Abstract
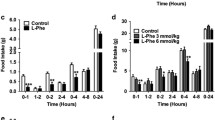
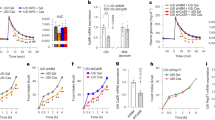
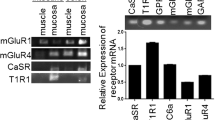
Food contamination by the trichothecene mycotoxin deoxynivalenol (DON, vomitoxin) has the potential to adversely affect animal and human health by suppressing food intake and impairing growth. In mice, the DON-induced anorectic response results from aberrant satiety hormone secretion by enteroendocrine cells (EECs) of the gastrointestinal tract. Recent in vitro studies in the murine STC-1 EEC model have linked DON-induced satiety hormone secretion to activation of calcium-sensing receptor (CaSR), a G-coupled protein receptor, and transient receptor potential ankyrin-1 (TRPA1), a TRP channel. However, it is unknown whether similar mechanisms mediate DON’s anorectic effects in vivo. Here, we tested the hypothesis that DON-induced food refusal and satiety hormone release in the mouse are linked to activation of CaSR and TRPA1. Oral treatment with selective agonists for CaSR (R-568) or TRPA1 (allyl isothiocyanate (AITC)) suppressed food intake in mice, and the agonist’s effects were suppressed by pretreatment with corresponding antagonists NPS-2143 or ruthenium red (RR), respectively. Importantly, NPS-2143 or RR inhibited both DON-induced food refusal and plasma elevations of the satiety hormones cholecystokinin (CCK) and peptide YY3–36 (PYY3–36); cotreatment with both antagonists additively suppressed both anorectic and hormone responses to DON. Taken together, these in vivo data along with prior in vitro findings support the contention that activation of CaSR and TRPA1 contributes to DON-induced food refusal by mediating satiety hormone exocytosis from EEC.











Similar content being viewed by others
References
Azcona-Olivera JI, Ouyang Y et al (1995) Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicol Appl Pharmacol 133(1):109–120
Chakravarti B, Chattopadhyay N, Brown EM (2012) Signaling through the extracellular calcium-sensing receptor (CaSR). Adv Exp Med Biol 740:103–142
Challis B, Pinnock S, Coll A et al (2003) Acute effects of PYY3–36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun 311(4):915–919
Della-Fera MA, Baile CA (1980) CCK-octapeptide injected in CSF decreases meal size and daily food intake in sheep. Peptides 1(1):51–54
Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H (2009a) Molecular cloning and characterization of dog TRPA1 and AITC stimulate the gastrointestinal motility through TRPA1 in conscious dogs. Eur J Pharmacol 617(1–3):124–129
Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H (2009b) TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn Schmiedebergs Arch Pharmacol 380(4):353–357
D’Souza-Li L (2006) The calcium-sensing receptor and related diseases. Arq Bras Endocrinol Metabol 50(4):628–639
Ebenezer I, De La Riva C, Baldwin B (1990) Effects of the CCK receptor antagonist MK-329 on food intake in pigs. Physiol Behav 47(1):145–148
EFSA (2013) Statement on the risks for public health related to a possible increase of the maximum level of deoxynivalenol for certain semi-processed cereal products. EFSA J 11(12):3490
Flannery BM, Wu W, Pestka JJ (2011) Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food Chem Toxicol 49(8):1863–1869
Flannery BM, Clark ES, Pestka JJ (2012) Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicol Sci 130(2):289–297
Girardet C, Bonnet MS, Jdir R et al (2011) The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS One 6(10):e26134
Hörsten S, Hoffmann T, Alfalah M et al (2004) PP, PYY and NPY: synthesis, storage, release and degradation. Neuropept Y relat reptides 162:23–44
Karra E, Batterham RL (2010) The role of gut hormones in the regulation of body weight and energy homeostasis. Mol Cell Endocrinol 316(2):120–128
Kim MJ, Son HJ, Song SH, Jung M, Kim Y, Rhyu MR (2013) The TRPA1 agonist, methyl syringate suppresses food intake and gastric emptying. PLoS One 8(8):e71603. doi:10.1371/journal.pone.0071603
Kohno D, Nakata M, Maejima Y et al (2008) Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149(3):1295–1301
le Roux CW, Batterham RL, Aylwin SJ et al (2006) Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinol 147(1):3–8
Liou AP (2013) Digestive physiology of the pig symposium: G protein-coupled receptors in nutrient chemosensation and gastrointestinal hormone secretion. J Anim Sci 91(5):1946–1956
Liu K, Samuel M, Ho M, Harrison RK, Paslay JW (2010) NPPB structure-specifically activates TRPA1 channels. Biochem Pharmacol 80(1):113–121
Maresca M (2013) From the gut to the brain: journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins (Basel) 5(4):784–820
Moran-Ramos S, Tovar AR, Torres N (2012) Diet: friend or foe of enteroendocrine cells—how it interacts with enteroendocrine cells. Adv Nutr 3(1):8–20
Nagata K, Duggan A, Kumar G, Garcia-Anoveros J (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25(16):4052–4061
Nemeth EF, Delmar EG, Heaton WL et al (2001) Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther 299(1):323–331
Nilius B, Owsianik G, Voets T et al (2007) Transient receptor potential cation channels in disease. Physiol Rev 87(1):165–217
Nilius B, Appendino G, Owsianik G (2012) The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch 464(5):425–458
Pestka JJ (2010a) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84(9):663–679
Pestka JJ (2010b) Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotox J 3(4):323–347
Reimann F, Tolhurst G, Gribble FM (2012) G-protein coupled receptors in intestinal chemosensation. Cell Metab 15(4):421–431
Schwartz MW (2006) Central nervous system regulation of food intake. Obesity 14(S2):1S–8S
Sloth B, Holst JJ, Flint A et al (2007) Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab 292(4):E1062–E1068
Steinert RE, Feinle-Bisset C, Geary N et al (2013) Digestive physiology of the pig symposium: secretion of gastrointestinal hormones and eating control. J Anim Sci 91(5):1963–1973
Talavera K, Gees M, Karashima Y et al (2009) Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci 12(10):1293–1299
Tapia R, Velasco I (1997) Ruthenium red as a tool to study calcium channels, neuronal death and the function of neural pathways. Neurochem Int 30(2):137–147
Terranegra A, Ferraretto A, Dogliotti E et al (2010) Calcimimetic R-568 effects on activity of R990G polymorphism of calcium-sensing receptor. J Mol Endocrinol 45(4):245–256
Wada M, Furuya Y, Sakiyama J et al (1997) The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest. 100(12):2977–2983
Wang Y, Chandra R, Samsa LA et al (2011) Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol 300(4):G528–G537
Ward BK, Magno AL, Walsh JP et al (2012) The role of the calcium-sensing receptor in human disease. Clin Biochem 45(12):943–953
Wu W, Flannery BM, Sugita-Konishi Y et al (2012) Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem Toxicol 50(6):2056–2061
Wu W, Bates MA, Bursian SJ et al (2013a) Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol Sci 131(1):279–291
Wu W, Bates MA, Bursian SJ et al (2013b) Peptide YY3–36 and 5-hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin). Toxicol Sci 133(1):186–195
Wu W, Zhou HR, He K et al (2014a) Role of cholecystokinin in anorexia induction following oral exposure to the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol Sci 138(2):278–289
Wu W, Zhou HR, Bursian SJ et al (2014b) Comparison of anorectic and emetic potencies of deoxynivalenol (vomitoxin) to the plant metabolite deoxynivalenol-3-glucoside and synthetic deoxynivalenol derivatives EN139528 and EN139544. Toxicol Sci 142(1):167–181
Wu W, Zhou HR, Pan X et al (2015a) Comparison of anorectic potencies of the trichothecenes T-2 Toxin, HT-2 Toxin and satratoxin G to the ipecac alkaloid emetine. Toxicol Rep 2:238–251
Wu W, Zhou HR, Bursian SJ et al (2015b). Emetic responses to T-2 toxin, HT-2 toxin and emetine correspond to plasma elevations of peptide YY and 5-hydroxytryptamine. Arch Toxicol [Epub ahead of print]
Montell C (2005) The TRP superfamily of cation channels. Sci STKE 272:re3
Zhang DM, Bula W, Stellar E (1986) Brain cholecystokinin as a satiety peptide. Physiol Behav 36(6):1183–1186
Zhou HR, Pestka JJ (2015) Deoxynivalenol (Vomitoxin)-induced cholecystokinin and glucagon-like peptide-1 release in the STC-1 enteroendocrine cell model is mediated by calcium-sensing receptor and transient receptor potential ankyrin-1 channel. Toxicol Sci 145(2):407–417
Acknowledgments
We would like to acknowledge the assistance of Dr. Erica Clark and Melissa Bates. This study was supported by USDA NIFA Award (2011-0635), by USDA Wheat and Barley SCAB Initiative Award 59-0206-9-058, and by Public Health Service Grant ES03553 from the National Institutes of Health. Wenda Wu was supported by National Natural Science Foundation of China (31402268), Fundamental Research Funds for the Central Universities (KJQN201526), Natural Science Foundation of Jiangsu Province of China (BK20140691), National Natural Science Foundation of China (31572576), and Priority Academic Development Program of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, W., Zhou, HR. & Pestka, J.J. Potential roles for calcium-sensing receptor (CaSR) and transient receptor potential ankyrin-1 (TRPA1) in murine anorectic response to deoxynivalenol (vomitoxin). Arch Toxicol 91, 495–507 (2017). https://doi.org/10.1007/s00204-016-1687-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1687-x