Abstract
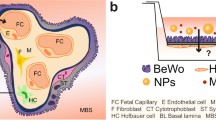
The human ex vivo placental perfusion model has regularly been used to study the transplacental transport of compounds. However, this method is laborious and dependent on the presence of fresh human placenta, hampering its use for the assessment of large numbers of compounds. An in vitro model for the placental barrier using BeWo b30 cells may provide an alternative to the ex vivo system. The present study aims to assess whether such an in vitro model could be used to reliably predict placental transfer. To this end, BeWo b30 cells, derived from a human choriocarcinoma, were grown on transwell insert to form a cell layer, separating an apical maternal compartment from a basolateral fetal compartment. For a set of nine selected model compounds, including the reference compound antipyrine, the transport velocity from the apical to the basolateral compartment was determined. Relative transport rates obtained were compared with the transfer indices (a measure for the transport relative to antipyrine) of these compounds obtained in ex vivo placental perfusion studies as reported in the literature. The relative transport rates in the in vitro BeWo model were in good correlation (R 2 = 0.95) with the transfer indices reported for the ex vivo model. This indicates that the BeWo model could be a valuable in vitro model for prediction of placental transfer of compounds.




Similar content being viewed by others
References
Adler S, Basketter D, Creton S, Pelkonen O, van Benthem J, Zuang V, Andersen K, Angers-Loustau A, Aptula A, Bal-Price A, Benfenati E, Bernauer U, Bessems J, Bois F, Boobis A, Brandon E, Bremer S, Broschard T, Casati S, Coecke S, Corvi R, Cronin M, Daston G, Dekant W, Felter S, Grignard E, Gundert-Remy U, Heinonen T, Kimber I, Kleinjans J, Komulainen H, Kreiling R, Kreysa J, Leite S, Loizou G, Maxwell G, Mazzatorta P, Munn S, Pfuhler S, Phrakonkham P, Piersma A, Poth A, Prieto P, Repetto G, Rogiers V, Schoeters G, Schwarz M, Serafimova R, Tähti H, Testai E, van Delft J, van Loveren H, Vinken M, Worth A, Zaldivar J-M (2011) Alternative (non-animal) methods for cosmetics testing: current status and future prospects—2010. Arch Toxicol 85(5):367–485
Akbaraly J, Guibert S, Leng J, Auzerie J (1985) Transplacental transfer of 5 antibiotics by in vitro human placental perfusion. Pathol Biol (Paris) 33(5):368–372
Avery ML, Meek CE, Audus KL (2003) The presence of inducible cytochrome P450 types 1A1 and 1A2 in the BeWo cell line. Placenta 24(1):45–52
Bassily M, Ghabrial H, Smallwood RA, Morgan DJ (1995) Determinants of placental drug transfer: studies in the isolated perfused human placenta. J Pharm Sci 84(9):1054–1060
Bode C, Soares M, Hunt J, Jin H, Rytting E, Silverstein P, Young A, Audus K (2006) In vitro models for studying trophoblast transcellular transport. In: Soares MJ, Hunt JS (eds) Placenta and trophoblast, vol 122. Methods in molecular medicine™. Humana Press, New York, pp 225–239
Eisenbrand G, Pool-Zobel B, Baker V, Balls M, Blaauboer BJ, Boobis A, Carere A, Kevekordes S, Lhuguenot JC, Pieters R, Kleiner J (2002) Methods of in vitro toxicology. Food Chem Toxicol 40(2–3):193–236
Enke U, Jaudszus A, Schleussner E, Seyfarth L, Jahreis G, Kuhnt K (2011) Fatty acid distribution of cord and maternal blood in human pregnancy: special focus on individual trans fatty acids and conjugated linoleic acids. Lipids Health Dis 10(1):247
Evseenko DA, Paxton JW, Keelan JA (2006) ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol 290(5):R1357–R1365
He Y-L, Seno H, Sasaki K, Tashiro C (2002) The influences of maternal albumin concentrations on the placental transfer of propofol in human dually perfused cotyledon in vitro. Anesth Analg 94(5):1312–1314
Heikkinen T, Ekblad U, Laine K (2002) Transplacental transfer of citalopram, fluoxetine and their primary demethylated metabolites in isolated perfused human placenta. BJOG Int J Obstet Gynaecol 109(9):1003–1008
Höfer T, Gerner I, Gundert-Remy U, Liebsch M, Schulte A, Spielmann H, Vogel R, Wettig K (2004) Animal testing and alternative approaches for the human health risk assessment under the proposed new European chemicals regulation. Arch Toxicol 78(10):549–564
Holcberg G, Sapir O, Tsadkin M, Huleihel M, Lazer S, Katz M, Mazor M, Ben-Zvi Z (2003) Lack of interaction of digoxin and P-glycoprotein inhibitors, quinidine and verapamil in human placenta in vitro. Eur J Obstet Gynecol Reprod Biol 109(2):133–137
Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C (1999) Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstet Gynecol 93(1):25–29
Johnson RF, Herman N, Arney TL, Olenick M, Paschall RL, Johnson HV, Downing JW (1999) Transfer of lidocaine across the dual perfused human placental cotyledon. Int J Obstet Anesth 8(1):17–23
Lagrange F, Pehourcq F, Bannwarth B, Leng JJ, Saux MC (1998) Passage of S-(+)- and R-(−)-ketoprofen across the human isolated perfused placenta. Fundam Clin Pharmacol 12(3):286–291
Lampela ES, Nuutinen LH, Ala-Kokko TI, Parikka RM, Laitinen RS, Jouppila PI, Vähäkangas KH (1999) Placental transfer of sulindac, sulindac sulfide, and indomethacin in a human placental perfusion model. Am J Obstet Gynecol 180(1):174–180
Liu F, Soares MJ, Audus KL (1997) Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol Cell Physiol 273(5):C1596–C1604
Louisse J, de Jong E, van de Sandt JJM, Blaauboer BJ, Woutersen RA, Piersma AH, Rietjens IMCM, Verwei M (2010) The use of in vitro toxicity data and physiologically based kinetic modeling to predict dose-response curves for in vivo developmental toxicity of glycol ethers in rat and man. Toxicol Sci 118(2):470–484
Louisse J, Verwei M, Woutersen RA, Blaauboer BJ, Rietjens IM (2012) Toward in vitro biomarkers for developmental toxicity and their extrapolation to the in vivo situation. Expert Opin Drug Metab Toxicol 8(1):11–27
Macklon NS, Geraedts JPM, Fauser BCJM (2002) Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update 8(4):333–343
Myllynen P, Vähäkangas K (2002) An examination of whether human placental perfusion allows accurate prediction of placental drug transport: studies with diazepam. J Pharmacol Toxicol Methods 48(3):131–138
Obeid R, Munz W, Jäger M, Schmidt W, Herrmann W (2005) Biochemical indexes of the B vitamins in cord serum are predicted by maternal B vitamin status. Am J Clin Nutr 82(1):133–139
Parry S, Zhang J (2007) Multidrug resistance proteins affect drug transmission across the placenta. Am J Obstet Gynecol 196(5):476.e471–476.e476
Peters Jr, T (1995) 6-clinical aspects: albumin in medicine. In: All about albumin. Academic Press, San Diego, pp 251–284
Polachek H, Holcberg G, Sapir G, Tsadkin-Tamir M, Polachek J, Katz M, Ben-Zvi Z (2005) Transfer of ciprofloxacin, ofloxacin and levofloxacin across the perfused human placenta in vitro. Eur J Obstet Gynecol Reprod Biol 122(1):61–65
Polin RA, Fox WW, Abman SH (2011) Fetal and neonatal physiology. Expert consult-online and print. Elsevier Limited, Oxford
Poulsen MS, Rytting E, Mose T, Knudsen LE (2009) Modeling placental transport: correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol In Vitro 23(7):1380–1386
Quentin C, Besnard R, Bonnard O, Akbaraly R, Brachet-Liermain A, Leng J, Bebear C (1983) Preliminary in vitro study of the transplacental passage of spiramycin. Pathol Biol (Paris) 31(5):425–428
Rietjens IMCM, Louisse J, Punt A (2011) Tutorial on physiologically based kinetic modeling in molecular nutrition and food research. Mol Nutr Food Res 55:941–956
Scholl TO, Chen X, Sims M, Stein TP (2006) Vitamin E: maternal concentrations are associated with fetal growth. Am J Clin Nutr 84(6):1442–1448
Syme MR, Paxton JW, Keelan JA (2004) Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 43(8):487–514
Takeuchi Y, Sakakibara R, Ishiguro M (1990) Synthesis and secretion of human chorionic gonadotropin and its subunits in choriocarcinoma cells: a comparative study with normal placental cells. Mol Cell Endocrinol 69(2–3):145–156
The European parliament and the council (2006) Regulation (EC) NO 1907/2006 of the European parliament and of the council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Official Journal of the European Union
Utoguchi N, Chandorkar GA, Avery M, Audus KL (2000) Functional expression of P-glycoprotein in primary cultures of human cytotrophoblasts and BeWo cells. Reprod Toxicol 14(3):217–224
Vardhana PA, Illsley NP (2002) Transepithelial glucose transport and metabolism in BeWo choriocarcinoma cells. Placenta 23(8–9):653–660
Acknowledgments
This work was supported by BASF SE (Grant number 6153511230).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., van Ravenzwaay, B., Rietjens, I.M.C.M. et al. Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch Toxicol 87, 1661–1669 (2013). https://doi.org/10.1007/s00204-013-1074-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1074-9