the herbicidal activity is usually not linked to the toxicological profile in mammals.
Surfactants are frequently used in herbicide formulations, including glyphosate. Polyethoxylated tallowamines are several orders of magnitude more cytotoxic than glyphosate (Mesnage et al. 2013); the mode of action is cell death with inhibition of the mitochondrial succinate dehydrogenase activity and membrane damage leading to necrosis. This mode of action is different from glyphosate, while similar to that observed for glyphosate-based formulations (Benachour and Seralini 2009). These tallowamines also produce oxidative and DNA damage (Nobels et al. 2011), and increase the apoptotic potential of glyphosate (Kim et al. 2013). Other surfactants as well as solvents used in pesticides formulations are cytotoxic and, possibly, genotoxic (Nobels et al. 2011).
The cytotoxicity and potential genotoxicity of other ingredients should be considered before assuming that the effects observed for a formulated product are linked to the active substance. Secondary genotoxic effects produced by cytotoxicity should also be distinguished from true genotoxic potential (Bryce et al. 2014; Kitamoto et al. 2015). In fact, the UN and EU guidance recommends carcinogenicity and genotoxicity studies to be conducted on individual chemicals, limiting testing of mixtures/formulations to cases where synergistic effects are expected (United Nations 2015).
From hazard assessment to public health risk assessment
While IARC focuses exclusively on the hazard identification, regulatory assessments also include the estimation of the toxicological potency of the substance and the setting of toxicological reference values to be used in the human health risk assessment. The toxicological reference values offer quantitative indications of the toxicity of a chemical, indicating the levels of human exposure that, according to the current scientific knowledge, are considered acceptable from a regulatory perspective. The recent EFSA evaluation has changed significantly the toxicological profile of glyphosate, compared to the previous EU assessment (Table 8).
The Acute Reference Dose (ARfD) and Acceptable Daily Intake (ADI) represent oral doses that should not be exceeded in a single event (or repeated within 24 h) or daily in long term exposures, respectively. The Acceptable Operator Exposure Level (AOEL) represents a systemic daily dose that should not be exceeded in non-dietary exposures. Figure 1 visualises the current and previous EU toxicological reference values for glyphosate, compared with those established for the entire group of herbicides assessed in the EU. The ranking and percentile within the distribution of ca. 150 herbicides assessed in the EU (data extracted from the EU pesticides database http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN) gives an indication of the relative toxicity of glyphosate to humans compared to the other herbicides. In contrast with previous evaluations, effects produced after acute exposures were considered relevant, requiring an ARfD and an acute risk assessment (European Food Safety Authority 2015b). The human, animal and mechanistic evidence indicates that glyphosate cannot be considered as a potent DNA reactive tumour-initiating chemical, and that a risk assessment based on threshold toxicological reference values is scientifically valid (SCOEL 2013). The data summarised in Tables 4, 5 and 6 confirms that the proposed reference values (Table 8) provide sufficient protection for all effects observed in the carcinogenicity and long-term toxicity studies, including the trends for tumour induction considered as sufficient evidence by IARC.
Graphical representation of the EFSA proposed changes in the glyphosate toxicological profile expressed as the relative toxicity ranking. This ranking represents the percentile of each glyphosate’s Toxicological Reference Value within the distribution of 141 herbicides assessed in the EU (data extracted from the EU pesticides database. http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.selection&language=EN on 25 May 2016)
Glyphosate has a relative low long-term dietary toxicity, being within the 10% of herbicides with higher ADI. Regarding short-term dietary exposure, the EU assessment proposed an ARfD which ranks glyphosate as slightly more toxic (45th percentile) than the average for herbicides. This new toxicological profile requires the re-assessment of health risks, which had only considered chronic exposure until now (Shao-Wen and Chun-Hong 2015). The need for personal protective equipment for glyphosate applicators is identified in the EFSA Conclusion. The need for an ARfD triggers also new considerations regarding the role of sporadic AOEL exceedance when addressing the risk of short-term inhalation and dermal exposures during application, including bystander and resident exposure in aerial applications, which are standard practice outside the EU in forest (Rolando et al. 2013) and for the control of illegal crops (Benner et al. 2016). Exposure estimations for children entering the area after application (Solomon et al. 2007) are higher than the proposed toxicological threshold.
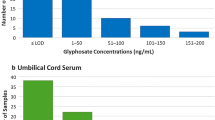
Regarding residues in food, a comprehensive update of the dietary risk assessment will be performed in the EU, following the decision on the approval of glyphosate, covering all EU uses and the residues expected on imported food. Meanwhile, Niemann et al. (2015) have compiled information on human biomonitoring data, and concluded that current exposures are well below the toxicological references values; exposure of European citizens seems to be lower than that of Americans. To complement these estimations, an indicative consumer exposure assessment based on EU monitoring data for glyphosate residues in food generated by competent authorities in the EU Member States is described below. The assessment covers over 10,000 samples of different types of food analysed for glyphosate residues between 2012 and 2014 (Fig. 2). Member States focussed the control activities for glyphosate mainly on crops relevant for human consumption, where the presence of glyphosate was expected, such as cereals (almost 4000 samples), followed by fruits, vegetables, pulses and oilseeds; it should be noted that only limited information is available on feed products such as soya beans (only nine soya beans samples were analysed). Overall glyphosate was detected in 6.3% of the samples, mostly in cereals (11.7% of the samples analysed contained residues above the Limit of Quantification), but also in lentils, linseed and table grapes, mostly from outside the EU. The legal limits were exceeded in 0.2% of the samples analysed for glyphosate. A very conservative risk assessment screening has been conducted with the EFSA PRIMO model (European Food Safety Authority 2007), using conservative assumptions. Table 9 summarises the residue levels measured in food items which were identified as main contributors in the risk assessment using European food consumption data. The data have been extracted from the EU pesticides residues monitoring programme (European Food Safety Authority 2016). Detailed information is provided in the supporting information.
The acute risk assessment used the maximum reported result. The chronic risk assessment used mean residue concentrations, assuming that residues below the Limit of Quantification (LOQ) actually occurred in concentrations equivalent to the LOQ; considering that over 94% of the samples analysed did not contain residues above the LOQ, this assumption contributes to the conservatism of the estimated exposure. The chronic exposure was well below the ADI (0.5% for unprocessed products and 0.6% of the ADI when processed foods are included). In the acute risk assessment, the highest exposure was calculated for lentils (23.4% of the ARfD), followed by beans (14.6%) and wheat (11.6%). Pending on the on-going EFSA assessment, these estimations further support the conclusion that glyphosate residues in food do not represent a public health concern for European citizens.
Conclusions
The following main factors should be considered when explaining the differences between IARC and the EU evaluations: the evidence and information sources, the methodology and the overall aim. The comparison is summarised in Table 10.
Evidence in humans
The same epidemiological studies were used in both assessments; all studies focussed on farmers exposed to formulations. For pesticides, the regulatory dossier may include information on medical surveillance and epidemiological studies on manufacturing plant personnel directly exposed to the active substance; but this was not the case for glyphosate. The key IARC role in compiling and evaluating human evidence is well proven, and the EU assessment was updated to consider recent publications included in the IARC monograph. The same weak evidence in humans for the carcinogenicity of glyphosate was interpreted differently by IARC and EFSA. IARC considered the association between exposure to glyphosate and non-Hodgkin lymphoma as “limited evidence in humans”; while in the EU assessment, most experts considered the evidence as “very limited” and insufficient for triggering the classification. The difference in the interpretation between IARC and the EU is mainly related to the fact that IARC is because IARC considered that glyphosate is carcinogenic in animals, and concluded that strong evidence for two mechanisms, genotoxicity and oxidative stress, supported the plausibility of the weak association in humans.
Evidence on carcinogenicity in experimental animal models
Regarding animal carcinogenicity, three main aspects should be considered for understanding the different conclusions from IARC and EFSA. Lack of consistency among studies on the same species and strain at equivalent doses supported the conclusion of chance results in the EU evaluation. IARC, however, could not use some studies included in the EU evaluation, since the EU assessment was on-going and only a draft was available at the time of the IARC Working Group meeting, limiting the capacity for checking consistency among studies. Second, the lack of consistency between sexes; according to the UN-GHS criteria, a plausible sex-related mechanism should be investigated in these cases, and was not identified in the EU assessment. No specific guidance is provided in the IARC evaluation and no indication is provided in the monograph. Third, the role of secondary effects observed at doses with excessive toxicity. For regulatory assessments, when classification is linked to labelling and risk management options, secondary effects due to excessive doses are excluded as the assessment focuses on the intrinsic capacity of the chemical to induce tumours at lower, less toxic doses. This element is not described in the IARC methodology, and the IARC Working Group considered as positive trends those triggered by tumour incidences at doses with demonstrated excessive toxicity. Regulatory assessments have access to full study reports; for IARC, unpublished industry-sponsored studies are secondary information sources, and their use is limited to the study summaries from previous assessments published by other agencies. Despite not having access to the original study reports, the IARC Working Group was able to run new statistical analyses, although its capacity for verifying details relevant for assessing the biological relevance was limited by the level of detail provided in the reports published by the regulatory agencies. The comparison with the WHO expert group JMPR assessments for glyphosate, conducted in 2004 and 2016, is informative regarding the value of granting the experts access to the full study reports.
Evidence on genotoxicity and other mechanisms of carcinogenicity
Regarding sources of mechanistic information, genotoxicity/mutagenicity should be discussed independently of other possible mechanisms. As observed for glyphosate, both industry-sponsored and scientific publications offer relevant information on the genotoxicity potential of pesticides that has raised interest among the scientific community. On one hand, IARC included one industry-sponsored study reported by the US-EPA but not those reported by JMPR (JMPR 2006); on the other hand, IARC reviewed effects observed in non-mammalian systems, which were considered of limited relevance for the assessment of carcinogenicity in humans in the regulatory assessment. IARC also assessed glyphosate-based formulations.
An important difference among IARC and regulatory assessment is the identification of a non-threshold genotoxic mode of action for carcinogenicity. This is not part of the IARC evaluation, while for regulatory assessment this is a key element triggering the risk assessment methodology. The IARC monograph used genotoxicity and oxidative stress as supporting mechanistic evidence; according to IARC principles, no indication is provided regarding threshold or non-threshold modes of action. The IARC allocation in group 2A may suggest that for the IARC Working Group the evidence on genotoxicity was insufficient for considering glyphosate as a potent DNA reactive non-threshold genotoxic human carcinogen. In fact, all oral studies, even at very high doses, are negative and the only in vivo mammalian positive evidence was for intraperitoneal studies at very high doses at which (cyto)toxicity is expected. This is again linked to the consideration of secondary effects due to severe systemic toxicity described above for the animal studies, which should be excluded for the classification of genotoxicity and carcinogenicity according to the UN-GHS criteria.
Other mechanistic studies should be discussed in connection with the methodological approach. With the exception of genotoxicity, mechanistic data on the mode of action are used in the regulatory context for assessing the relevance for humans, and are mostly used to downgrade the classification (Boobis et al. 2006; Clewell 2005; Meek et al. 2014a). Mechanistic data can be pivotal in IARC evaluations with inconclusive evidence in humans (Cogliano et al. 2008; Lauby-Secretan et al. 2016); and IARC has used mechanistic data for upgrading 52 agents and downgrading 8 agents (Cogliano et al. 2008). The recent review of the IARC approach for assessing mechanistic information may further change this picture. Strong evidence on non-genotoxic mechanisms is included in the recent IARC assessments for lindane, DDT and 2,4-D (Loomis et al. 2015). Moreover, mechanistic information is essential in the assessment of causality versus chance and bias.
To summarise, definitions for limited and sufficient evidence in humans and animals are identical for IARC and the UN-GHS; however, differences in criteria and methodological considerations for weighing and assessing the evidence can lead to divergent interpretations between the IARC assessment and regulatory evaluations following the UN-GHS criteria, even when based on the same evidence.
The differences between IARC and regulatory assessments are related not only to parallel historical developments, but to the different overall scope. IARC classifications represent a first step, alerting on the carcinogenicity potential of a broad range of agents; scientific regulatory assessments are connected to specific risk management recommendations, such as labelling, packaging requirements, use restrictions, etc., and produce the basis to be used in the risk assessment. In this different context, the focus and role of conservativeness is very different. While IARC assessments are not connected to risk management decisions, and are based exclusively on published information, without access to the full study reports for regulated products, regulatory assessments may identify data gaps and request additional studies to confirm or exclude potential concerns identified during their evaluation.
Human health safety is a critical issue for understanding the consequences of scientific divergences regarding the carcinogenicity classification of glyphosate. Regulatory assessments cover all relevant effects, not only carcinogenicity. Effects other than tumour induction were responsible for setting the NOAELs of the long-term toxicity–carcinogenicity studies, and the toxicological reference values were established from critical effects observed at lower dose levels in other studies. From a health assessment perspective, the IARC-EFSA scientific divergence is at lower dose levels that are in reality of limited, if any, relevance. The toxicological reference values proposed by EFSA provide a margin of protection of about four orders of magnitude for the trends in tumour induction and genotoxic damage at toxic levels reported by IARC. Those effects are expected only in concomitance with other signs of toxicity and at exposure levels orders of magnitude higher than the toxicological reference values recommended by EFSA. Risk assessments based on human biomonitoring and monitoring of levels of glyphosate residues in food have not identified concerns for consumers, and a full consumers’ risk assessment of all EU uses is on-going.
References
Barlow S, Schlatter J (2010) Risk assessment of carcinogens in food. Toxicol Appl Pharmacol 243:180–190
Becker RA, Patlewicz G, Simon TW, Rowlands JC, Budinsky RA (2015) The adverse outcome pathway for rodent liver tumor promotion by sustained activation of the aryl hydrocarbon receptor. Regul Toxicol Pharmacol 73:172–190
Benachour N, Seralini G-E (2009) Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem Res Toxicol 22:97–105
Benigni R, Bossa C, Tcheremenskaia O (2013) Nongenotoxic carcinogenicity of chemicals: mechanisms of action and early recognition through a new set of structural alerts. Chem Rev 113:2940–2957
Benner P, Mena H, Schneider R (2016) Modeling glyphosate aerial spray drift at the ecuador-colombia border. Appl Math Modell 40:373–387
Beyer LA, Beck BD, Lewandowski TA (2011) Historical perspective on the use of animal bioassays to predict carcinogenicity: evolution in design and recognition of utility. Crit Rev Toxicol 41:321–338
Blaylock RL (2015) Civility in scientific publishing: the glyphosate paper. Surg Neurol Int 6:163–163
Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C et al (2006) Ipcs framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol 36:781–792
Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M et al (2008) Ipcs framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol 38:87–96
Bryce SM, Bemis JC, Mereness JA, Spellman RA, Moss J, Dickinson D et al (2014) Interpreting in vitro micronucleus positive results: simple biomarker matrix discriminates clastogens, aneugens, and misleading positive agents. Environ Mol Mutagen 55:542–555
Cavas T (2011) In vivo genotoxicity evaluation of atrazine and atrazine-based herbicide on fish carassius auratus using the micronucleus test and the comet assay. Food Chem Toxicol 49:1431–1435
Choi SJ, Lim KC (2010) A study on classification and management system for carcinogens. J Korea Safety Manage Sci 12:107–119
Chruscielska KGB, Brzezinski J, Kita K et al (2000) Glyphosate: evaluation of chronic activity and possible far-reaching effects-Part 1. Studies on chronic toxicity. Pestycydy (3–4):11–20
Clewell H (2005) Use of mode of action in risk assessment: Past, present, and future. Regul Toxicol Pharmacol 42:3–14
Coalova I, de Molina MdCR, Chaufan G (2014) Influence of the spray adjuvant on the toxicity effects of a glyphosate formulation. Toxicol Vitro 28:1306–1311
Cogliano VJ, Boan RA, Straif K, Grosse Y, Secretan B, Ghissassi FE (2008) Use of mechanistic data in iarc evaluations. Environ Mol Mutagen 49:100–109
Collier ZA, Gust KA, Gonzalez-Morales B, Gong P, Wilbanks MS, Linkov I et al. (2016) A weight of evidence assessment approach for adverse outcome pathways. Regul Toxicol Pharmacol RTP 75:46–57.
Cox C, Surgan M (2006) Unidentified inert ingredients in pesticides: Implications for human and environmental health. Environ Health Perspect 114:1803–1806
Dinse GE, Peddada SD (2011) Comparing tumor rates in current and historical control groups in rodent cancer bioassays. Stat Biopharm Res 3:97–105.
Doktorova TY, Pauwels M, Vinken M, Vanhaecke T, Rogiers V (2012) Opportunities for an alternative integrating testing strategy for carcinogen hazard assessment? Crit Rev Toxicol 42:91–106
Downes N, Foster J (2015) Regulatory forum opinion piece: Carcinogen risk assessment: the move from screens to science. Toxicol Pathol 43:1064–1073
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64:319–325
Dybing E, Sanner T, Roelfzema H, Kroese D, Tennant RW (1997) T25: a simplified carcinogenic potency index: description of the system and study of correlations between carcinogenic potency and species/site specificity and mutagenicity. Pharmacol Toxicol 80:272–279
Eastmond DA (2012) Factors influencing mutagenic mode of action determinations of regulatory and advisory agencies. Mutat Res Rev Mutat Res 751:49–63
Edwards SW, Tan Y-M, Villeneuve DL, Meek ME, McQueen CA (2016) Adverse outcome pathways-organizing toxicological information to improve decision making. J Pharmacol Exp Ther 356:170–181
Elmore SA, Peddada SD (2009) Points to consider on the statistical analysis of rodent cancer bioassay data when incorporating historical control data. Toxicol Pathol 37:672–676
European Chemicals Agency (2015) Guidance on the application of clp criteria. European chemicals agency echa-15-g-05-en
European Food Safety Authority (2007) Pesticide residue intake model (primo) rev. 2
European Food Safety Authority (2015a) Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance glyphosate
European Food Safety Authority (2015b) Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J 13:4302–4302
European Food Safety Authority (2016) The 2014 European union report on pesticide residues in food. EFSA J 14:4611, p 139. doi:10.2903/j.efsa.2016.4611
European Food Safety Authority (2012) Final review of the Séralini et al. (2012a) publication on a 2-year rodent feeding study with glyphosate formulations and GM maize NK603 as published online on 19 september 2012 in food and chemical toxicology. EFSA J 10(11):2986. doi:10.2903/j.efsa.2012.2986
Fagan J, Traavik T, Bohn T (2015) The seralini affair: degeneration of science to re-science? Environmental Sciences Europe 27:(29 August 2015)-(2029 August 2015)
Faria MA (2015) Glyphosate, neurological diseases—and the scientific method. Surg Neurol Int 6:132–132
Gaylor DW (2005) Are tumor incidence rates from chronic bioassays telling us what we need to know about carcinogens? Regul Toxicol Pharmacol 41:128–133
George J, Prasad S, Mahmood Z, Shukla Y (2010) Studies on glyphosate-induced carcinogenicity in mouse skin: a proteomic approach. J Proteomics 73:951–964
Germany (2015) Final addendum to the renewal assessment report on glyphosate, compiled by efsa
Ghisi NdC, de Oliveira EC, Prioli AJ (2016) Does exposure to glyphosate lead to an increase in the micronuclei frequency? A systematic and meta-analytic review. Chemosphere 145:42–54
Greim H, Gelbke HP, Reuter U, Thielmann HW, Edler L (2003) Evaluation of historical control data in carcinogenicity studies. Human Exp Toxicol 22:541–549
Greim H, Saltmiras D, Mostert V, Strupp C (2015) Evaluation of carcinogenic potential of the herbicide glyphosate, drawing on tumor incidence data from fourteen chronic/carcinogenicity rodent studies. Crit Rev Toxicol 45:185–208
Guyton KZ, El Ghissassi F, Benbrahim-Talaa L, Grosse Y, Loomis D, Straif K (2015a) Recent progress in mechanistic data evaluation: the iarc monographs perspective. Environ Mol Mutagen 56:S84–S84
Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N et al (2015b) Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol 16:490–491
Hernandez LG, van Steeg H, Luijten M, van Benthem J (2009) Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutation Res Rev Mutation Res 682:94–109
Heydens WF, Healy CE, Hotz KJ, Kier LD, Martens MA, Wilson AGE et al (2008) Genotoxic potential of glyphosate formulations: mode-of-action investigations. J Agric Food Chem 56:1517–1523
Hothorn LA (2014) Statistical evaluation of toxicological bioassays—a review. Toxicol Res 3:418–432.
IARC (2015) Monographs, volume 112: some organophosphate insecticides and herbicides: tetrachlorvinphos, parathion, malathion, diazinon and glyphosate. Iarc working group. Lyon; 3–10 march 2015. Iarc monogr eval carcinog risk chem hum
Ibrahim YA (2015) A regulatory perspective on the potential carcinogenicity of glyphosate. J Toxicol Health 2:1
JMPR (2006) Pesticide residues in food – 2004. Joint fao/who meeting on pesticide residues evaluations 2004 part ii—toxicological. Who/pcs/06.1. Who, malta.
JMPR (2016) Pesticide residues in food—2016. Special Session of the Joint FAO/WHO Meeting on Pesticide Residues. FAO Plant Protection Paper 227. Rome
Keenan C, Elmore S, Franckecarroll S, Kemp R, Kerlin R, Pletcher J et al (2008) Stp working group for historical control data of proliferative rodent lesions. Toxicol Pathol 36:157–157
Keenan C, Elmore S, Francke-Carroll S, Kemp R, Kerlin R, Peddada S et al (2009) Best practices for use of historical control data of proliferative rodent lesions. Toxicol Pathol 37:679–693
Kier LD (2015) Review of genotoxicity biomonitoring studies of glyphosate-based formulations. Crit Rev Toxicol 45:209–218
Kim Y-h, Hong J-r, Gil H-w, Song H-y, Hong S-y (2013) Mixtures of glyphosate and surfactant tn20 accelerate cell death via mitochondrial damage-induced apoptosis and necrosis. Toxicol in Vitro 27:191–197
Kitamoto S, Matsuyama R, Uematsu Y, Ogata K, Ota M, Yamada T et al (2015) Optimal dose selection of n-methyl-n-nitrosourea for the rat comet assay to evaluate DNA damage in organs with different susceptibility to cytotoxicity. Mutation Res Genetic Toxicol Environ Mutagen 786:129–136
Knight A, Bailey J, Balcombe J (2006) Animal carcinogenicity studies: 2. Obstacles to extrapolation of data to humans. Atla-Alternatives Lab Anim 34:29–38
Kobayashi K, Sakuratani Y, Abe T, Nishikawa S, Yamada J, Hirose A et al (2010) Relation between statistics and treatment-related changes obtained from toxicity studies in rats: if detected a significant difference in low or middle dose for quantitative values, this change is considered as incidental change? J Toxicol Sci 35:79–85
Lauby-Secretan B, Loomis D, Baan R, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L et al (2016) Use of mechanistic data in the iarc evaluations of the carcinogenicity of polychlorinated biphenyls and related compounds. Environ Sci Pollut Res 23:2220–2229
Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L et al (2015) Carcinogenicity of lindane, ddt, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol 16:891–892
Lutter R, Abbott L, Becker R, Borgert C, Bradley A, Charnley G et al (2015) Improving weight of evidence approaches to chemical evaluations. Risk Anal 35:186–192
Ma YP, Guo JH, Shi NZ, Tang ML (2002) On the use of historical control information for trend test in carcinogenesis. Biometrics 58:917–927
Marone PA, Hall WC, Hayes AW (2014) Reassessing the two-year rodent carcinogenicity bioassay: a review of the applicability to human risk and current perspectives. Regul Toxicol Pharmacol 68:108–118
Massarelli R, Adamou A, Henning G, Kangas L (2013) Comparison of historical control data in two strains of rat used in carcinogenicty studies. Int J Toxicol 32:71–71
McGregor D, Boobis A, Binaglia M, Botham P, Hoffstadt L, Hubbard S et al (2010) Guidance for the classification of carcinogens under the globally harmonised system of classification and labelling of chemicals (ghs). Crit Rev Toxicol 40:245–285
McInnes EF, Scudamore CL (2014) Review of approaches to the recording of background lesions in toxicologic pathology studies in rats. Toxicol Lett 229:134–143
Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S et al (2014a) New developments in the evolution and application of the who/ipcs framework on mode of action/species concordance analysis. J Appl Toxicol 34:1–18
Meek ME, Palermo CM, Bachman AN, North CM, Lewis RJ (2014b) Mode of action human relevance (species concordance) framework: evolution of the bradford hill considerations and comparative analysis of weight of evidence. J Appl Toxicol 34:595–606
Mesnage R, Bernay B, Seralini GE (2013) Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 313:122–128
Niemann L, Sieke C, Pfeil R, Solecki R (2015) A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. Journal Fur Verbraucherschutz Und Lebensmittelsicherheit-Journal of Consumer Protection and Food Safety 10:3–12
Nobels I, Spanoghe P, Haesaert G, Robbens J, Blust R (2011) Toxicity ranking and toxic mode of action evaluation of commonly used agricultural adjuvants on the basis of bacterial gene expression profiles. PLOS ONE 6(11):e24139. doi:10.1371/journal.pone.0024139
Ntzani EECM, Ntritsos G, Evangelou E, Tzoulaki I (2013) Literature review on epidemiological studies linking exposure to pesticides and health effects. Efsa supporting publication 2013:En-497, pp 159
OECD (2012) Guidance document 116 on the conduct and design of chronic toxicity and carcinogenicity studies, supporting test guidelines 451, 452 and 453 2nd edition. Series on testing and assessment no. 116. Env/jm/mono(2011)47.
Osimitz TG, Droege W, Boobis AR, Lake BG (2013) Evaluation of the utility of the lifetime mouse bioassay in the identification of cancer hazards for humans. Food Chem Toxicol 60:550–562
Pearce N, Blair A, Vineis P, Ahrens W, Andersen A, Anto JM et al (2015) Iarc monographs: 40 years of evaluating carcinogenic hazards to humans. Environ Health Perspect 123:507–514
Peddada SD, Dinse GE, Kissling GE (2007) Incorporating historical control data when comparing tumor incidence rates. J Am Stat Assoc 102:1212–1220
Portier CJ, Goldman LR, Goldstein BD (2014) Inconclusive findings: now you see them, now you don’t! Environ Health Perspect 122:A36–A36
Pratt IS (2002) Global harmonisation of classification and labelling of hazardous chemicals. Toxicol Lett 128:5–15
Rhomberg L (2015a) Hypothesis-based weight of evidence: an approach to assessing causation and its application to regulatory toxicology. Risk Anal 35:1114–1124
Rhomberg LR (2015b) Contrasting directions and directives on hazard identification for formaldehyde carcinogenicity. Regul Toxicol Pharmacol 73:829–833
Rhomberg LR, Goodman JE, Bailey LA, Prueitt RL, Beck NB, Bevan C et al (2013) A survey of frameworks for best practices in weight-of-evidence analyses. Crit Rev Toxicol 43:753–784
Rolando CA, Garrett LG, Baillie BR, Watt MS (2013) A survey of herbicide use and a review of environmental fate in new zealand planted forests. N Z J For Sci 43(1):17. doi:10.1186/1179-5395-43-17
SCOEL (2013) Methodology for the derivation of occupational exposure limits (version 7). Scientific committee on occupational exposure limits, scoel
Seralini G-E, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M et al (2014) Republished study: long-term toxicity of a roundup herbicide and a roundup-tolerant genetically modified maize. Environ Sci Eur 26:14. doi:10.1186/s12302-014-0014-5
Shao-Wen H, Chun-Hong L (2015) Toxic effects and exposure risk assessment of glyphosate. J Food Saf Quality 6:880–885
Smith MTGK, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavlock RJ, Lambert P, Hecht SS, Bucher JR, Stewart BW, Baan R, Cogliano VJ, Straif K (2016) Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect 124:713–721. doi:10.1289/ehp.1509912
Solomon KR, Anadon A, Carrasquilla G, Cerdeira AL, Marshall J, Sanin L-H (2007) Coca and poppy eradication in colombia: environmental and human health assessment of aerially applied glyphosate. In: Reviews of environmental contamination and toxicology, vol 190, (Ware GW (ed)), 43–125
Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp R et al (2001) Ipcs conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol 34:146–152
Straif K, Loomis D, Guyton K, Grosse Y, Lauby-Secretan B, El Ghissassi F et al (2014) Future priorities for the iarc monographs. Lancet Oncol 15:683–684
United Nations (2003) Globally harmonized system of classification and labelling of chemicals (ghs), first edition. United nations, new york and geneva.
United Nations (2015) Globally harmonized system of classification and labelling of chemicals (ghs), revision 6. United nations, new york and geneva
Wakeford R (2015) Association and causation in epidemiology—half a century since the publication of bradford hill’s interpretational guidance. J R Soc Med 108:4–6
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31:117–165
Wood CE, Hukkanen RR, Sura R, Jacobson-Kram D, Nolte T, Odin M et al (2015) Scientific and regulatory policy committee (srpc) review*: interpretation and use of cell proliferation data in cancer risk assessment. Toxicol Pathol 43:760–775
Yauk CL, Aardema MJ, van Benthem J, Bishop JB, Dearfield KL, DeMarini DM et al (2015) Approaches for identifying germ cell mutagens: report of the 2013 iwgt workshop on germ cell assays. Mutation Res Genetic Toxicol Environ Mut 783:36–54
Zhou B (2015) Adverse outcome pathway: framework, application, and challenges in chemical risk assessment. J Environ Sci 35:191–193
Acknowledgements
The authors are staff personnel of the European Food Safety Authority (EFSA) and the German Federal Institute for Risk Assessment (BfR). The authors declare no competing financial interests. The content of this paper is the exclusive responsibility of the authors; the opinion of the Authority is that presented in the EFSA Conclusion and institutional documents. The authors want to thank the contribution of all experts that participated in the European peer review of glyphosate, particularly the experts from the (co)rapporteur Member State, as well as the comments and support from other EFSA colleagues and the reviewers that have improved the original manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tarazona, J.V., Court-Marques, D., Tiramani, M. et al. Glyphosate toxicity and carcinogenicity: a review of the scientific basis of the European Union assessment and its differences with IARC. Arch Toxicol 91, 2723–2743 (2017). https://doi.org/10.1007/s00204-017-1962-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-1962-5