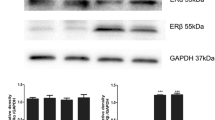
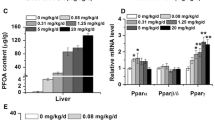
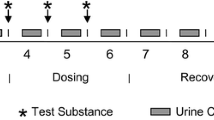
Abstract
Perfluorodecanoic acid (PFDA) is widely used in production of many daily necessities based on their surface properties and stability. It was assigned as a Persistent Organic Pollutant in 2009 and became a public concern partly because of its potential for activation of the peroxisome proliferator-activated receptor alpha (PPARα). In this study, wild-type and Ppara-null mice were administered PFDA (80 mg/kg). Blood and liver tissues were collected and subjected to systemic toxicological and mechanistic analysis. UPLC-ESI-QTOFMS-based metabolomics was used to explore the contributing components of the serum metabolome that led to variation between wild-type and Pparα-null mice. Bile acid homeostasis was disrupted, and slight hepatocyte injury in wild-type mice accompanied by adaptive regulation of bile acid synthesis and transport was observed. The serum metabolome in wild-type clustered differently from that in Pparα-null, featured by sharp increases in bile acid components. Differential toxicokinetic tendency was supported by regulation of UDP-glucuronosyltransferases dependent on PPARα, but it did not contribute to the hepatotoxic responses. Increase in Il-10 and activation of the JNK pathway indicated inflammation was induced by disruption of bile acid homeostasis in wild-type mice. Inhibition of p-p65 dependent on PPARα activation by PFDA stopped the inflammatory cascade, as indicated by negative response of Il-6, Tnf-α, and STAT3 signaling. These data suggest disruptive and protective role of PPARα in hepatic responses induced by PFDA.








Similar content being viewed by others
References
Arand M, Coughtrie MW, Burchell B, Oesch F, Robertson LW (1991) Selective induction of bilirubin UDP-glucuronosyl-transferase by perfluorodecanoic acid. Chem Biol Interact 77(1):97–105
Bjerregaard-Olesen C, Bach CC, Long M et al (2016) Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008-2013. Environ Int 91:14–21. doi:10.1016/j.envint.2016.02.010
Chang SC, Das K, Ehresman DJ et al (2008) Comparative pharmacokinetics of perfluorobutyrate in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol Sci 104(1):40–53
Cheng X, Klaassen CD (2008a) Critical role of PPAR- in perfluorooctanoic acid- and perfluorodecanoic acid-induced downregulation of oatp uptake transporters in mouse livers. Toxicol Sci 106(1):37–45
Cheng X, Klaassen CD (2008b) Perfluorocarboxylic acids induce cytochrome P450 enzymes in mouse liver through activation of PPAR-alpha and CAR transcription factors. Toxicol Sci 106(1):29–36
Delerive P, Gervois P, Fruchart JC, Staels B (2000) Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem 275(47):36703–36707
Donner MG, Keppler D (2001) Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology 34(2):351–359
Foreman JE, Chang SC, Ehresman DJ et al (2009) Differential hepatic effects of perfluorobutyrate mediated by mouse and human PPAR-alpha. Toxicol Sci 110(1):204–211
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40(11):3463–3473
Johnson DR, Klaassen CD (2002) Regulation of rat multidrug resistance protein 2 by classes of prototypical microsomal enzyme inducers that activate distinct transcription pathways. Toxicol Sci 67(2):182–189
Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y (2001) Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem Biol Interact 134(2):203–216
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394
Li F, Patterson AD, Krausz KW, Tanaka N, Gonzalez FJ (2012) Metabolomics reveals an essential role for peroxisome proliferator-activated receptor in bile acid homeostasis. J Lipid Res 53(8):1625–1635
Liu A, Krausz KW, Fang Z-Z, Brocker C, Qu A, Gonzalez FJ (2014a) Gemfibrozil disrupts lysophosphatidylcholine and bile acid homeostasis via PPARα and its relevance to hepatotoxicity. Arch Toxicol 88(4):983–996
Liu A, Tanaka N, Sun L et al (2014b) Saikosaponin d protects against acetaminophen-induced hepatotoxicity by inhibiting NF-kappaB and STAT3 signaling. Chem Biol Interact 223C:80–86. doi:10.1016/j.cbi.2014.09.012
Maher JM, Aleksunes LM, Dieter MZ et al (2008) Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol Sci 106(2):319–328
Marx N, Sukhova GK, Collins T, Libby P, Plutzky J (1999) PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 99(24):3125–3131
Ohmori K, Kudo N, Katayama K, Kawashima Y (2003) Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology 184(2–3):135–140
Patterson AD, Shah YM, Matsubara T, Krausz KW, Gonzalez FJ (2012) Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 56(1):281–290
Peters JM, Cheung C, Gonzalez FJ (2005) Peroxisome proliferator-activated receptor-alpha and liver cancer: where do we stand? J Mol Med (Berl) 83(10):774–785
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH (2006) Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40(1):32–44
Russell DW (2003) The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72:137–174. doi:10.1146/annurev.biochem.72.121801.161712
Senekeo-Effenberger K, Chen S, Brace-Sinnokrak E et al (2007) Expression of the human UGT1 locus in transgenic mice by 4-chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic acid (WY-14643) and implications on drug metabolism through peroxisome proliferator-activated receptor alpha activation. Drug Metab Dispos Biol Fate Chem 35(3):419–427
Shelby MK (2006) Induction of rat UDP-glucuronosyltransferases in liver and duodenum by microsomal enzyme inducers that activate various transcriptional pathways. Drug Metab Dispos 34(10):1772–1778
Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD (2009) ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol Sci 108(2):247–257
Van Rafelghem MJ, Vanden Heuvel JP, Menahan LA, Peterson RE (1988) Perfluorodecanoic acid and lipid metabolism in the rat. Lipids 23(7):671–678
Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ (2006) Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92(2):476–489
Wagner M, Halilbasic E, Marschall H-U et al (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42(2):420–430
Woolbright BL, Li F, Xie Y et al (2014) Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol Lett 228(1):56–66
Yu SJ, Bae S, Kang JS et al (2015) Hepatoprotective effect of vitamin C on lithocholic acid-induced cholestatic liver injury in Gulo(−/−) mice. Eur J Pharmacol 762:247–255. doi:10.1016/j.ejphar.2015.06.008
Zeng H, Li D, Qin X et al (2016) Hepatoprotective effects of schisandra sphenanthera extract against lithocholic acid-induced cholestasis in male mice are associated with activation of the pregnane X receptor pathway and promotion of liver regeneration. Drug Metab Dispos Biol Fate Chem 44(3):337–342
Zhou X, Cao L, Jiang C et al (2014) PPARalpha-UGT axis activation represses intestinal FXR-FGF15 feedback signalling and exacerbates experimental colitis. Nat Commun 5:4573. doi:10.1038/ncomms5573
Acknowledgments
This work was supported by National Natural Science Foundation of China [Grant 81273582, 81302848], Public Projects of Zhejiang Province (2015C37110, 2015R405055), K.C.Wong Magna Fund in Ningbo University and the National Cancer Institute Intramural Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
204_2016_1779_MOESM1_ESM.tif
Supplementary Figure 1 Analysis of total bile acid (TBA) and toxicological responses in livers of wild-type (WT) and Pparα-null (KO) mice treated with PFDA. a Effect of PFDA (40 mg/kg, 80 mg/kg) on serum TBA in WT mice. b Serum TBA level of on days 0, 3, 5, 7, 10 in WT and KO mice, PFDA (80 mg/kg). c, d Activity of serum ALP and ALT, PFDA (80 mg/kg). Data were expressed as mean ± SD (n = 5; *p < 0.05, **p < 0.01, ***p < 0.001) (TIFF 567 kb)
204_2016_1779_MOESM2_ESM.tif
Supplementary Figure 2 Identification of tauroursodeoxycholic acid (TUDCA) by fragmentation profile and retention time on UPLC-ESI-QTOF-MS. a Total ion count (TIC) chromatogram of MS 498.29 of PFDA-treated wild-type (WT) mouse serum sample when fragmentation was performed. b MS/MS spectra of the peak with retention time 3.809 in WT mouse serum in panel a. c TIC chromatogram of authentic TUDCA when fragmentation performed at MS 498.29. D, MS/MS spectra of the peak with retention time 3.80 in panel c. (TIFF 594 kb)
204_2016_1779_MOESM3_ESM.tif
Supplementary Figure 3 Identification of TCA by fragmentation profile and retention time on UPLC-ESI-QTOF-MS. a Total ion count (TIC) chromatogram of MS 514.28 of PFDA-treated wild-type (WT) mouse serum sample when fragmentation was performed. b MS/MS spectra of the peak of WT mouse serum sample with retention time 3.83 in panel a. c, TIC chromatogram of authentic TCA when fragmentation was performed at MS 514.28. d MS/MS spectra of the peak with retention time 3.86 in panel c (TIFF 448 kb)
204_2016_1779_MOESM4_ESM.tif
Supplementary Figure 4 Identification of tauro-α-muricholic (Tα/β/ωMCA) by fragmentation profile and retention time on UPLC-ESI-QTOF-MS. a Total ion count (TIC) chromatogram of MS 514.28 of PFDA-treated wild-type (WT) mouse serum sample when fragmentation performed. b MS/MS spectra of the peak of with retention time 3.45 in WT mouse serum in panel a. c, TIC chromatogram of authentic TαMCA when fragmentation was performed at MS 514.28. d MS/MS spectra of the peak with retention time 3.41 in panel c (TIFF 474 kb)
Rights and permissions
About this article
Cite this article
Luo, M., Tan, Z., Dai, M. et al. Dual action of peroxisome proliferator-activated receptor alpha in perfluorodecanoic acid-induced hepatotoxicity. Arch Toxicol 91, 897–907 (2017). https://doi.org/10.1007/s00204-016-1779-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1779-7