Abstract
Background and Objective
Although acetaminophen is frequently used during pregnancy, little is known about fetal acetaminophen pharmacokinetics. Acetaminophen safety evaluation has typically focused on hepatotoxicity, while other events (fetal ductal closure/constriction) are also relevant. We aimed to develop a fetal–maternal physiologically based pharmacokinetic (PBPK) model (f-m PBPK) to quantitatively predict placental acetaminophen transfer, characterize fetal acetaminophen exposure, and quantify the contributions of specific clearance pathways in the term fetus.
Methods
An acetaminophen pregnancy PBPK model was extended with a compartment representing the fetal liver, which included maturation of relevant enzymes. Different approaches to describe placental transfer were evaluated (ex vivo cotyledon perfusion experiments, placental transfer prediction based on Caco-2 cell permeability or physicochemical properties [MoBi®]). Predicted maternal and fetal acetaminophen profiles were compared with in vivo observations.
Results
Tested approaches to predict placental transfer showed comparable performance, although the ex vivo approach showed highest prediction accuracy. Acetaminophen exposure in maternal venous blood was similar to fetal venous umbilical cord blood. Prediction of fetal acetaminophen clearance indicated that the median molar dose fraction converted to acetaminophen-sulphate and N-acetyl-p-benzoquinone imine was 0.8% and 0.06%, respectively. The predicted mean acetaminophen concentration in the arterial umbilical cord blood was 3.6 mg/L.
Conclusion
The median dose fraction of acetaminophen converted to its metabolites in the term fetus was predicted. The various placental transfer approaches supported the development of a generic f-m PBPK model incorporating in vivo placental drug transfer. The predicted arterial umbilical cord acetaminophen concentration was far below the suggested postnatal threshold (24.47 mg/L) for ductal closure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A fetal–maternal physiologically based pharmacokinetic (f-m PBPK) model has been developed to quantitatively predict placental transfer of acetaminophen and characterize fetal acetaminophen exposure and metabolic clearance in the fetus at term delivery. |
Different approaches for describing placental drug transfer (estimation of placental transfer parameters from ex vivo cotyledon perfusion experiments, scaling of placental transfer via Caco-2 cell permeability, and via physicochemical properties [MoBi® default method]) showed broadly comparable performance, although the ex vivo approach achieved highest prediction accuracy. |
Acetaminophen exposure in the maternal venous blood was similar to that in the fetal venous umbilical cord blood. In addition, the predicted mean acetaminophen concentration, after a maternal dose of 1000 mg, in arterial umbilical cord blood, suspected to be involved in ductus arteriosus closure/constriction, was 3.6 mg/L and therefore far below the suggested postnatal threshold (24.47 mg/L). |
Prediction of acetaminophen clearance in the fetus indicated that the median molar dose fraction of acetaminophen converted to acetaminophen-sulphate and N-acetyl-p-benzoquinone imine was 0.8% and 0.06%, respectively. |
1 Introduction
Pregnant women frequently and increasingly take medication [1]. Irrespective of whether the fetus is the target of pharmacotherapy, the fetus is probably exposed to any drug taken by the mother [2]. Adequate models to predict fetal pharmacokinetic profiles and drug exposure are scarce. Physiologically based pharmacokinetic (PBPK) modeling can be a valuable tool facilitating the prediction of fetal drug exposure [3, 4].
Although 60% of pregnant women take acetaminophen (paracetamol) [5], little is known about acetaminophen pharmacokinetics after therapeutic dosing and the contributions of specific metabolic clearance pathways to total clearance in the fetus. Acetaminophen is metabolized through different metabolic pathways in the liver [6]. In adults, acetaminophen is predominantly metabolized via glucuronidation (55%) and sulphation (30%). To a smaller extent, acetaminophen is excreted unchanged (2–5%) in urine. In addition, a minor fraction is metabolized through cytochrome P450 (CYP) enzyme-mediated oxidation forming the toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) (5–10%). Under normal conditions, NAPQI is immediately neutralized by conjugation with glutathione [7]. At high dosages, however, glutathione will become depleted and NAPQI is held responsible for acetaminophen-induced hepatotoxicity [8]. Research on acetaminophen toxicity has typically been concerned with hepatotoxicity, whereas other adverse events might also be relevant for the fetus and may relate to fetal exposure to acetaminophen or its metabolites. Therefore, it is important to explore the separate contributions of the different metabolic pathways. Several epidemiological studies [9,10,11,12] report that perinatal acetaminophen exposure might be associated with pulmonary (e.g., atopy) and neurodevelopmental (e.g., attention deficit hyperactivity disorder) toxicology. In addition, a recent case series analysis describes an association between maternal acetaminophen intake and fetal ductus arteriosus constriction or closure [13]. It seems unlikely that both hepatic and extrahepatic adverse events can be attributed to NAPQI alone, since adverse events can also be attributed to acetaminophen [5, 14].
Different approaches to integrate placental drug transfer in a fetal–maternal PBPK (f-m PBPK) framework have been previously presented [4]. Mathematically, placental transfer can be described by a modified form of Fick’s first law of diffusion with the two key parameters being the transplacental passive diffusion clearance (Dpl) and partition coefficient between the fetal and maternal compartment (Kf,m). Informing these parameters is difficult since they are not readily measurable in the in vivo system and hence several approaches to estimate these parameters were previously reported [4]. These approaches relied on informing the placental transfer rate and partition coefficient through ex vivo cotyledon perfusion experiments [15] or scaling placental transfer via Caco-2 cell permeability or physicochemical properties [MoBi® default method] while fixing the partition coefficient at a value of 1.0 [2, 16]. While some of these approaches were evaluated for a limited number of specific drugs, they have not yet been carefully compared with each other.
This study has two objectives: first, to develop a f-m PBPK model that can quantitatively predict placental transfer and drug exposure of acetaminophen in the term fetus at delivery and compare the predictive performance of the three different approaches to integrate placental drug transfer in a PBPK framework; second, to quantify the hitherto unknown contributions of specific metabolic clearance pathways to total clearance in the term fetus.
2 Methods
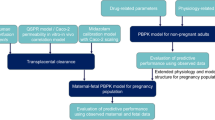
Figure 1 shows the workflow of the present study. Previously, a pregnancy PBPK model for acetaminophen and its metabolites has been developed and verified [17]. To this model structure, we added a fetal liver compartment (Fig. 2). Three different methods for informing the two unknown parameters of placental transfer (the Dpl and partition coefficient) in a PBPK model were evaluated: (i) estimation of the Dpl and partition coefficient from the ex vivo cotyledon perfusion experiment [18]; (ii) estimation of the Dpl from a previously reported scaling approach via Caco-2 cells while fixing the partition coefficient to a value of 1.0 [2, 16]; and (iii) estimation of the Dpl from physicochemical properties of the drug (default method implemented in MoBi®) while fixing the partition coefficient to a value of 1.0 [3]. Predicted acetaminophen pharmacokinetic profiles were compared to observed data obtained from the umbilical vein at delivery [19]. In addition, acetaminophen (mean) concentrations in the arterial umbilical cord blood were predicted for evaluating the risk of possible constriction/closure of ductus arteriosus. Finally, the contributions of specific metabolic clearance pathways in the term fetus to total clearance were both predicted but could not be evaluated due to lack of in vivo data.
Schematic workflow of fetal–maternal physiologically based pharmacokinetic (f-m PBPK) model development and evaluation. Dcot transcotyledon passive diffusion clearance, Dpl transplacental passive diffusion clearance, Kf,m partition coefficient between the fetal and maternal compartment, PBPK physiologically based pharmacokinetic, PK pharmacokinetics
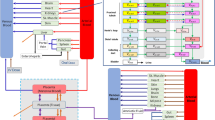
Structure of the fetal–maternal physiologically based pharmacokinetic (f-m PBPK) model. The four sub-compartments (blood cells, plasma, interstitial, and intracellular) for acetaminophen distribution in the fetal liver have been visualized separately. Solid lines and closed arrows indicate blood flow process, dash–dotted lines and closed arrows indicate biliary secretion or movement along the intestine through gastrointestinal motility, solid lines and open arrows indicate transport across the placenta through passive diffusion, boxes with solid frame indicate compartments representing organs available in both non-pregnant and pregnant women, and boxes with dashed frame indicate compartments representing organs exclusively available in pregnant women
2.1 Software
PBPK models were built in MoBi®, part of the Open Systems Pharmacology software suite (http://www.open-systems-pharmacology.org). R Studio (version 3.3.0) was used for graphics creations and statistical analysis. MONOLIX version 4.4.0 (Lixoft, Orsey, France) was used to estimate placental transfer parameters of acetaminophen from the ex vivo cotyledon perfusion experiment.
2.2 Development of Fetal–Maternal Physiologically Based Pharmacokinetic (f-m PBPK) Models
In a previous study, the development of pregnancy PBPK models was successfully verified for the three trimesters of pregnancy [17]. In brief, first, a PBPK model was previously developed for both intravenous and oral acetaminophen administration in non-pregnant women. The predictive performance of the model was evaluated by comparing simulations with observed in vivo pharmacokinetic profiles of acetaminophen, acetaminophen-glucuronide, acetaminophen-sulphate, and unchanged acetaminophen after both oral and intravenous acetaminophen administration of standard dosages [20,21,22]. Once the non-pregnant PBPK model captured the observed pharmacokinetics adequately, all drug-specific parameters were fixed and pregnancy-specific changes were incorporated. Model performance was evaluated by comparing simulations with observed in vivo pharmacokinetic profiles of acetaminophen, acetaminophen-glucuronide, acetaminophen-sulphate, and unchanged acetaminophen obtained from third-trimester pregnant women [20]. For more detailed information about parameterization and validation of the previously developed pregnancy PBPK model we refer to Mian et al. [17]. Here, the structure of the pregnancy PBPK model was extended by incorporating a separate well-stirred compartment representing the fetal liver. The fetal liver was connected through the blood flow from the venous blood pool of the umbilical cord and that to the fetal body. This fetal liver compartment was subdivided into four sub-compartments, namely plasma (\({\text{pls}}\)), blood cells (\({\text{bc}}\)), interstitial (\({\text{int}}\)), and intracellular (\({\text{cell}}\)) space. The volume of the fetal body compartment was then reduced by the volume (0.14 L) of the compartment representing the fetal liver. The full structure of the f-m PBPK model is depicted in Fig. 2. The expressions of relevant enzymes and acetaminophen metabolism were implemented in the intracellular space of the fetal liver. Equations (1–4) were used to describe the molar drug amount in each of the four sub-compartments of the fetal liver (\(N_{{\left\{ {{\text{pls}},{\text{bc}},{\text{int}},{\text{cell}}} \right\}}}\)) (µmol):
where \(C_{{\left\{ {{\text{pls}},{\text{bc}},{\text{int}},{\text{cell}}} \right\}}}\) denotes the molar drug concentration in each of the four sub-compartments (µmol/L); \(Q\) the absolute organ blood flow (L/min); \({\text{HCT}}\) the fetal hematocrit; \(C_{{\left\{ {{\text{bc}},{\text{pls}}} \right\}}}^{\text{venUC}}\) the molar drug concentration in blood cells and plasma in the venous blood of the umbilical cord (µmol/L); \(f_{\text{u}}\) the fraction unbound of the drug; \(P\) the local permeability between the respective sub-compartments (cm/min); \({\text{SA}}\) the surface area at the interface between the respective sub-compartments (cm²); \(K\) the partition coefficient between the respective sub-compartments; \(K_{{{\text{prot}}:{\text{water}}}}\) the partition coefficient between protein and water; \(C_{\text{cell}}^{\text{Enzyme}}\) the concentration of the drug-metabolizing enzyme in the intracellular space of the fetal liver (µmol/L); \(V_{\text{cell}}\) the volume of the intracellular space of the fetal liver (L); \(k_{\text{cat}}\) the turnover number of the specific enzyme (min–1); and \(K_{\text{m}}\) the Michaelis-Menten constant (µmol/L).
The fraction of unbound acetaminophen was assumed to be similar between maternal and fetal plasma. The local permeabilities, the volume of the intracellular space of the fetal liver, and the surface area at the interface between the respective sub-compartments were automatically estimated using scaling approaches implemented in the software [3]. It was assumed that the fetal liver is geometrically similar to the adult liver (e.g., that the intracellular volume fraction of total liver is similar between fetus and adult). The partition coefficients between the respective sub-compartments as well as those between protein and water were estimated from equations described elsewhere [3]. Based on literature information discussed later, the enzyme concentration in the fetal liver was estimated relative to adult levels. Finally, values for \(k_{\text{cat}}\) and \(K_{\text{m}}\) were taken from the PK-Sim® template [17, 23], assuming that acetaminophen and its metabolites display the same affinity to fetal and adult enzymes. An exception was fetal sulfotransferase (SULT) 1A1, for which a Km value of 2.4 mmol/L [24] was used in the model. Renal excretion of unchanged acetaminophen by the fetus was not accounted for in the model, as we assumed that this is very limited anyhow [25]
2.3 Acetaminophen Absorption During Labor and Fetal Metabolism in the f-m PBPK Model
The different elimination pathways of acetaminophen and its metabolites were implemented in the model as described previously for a pregnancy PBPK in non-laboring women [17]. In the present study, the maternal pharmacokinetics were predicted in term pregnant women during labor. There is some evidence that gastric emptying, gastrointestinal motility, and hence drug absorption from the gastrointestinal tract, are slower during labor [26, 27]. This is probably due to analgesic treatment with opioids [28], vomiting during labor, absent of food intake for a long time [29,30,31], or extreme physical exercise, which in particular delays gastric emptying time during labor [32, 33]. Whitehead et al. [29] reported a threefold delay in time to maximum concentration (tmax) in pregnant women (n = 36) during labor when compared with 2 h post-delivery women (n = 17) [29]. Based on this observation, we applied a threefold increase in gastric emptying in the maternal PBPK model.
2.4 Maturation of Enzymes in the Fetus
One aim of the present study was to characterize the contributions of specific metabolic clearance pathways to total clearance in the term fetus, including metabolism by uridine 5ʹ-diphospho-glucuronosyltransferase (UGT), SULT and CYP. In the PK-Sim® template for acetaminophen, UGT1A1, SULT1A1, and CYP2E1 were implemented as the main isoforms for the respective metabolic pathway. Due to missing information, detoxification kinetics of NAPQI could not be parameterized and it was therefore assumed that the concentrations of cysteine and mercapturate together are equivalent to that of NAPQI [23]. In the PBPK model, fetal enzyme expression, and hence fetal metabolism, was accounted for only in the fetal liver, not in other fetal tissues.
2.4.1 Uridine 5′-Diphospho-Glucuronosyltransferase (UGT) 1A1
Studies [34, 35] have shown very low expression and activity of UGT1A1 in human fetal liver microsomes in the second half of gestation (0.1–1% of the adult level). Therefore, no fetal UGT1A1 expression was implemented in the PBPK model, and hence glucuronidation was not modeled in the fetus.
2.4.2 Sulfotransferase (SULT) 1A1 and 1A3
There is a broad consensus that, throughout fetal life until birth, SULT1A1 is expressed at a relatively constant level of about 100% of the level in adult livers [24, 36,37,38,39,40]. SULT1A3 expression is 3- to 10-fold higher than adult liver values in fetal liver at term [24, 37, 38, 40]. In the PBPK model, SULT1A1 and SULT1A3 expression in the fetal liver were lumped together and implemented as a 6.5-fold higher than the adult value.
2.4.3 Cytochrome P450 (CYP) 2E1
Contradictory information (see Sect. 4) on fetal changes in CYP2E1 has been reported [36]. Most studies reported detectable CYP2E1 expression or activity in the third trimester [36, 41]. Johnsrud et al. [42] reported detectable CYP2E1 amounts in 80% of third-trimester liver microsomes (16.2% of the adult level); therefore, this value was implemented in the PBPK model [42].
2.5 Evaluation of Various Approaches for Estimating Unknown Parameters of Placental Transfer and Integration in Physiologically Based Pharmacokinetic Models
Three different methods for informing the two unknown parameters (Dpl, Kf,m) of placental transfer in a PBPK model were evaluated.
2.5.1 Estimation of Placental Permeability and Partition Coefficient from Ex Vivo Cotyledon Perfusion Experiment
2.5.1.1 Placenta Perfusion
The study protocol was approved by the ethics review board of the University Hospitals (UZ) Leuven (s54819), Eudra-CT number 2012-004580-51, ClinicalTrials.gov identifier NCT02622802 [43]. The experimental setup and methodology have been published previously [18]. Placentas were perfused in a recirculating (closed–closed) circuit within 30 min after delivery. An intact cotyledon was selected for perfusion and the corresponding chorionic artery and vein were cannulated. To test maternal–fetal transport, acetaminophen 10 µg/mL was perfused in the maternal circulation; this is the concentration that corresponds with the steady-state concentration at clinical use. The fetal (Qf) and maternal (Qm) circulations were established at a flow of 6 and 14 mL/min, respectively. The mean maternal and fetal reservoir volumes (Vm and Vf) were 280 and 284 mL, respectively. Samples from both maternal and fetal sides were collected at 0, 3, 6, 10, 15, 20, and 30 min, then every 15 min until 150 min, and thereafter every 30 min until 210 min after addition of acetaminophen to the respective reservoir.
2.5.1.2 Estimation of Placental Transfer Parameters
Acetaminophen maternal to fetal transfer across the placenta was estimated using a four-compartment model structure with the cotyledon being split into a maternal and a fetal compartment (Fig. 3). The transcotyledon passive diffusion clearance (Dcot) was assumed to be equal in both fetal-to-maternal and maternal-to-fetal direction (i.e., no polarity was assumed). Although acetaminophen-glucuronide and acetaminophen-sulphate transfer have been investigated [18], both metabolites were not implemented as verification with in vivo data was not possible. The cotyledon volume was assumed to be 58 mL on average [44]. The maternal cotyledon volume (Vmp) was assumed to be 23 mL and the fetal cotyledon volume (Vfp) 35 mL [44]. Loss of volume related to sampling was not corrected for as it was < 10%. The model was built in two steps: building (1) a structural model and (2) a statistical sub-model [45]. Discrimination between different models was made by the likelihood ratio test using the Objective Function Value (OFV) (i.e., 2* log likelihood), where a decrease in OFV of 3.84 points (p < 0.05 based on a Chi-squared [χ2] distribution) was considered statistically significant, between nested models with one additional degree of freedom. Furthermore, goodness-of-fit plots, individual plots, and relative standard error (RSE) were evaluated. Several placental transfer models for acetaminophen were tested (e.g., simple passive diffusion, linear elimination). For the statistical model, inter-individual variability was tested for significance on all parameters except Vmp and Vfp, as the latter were fixed. Error models (proportional, constant, mixed) were investigated to describe the residual unexplained variability. Simulated concentrations in the maternal and fetal reservoirs were compared to the ex vivo experiment concentrations [18].
Schematic representation of the ex vivo cotyledon perfusion model. Dcot transcotyledon passive diffusion clearance, Kf,m partition coefficient between the fetal and maternal compartment, Kpe placental elimination, Qf fetal flow rate, Qm maternal flow rate, Vf volume of fetal reservoir, Vm volume of maternal reservoir, Vfp volume of fetal part of the cotyledon, Vmp volume of maternal part of cotyledon
Equations (5–8) describing the time-dependent change of the molar drug amount in the respective compartment (N [µmol]) were used to estimate Dcot (mL/min), Kf,m and placental elimination (Kpe) (min–1).
Equation (5): maternal reservoir
Equation (6): maternal part of cotyledon
Equation (7): fetal part of cotyledon
Equation (8): fetal reservoir
where Q is the flow rate (mL/min) and V the volume (mL), m indicates maternal, mp indicates maternal part of placenta, f indicates fetus, and fp indicates fetal part of placenta.
2.5.1.3 Upscaling of Transfer Parameters from the Ex Vivo Perfusion Experiment
The estimates of the fitted ex vivo transcotyledon transfer parameters (Dcot, Kf,m) were implemented in the f-m PBPK model after scaling the Dcot to the Dpl using Eq. 9:
where Dpl (mL/min) and Dcot (mL/min) represent the Dpl and transcotyledon passive diffusion clearance, respectively, and Vpl (mL) and Vcot (mL) represent the placental and cotyledon volumes, respectively.
2.5.2 Scaling Placental Permeability from Caco-2 Cell Permeability
To estimate placental drug transfer [2, 16], Dpl was scaled from the apparent permeability measured across Caco-2 monolayers and total diffusion parameter of a reference substance (midazolam). The mean apparent permeability of acetaminophen across Caco-2 monolayers (256 nm/s) [46,47,48,49,50] was normalized to that of midazolam and subsequently multiplied with the total diffusion parameter of midazolam at term, which had been determined previously [2, 16]. Since this method was only evaluated for drugs transferring the placenta via passive diffusion drugs, a Kf,m of 1.0 was assumed for acetaminophen [2, 16].
2.5.3 Scaling Placental Permeability from Physicochemical Properties
Another method to estimate placental diffusion is the default calculation method already implemented in MoBi®, which assumes that the placental permeability of the drug is the same as those across other organ membranes. According to this method, the permeability is estimated from physicochemical properties of the drug, such as lipophilicity and molecular weight [3]. Subsequently, the permeability is multiplied with the villi surface area (11.8 m2) at the specific fertilization age [51], yielding the Dpl. The Kf,m was assumed to be 1.0 [52].
It has to be noted that for all three placental transfer approaches, no polarity in Dpl was assumed, i.e., Dpl is similar in both (fetal–maternal [f-m] and maternal–fetal [m-f]) directions.
2.6 Evaluation of f-m PBPK Models
The f-m PBPK models were evaluated by comparing the predicted acetaminophen concentrations in the maternal and the fetal venous umbilical cord blood plasma with in vivo concentration data obtained from 34 women and their newborns (median gestational age 39 weeks, range 38–40 weeks) following single oral administration of acetaminophen 1000 mg at delivery [19]). Umbilical cord samples were collected at delivery, 0.5–5.8 h after maternal acetaminophen dosing [19]. The estimated pharmacokinetic parameters for all three approaches and the observed pharmacokinetic parameters were obtained from the Open Systems Pharmacology software. Furthermore, ratios of predicted to observed pharmacokinetic parameters of acetaminophen were estimated. A local sensitivity analysis was performed for the two key parameters relevant for placental transfer and the gastric emptying time. To this aim, the original value was divided or multiplied by 1.3, 2, and 5 [53] and the resulting pharmacokinetic profiles were predicted and compared with each other.
3 Results
3.1 Evaluation of Placental Transfer Informed by Various Approaches
Table 1 shows the estimated values for the Dpl and the partition coefficient obtained from the ex vivo cotyledon perfusion model. Figure 4 compares observed to simulated concentrations for the ex vivo perfusion model, showing good agreement between observed and simulated concentrations. Table 2 shows the values for Dpl and Kf,m from the different placental transfer approaches.
Ex vivo observed [19] fetal and maternal acetaminophen concentration compared with fetal and maternal simulated acetaminophen profiles in the ex vivo cotyledon perfusion experiment
3.2 Predictions of Maternal and Fetal In Vivo Concentrations
Values for the Dpl and the partition coefficient estimated by all three approaches were applied in the PBPK model. For the ex vivo cotyledon perfusion experiments, kpe was negligibly small (0.0126 min–1) and estimated with high imprecision (residual standard error 229%). Therefore, it was not implemented in the model. All placental transfer approaches adequately described the observed maternal venous blood concentrations (Fig. 5a, Table 3). Concerning the fetal acetaminophen predictions, the different approaches showed broadly comparable performance with respect to observed pharmacokinetics. Specifically, the ex vivo cotyledon perfusion approach described the fetal pharmacokinetic parameters more accurately (Fig. 5b, Tables 3, 4), while scaling Dpl via Caco-2 cell permeability or physicochemical properties and keeping Kf,m fixed at 1.0 resulted in predictions that were in slightly weaker agreement with observed concentrations for the acetaminophen fetal pharmacokinetic parameters (Fig. 5b, Tables 3, 4). All predicted umbilical cord concentrations resulting from each of the three approaches were within a 1.5-fold error range and 75% within the 1.25-fold error range (Table 4).
Predicted maternal (a) and fetal (b) acetaminophen pharmacokinetic profiles in venous umbilical cord plasma following administration of oral acetaminophen 1000 mg using the three different placental transfer approaches described in the text. Predicted maternal and fetal plasma acetaminophen pharmacokinetic profiles were compared with observed cord blood concentrations for the maternal dose of 1000 mg [19]
The predicted mean acetaminophen concentration in arterial umbilical cord blood—of relevance to potential ductus arteriosus constriction/closure—was 3.6 mg/L.
Because the ex vivo cotyledon perfusion approach described the fetal pharmacokinetic parameters most accurately, data from this approach were used to predict the molar dose fraction of acetaminophen converted to its metabolites. Prediction of acetaminophen clearance in the fetus indicated that the median molar dose fraction of acetaminophen converted to acetaminophen-sulphate and NAPQI were 0.8% and 0.06%, respectively (Fig. 6).
3.3 Sensitivity Analysis
The local sensitivity analyses for the two unknown parameters describing placental transfer (Kf,m and Dpl) are provided in Fig. 7. Changes in Kf,m substantially impacted the predicted fetal plasma concentrations and thereby the pharmacokinetic parameters of acetaminophen, while the predicted fetal acetaminophen concentration–time profile was relatively insensitive to changes in Dpl (Fig. 7). To further support the above-mentioned finding, the Kf,m for each of the placental transfer approaches was assumed to be the same and therefore set at 1.0. From the Electronic Supplementary Material (ESM_1) it can be seen that large variations in Dpl did not have a significant effect on the predicted maternal and fetal acetaminophen concentration–time profiles, but that Kf,m is the sensitive parameter. However, when fixing Kf,m at 1.0, all approaches slightly overestimated fetal acetaminophen concentrations. In addition, the sensitivity analysis for gastric emptying time revealed that this is a sensitive parameter as well, although the predicted pharmacokinetic profile in the mother was only moderately sensitive towards changes within a biologically plausible range. When dividing and multiplying the value of gastric emptying time by a factor of 5 (highest and lowest value in the sensitivity analysis), maximum concentration (Cmax) and tmax are affected by 47.3% and 55.1%, respectively
Sensitivity analyses illustrating how the predicted fetal acetaminophen concentrations responds to variations in either the transplacental passive diffusion clearance (Dpl) or partition coefficient between the fetal and maternal compartment (Kf,m). The parameter values for these two parameters were calculated from the three evaluated approaches: ex vivo cotyledon perfusion experiment (a, b), scaling of placental transfer rate via physicochemical properties (MoBi® default method) (c, d), and scaling of placental transfer rate via Caco-2 cell permeability (e, f)
4 Discussion
Acetaminophen is one of the most frequently used drugs throughout pregnancy. Nevertheless, little is known about the pharmacokinetics of acetaminophen and its metabolites in the fetus. Relevant issues concerning the two study objectives—estimating the placental transfer and pharmacokinetics of acetaminophen, and contributions of the metabolic pathways—are outlined in this section.
Placental transfer and fetal exposure were predicted by integrating parameter estimates obtained from three different approaches. Subsequently, maternal and fetal pharmacokinetic predictions were evaluated using published in vivo data. Only one study has been conducted in which maternal and fetal venous umbilical cord blood was collected to investigate acetaminophen pharmacokinetics, which were used for model evaluation [19]. Tested placental transfer approaches showed broadly comparable performance with respect to observed pharmacokinetics (Fig. 5, Tables 3, 4). All ratios of predicted to observed maternal and fetal pharmacokinetic parameters were within a 1.25- and 1.5-fold error range, respectively (Table 4). This indicates that the developed acetaminophen f-m PBPK models can adequately predict placental transfer of acetaminophen and the fetal pharmacokinetic profile. Specifically, the ex vivo cotyledon perfusion approach showed the highest prediction accuracy resulting from the lower Kf,m value, which was identified through local sensitivity analysis as a sensitive parameter driving fetal exposure (Fig. 7, ESM_1). This finding is intriguing in that it indicates that, within the range of tested parameter values, fetal acetaminophen concentrations are not primarily governed by the Dpl (probably because equilibrium is reached very fast), but rather by the partitioning between the maternal blood to maternal plasma and the fetal blood to fetal placenta. Hence, at least within the range of tested values, the maternal-to-fetal concentration ratio at equilibrium (i.e., the partition coefficient) is the critical parameter for adequately predicting placental transfer. This also implies that the placental barrier is a relatively efficient barrier inasmuch it has a lower affinity for acetaminophen than blood/plasma.
Since the partition coefficient refers to equilibrium concentrations, this finding also suggests that few measurements at steady state in the ex vivo cotyledon perfusion experiment may be sufficient for successful integration in a PBPK framework. Dpl can be informed on the basis of other approaches (e.g., the herein evaluated scaling approaches via apparent Caco-2 cell permeability or via physicochemical descriptors, such as lipophilicity and molecular weight). For example, this could indicate that the maternal-to-fetal ratios of the cord blood concentration combined with scaling methods for Dpl could be readily used to further investigate placental transfer and fetal exposure to drugs that have, similar to acetaminophen, a good permeability and are not actively transferred across the placenta by drug transporters. Importantly, scaling Dpl via Caco-2 cell permeability or molecular descriptors (as implemented per default in MoBi®) relies on information that is often already available. In contrast to the integration of information from the ex vivo cotyledon perfusion experiments, which is a laborious experiment, these two approaches enable rather simple and fast integration into a PBPK model—at least in situations where Kf,m can be assumed to be 1.0 and where placental transfer occurs relatively fast. However, the tenfold difference in Dpl estimates obtained from the ex vivo cotyledon perfusion experiment and the other two approaches requires further investigations. While Dpl was not a sensitive parameter for acetaminophen, future studies could test high permeability compounds with high Kf,m values to generate more knowledge about the validity of predicting Dpl via different approaches. Overall, the results of the comparison of the different approaches for estimating placental transfer are in line with a recently published study on emtricitabine and acyclovir that demonstrated that fetal exposure can be adequately predicted when placental transfer is informed either by the ex vivo cotyledon experiment or by the approach by Liu et al. [54]. Still, more research on other drugs with a Kf,m approximating 1.0 and high permeability is clearly needed to further build confidence.
The second objective was to quantitatively predict the hitherto unknown contributions of specific metabolic clearance pathways to total clearance in the term fetus. Of note, transfer of acetaminophen from the fetal organism over the placenta (i.e., diffusion back to the mother) can be considered the main elimination pathway for the fetus (Fig. 6). Yet the explicit description of the different fetal elimination pathways is highly relevant since one of the metabolic pathways (CYP2E1-mediated pathway) is involved in hepatotoxicity. However, since it seems unlikely that both hepatic and extrahepatic adverse events can be attributed to NAPQI alone, but also to, for example, acetaminophen itself [14], it is important to explore the separate contributions of the different metabolic pathways to the total acetaminophen (fetal) clearance. Expectedly, as can be seen from Fig. 6, the fetal contribution to the total acetaminophen (fetal) clearance (Fig. 6a) is, compared to that of the maternal organism (Fig. 6b), very low. As recently shown, fetal ductus arteriosus constriction or closure following acetaminophen intake by pregnant women is a potential safety concern [14]. The presented results show that the acetaminophen concentration at steady state achieved in the arterial umbilical cord blood plasma is 3.6 mg/L. When assuming a same target concentration for ductus constriction in the human fetus as documented in the fetal rat [55], the EC50 (concentration of drug producing 50% of maximum effect) is 24.47 mg/L. This indicates that a much higher concentration of acetaminophen is needed to constrict the ductus. However, as incidents have been reported [13], it is possible that other pathways or compartments (e.g., prostaglandins synthesis in placenta) are involved.
Some limitations and assumptions of this analysis need to be addressed. Firstly, the fetal sub-model of the PBPK model was extended by incorporating a separate compartment representing the fetal liver placed between the compartments representing venous cord blood and the fetal body. While the blood from the venous umbilical cord flows through the fetal liver through the ductus venosus, the liver parenchyma is mainly supplied by blood through the fetal arteria hepatica. Additionally, unchanged drug may be renally excreted in the amniotic fluid and subsequently swallowed and re-absorbed. Hence, it is important to note that the herein presented model structure does not fully reflect this physiologic reality. Further refinements may be necessary for more detailed applications. Furthermore, enzyme expression in the fetal liver was implemented and informed on the basis of available data from literature. Obviously, such a knowledge-driven approach is limited by the amount and quality of information available in the literature. Although most studies [42, 56, 57] reported the presence of CYP2E1 expression or activity during the third trimester of pregnancy, Vieira et al. [58] found no CYP2E1 expression in 66 fetal livers (ranging from 16 to 40 weeks of gestational age) in the second and third trimester. While a lower expression of CYP2E1 in the fetal liver may be physiologically plausible in the third trimester, the herein presented PBPK model incorporated a relative high expression (16% of the adult level in the liver) because this value translates into the maximum NAPQI exposure that might be expected in vivo. Hence, the presented model can be biased to overestimation of fetal exposure to NAPQI as a worst-case scenario. Secondly, a limitation of the presented model is that only one isoform of each enzyme sub-family (UGT1A1, CYP2E1) in the fetal liver is incorporated, while other isoforms may also be involved in acetaminophen metabolism. No study has reported cord blood concentrations of the metabolites of acetaminophen, which complicates proper evaluation of the predicted metabolite pharmacokinetic profiles. Thirdly, fetal and maternal protein binding of acetaminophen and its metabolites were assumed to be the same. For acetaminophen and its metabolites this may not be relevant, as all compounds are only marginally protein bound. Furthermore, due to missing information on glutathione-related enzymes in the fetus, the detoxification kinetics of NAPQI could not be parameterized and we assumed that the concentration of cysteine and mercapturate is equivalent to that of NAPQI. Here, more research is needed to inform PBPK models more accurately. Fourthly, enzyme expression in the placenta was not accounted for in the PBPK model. Syme et al. [59] systematically reviewed the expression of enzymes in the placenta, recognizing that UGT1A1 messenger RNA (mRNA) and protein expression have been undetectable at term. For CYP2E1, however, mRNA and protein levels have been detected in the placenta from the first trimester onwards. This observation raises the question whether NAPQI toxicity can also be expected in the placenta, an organ with a vitally important function for the fetus. For SULT, little is known on placental metabolism [59], although Weigand et al. [60] reported no acetaminophen-sulphate in placental tissue and maternal and fetal plasma concentrations. In addition, it should be emphasized that the current f-m PBPK model investigated placenta transfer at term pregnancy. Since placenta morphology and function change radically throughout gestation, the presented results are probably not readily scalable to earlier stages of pregnancy. Finally, placental transfer of all acetaminophen metabolites was not covered in this analysis because in vivo data from umbilical cord samples of metabolites are lacking throughout different weeks of gestational age, which precludes the investigation and evaluation of fetal exposure to the metabolites of acetaminophen.
5 Conclusion
The developed f-m PBPK model adequately captured maternal and fetal pharmacokinetic profiles of acetaminophen at term delivery. Taken together, this study provides important insights on placental drug transfer and fetal drug exposure that can support future efforts to develop more generic f-m PBPK models for different drugs. Ultimately, such f-m PBPK models can constitute powerful tools to support informed decision-making in the clinical setting when information from other sources is lacking or inconsistent.
References
Pisa FE, Casetta A, Clagnan E, Michelesio E, Vecchi Brumatti L, Barbone F. Medication use during pregnancy, gestational age and date of delivery: agreement between maternal self-reports and health database information in a cohort. BMC Pregnancy Childbirth. 2015;15:310. https://doi.org/10.1186/s12884-015-0745-3.
Zhang Z, Imperial MZ, Patilea-Vrana GI, Wedagedera J, Gaohua L, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model I: insights into factors that determine fetal drug exposure through simulations and sensitivity analyses. Drug Metab Dispos. 2017;45(8):920–38. https://doi.org/10.1124/dmd.117.075192.
Dallmann A, Ince I, Solodenko J, Meyer M, Willmann S, Eissing T, et al. Physiologically based pharmacokinetic modeling of renally cleared drugs in pregnant women. Clin Pharmacokinet. 2017;56(12):1525–41. https://doi.org/10.1007/s40262-017-0538-0.
Dallmann A, Pfister M, van den Anker J, Eissing T. Physiologically based pharmacokinetic modeling in pregnancy: a systematic review of published models. Clin Pharmacol Ther. 2018;104(6):1110–24. https://doi.org/10.1002/cpt.1084.
Allegaert K, van den Anker JN. Perinatal and neonatal use of paracetamol for pain relief. Semin Fetal Neonatal Med. 2017;22(5):308–13. https://doi.org/10.1016/j.siny.2017.07.006.
Flint RB, Mian P, van der Nagel B, Slijkhuis N, Koch BC. Quantification of acetaminophen and its metabolites in plasma using UPLC-MS: doors open to therapeutic drug monitoring in special patient populations. Ther Drug Monit. 2017;39(2):164–71. https://doi.org/10.1097/ftd.0000000000000379.
Forrest JAH, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet. 1982;7(2):93–107.
Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S–8S.
Sordillo JE, Scirica CV, Rifas-Shiman SL, Gillman MW, Bunyavanich S, Camargo CA, et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol. 2015;135(2):441–8. https://doi.org/10.1016/j.jaci.2014.07.065.
Shaheen SO, Newson RB, Ring SM, Rose-Zerilli MJ, Holloway JW, Henderson AJ. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allergy Clin Immunol. 2010;126(6):1141–8. https://doi.org/10.1016/j.jaci.2010.08.047.
Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–13. https://doi.org/10.1093/ije/dyt183.
Liew Z, Ritz B, Rebordosa C, Lee PC, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014;168(4):313–20. https://doi.org/10.1001/jamapediatrics.2013.4914.
Allegaert K, Mian P, Lapillonne A, van den Anker JN. Maternal paracetamol intake and fetal ductus arteriosus constriction or closure: a case series analysis. Br J Clin Pharmacol. 2019;85(1):245–51. https://doi.org/10.1111/bcp.13778.
McGill MR, Sharpe MR, Williams CD. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Investig. 2012;122(4):1574–83. https://doi.org/10.1172/JCI59755.
De Sousa Mendes M, Hirt D, Vinot C, Valade E, Lui G, Pressiat C, et al. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br J Clin Pharmacol. 2016;81(4):646–57. https://doi.org/10.1111/bcp.12815.
Zhang Z, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model II: verification of the model for passive placental permeability drugs. Drug Metab Dispos. 2017;45(8):939–46. https://doi.org/10.1124/dmd.116.073957.
Mian P, van den Anker JN, van Calsteren K, Annaert P, Tibboel D, Pfister M, et al. Physiologically based pharmacokinetic modeling to characterize acetaminophen pharmacokinetics and N-acetyl-p-benzoquinone imine (NAPQI) formation in non-pregnant and pregnant women. Clin Pharmacokinet. 2019 Jul 25. https://doi.org/10.1007/s40262-019-00799-5.
Conings S, Tseke F, Van den Broeck A, Qi B, Paulus J, Amant F, et al. Transplacental transport of paracetamol and its phase II metabolites using the ex vivo placenta perfusion model. Toxicol Appl Pharmacol. 2019;370:14–23. https://doi.org/10.1016/j.taap.2019.03.004.
Nitsche JF, Patil AS, Langman LJ, Penn HJ, Derleth D, Watson WJ, et al. Transplacental passage of acetaminophen in term pregnancy. Am J Perinatol. 2017;34(6):541–3. https://doi.org/10.1055/s-0036-1593845.
Allegaert K, Peeters MY, Beleyn B, Smits A, Kulo A, van Calsteren K, et al. Paracetamol pharmacokinetics and metabolism in young women. BMC Anesthesiol. 2015;15(1):163. https://doi.org/10.1186/s12871-015-0144-3.
Beaulac-Baillargeon L, Rocheleau S. Paracetamol pharmacokinetics during the first trimester. Eur J Clin Pharmacol. 1994;46(5):451–4.
Mitchell MC, Hanew T, Meredith CG, Schenker S. Effects of oral contraceptive steroids on acetaminophen metabolism and elimination. Clin Pharmacol Ther. 1983;34(1):48–53.
Krauss M, Schaller S, Borchers S, Findeisen R, Lippert J, Kuepfer L. Integrating cellular metabolism into a multiscale whole-body model. PLoS Comput Biol. 2012;8(10):e1002750. https://doi.org/10.1371/journal.pcbi.1002750.
Adjei AA, Gaedigk A, Simon SD, Weinshilboum RM, Leeder JS. Interindividual variability in acetaminophen sulfation by human fetal liver: Implications for pharmacogenetic investigations of drug-induced birth defects. Birth Defects Res A Clin Mol Teratol. 2008;82(3):155–65. https://doi.org/10.1002/bdra.20535.
Krekels EH, van Ham S, Allegaert K, de Hoon J, Tibboel D, Danhof M, et al. Developmental changes rather than repeated administration drive paracetamol glucuronidation in neonates and infants. Eur J Clin Pharmacol. 2015;71(9):1075–82. https://doi.org/10.1007/s00228-015-1887-y.
Galinsky RE, Levy G. Absorption and metabolism of acetaminophen shortly before parturition. Drug Intell Clin Pharm. 1984;18(12):977–9.
Davison JS, Davison MC, Hay DM. Gastric emptying time in late pregnancy and labour. J Obstet Gynaecol Br Commonw. 1970;77(1):37–41.
Kulo A, van Calsteren K, Verbesselt R, Smits A, Devlieger R, de Hoon J, et al. The impact of Caesarean delivery on paracetamol and ketorolac pharmacokinetics: a paired analysis. J Biomed Biotechnol. 2012;2012:437639.
Whitehead EM, Smith M, Dean Y, O’Sullivan G. Forum: an evaluation of gastic emptying times in pregnancy and the puerperium. Anaesthesia. 1993;48(1):53–7.
Holdsworth JD. Relationship between stomach contents and analgesia in labour. Br J Anaesth. 1978;50(11):1145–8.
Nimmo WS, Wilson J, Prescott LF. Narcotic analgesics and delayed gastric emptying during labour. Lancet. 1975;1(7912):890–3.
Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;8:CD003930. https://doi.org/10.1002/14651858.CD003930.pub3.
Marzio L, Formica P, Fabiani F, LaPenna D, Vecchiett L, Cuccurullo F. Influence of physical activity on gastric emptying of liquids in normal human subjects. Am J Gastroenterol. 1991;86(10):1433–6.
Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J. 1981;196(1):257–60.
Felsher BF, Maidman JE, Carpio NM, VanCouvering K, Woolley MM. Reduced hepatic bilirubin uridine diphosphate glucuronyl transferase and uridine diphosphate glucose dehydrogenase activity in the human fetus. Pediatr Res. 1978;12(8):838–40. https://doi.org/10.1203/00006450-197808000-00007.
Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–67. https://doi.org/10.1016/j.pharmthera.2008.02.005.
Vietri M, Pietrabissa A, Mosca F, Rane A, Pacific GM. Human adult and foetal liver sulphotransferases: inhibition by mefenamic acid and salicylic acid. Xenobiotica. 2001;31(3):153–61. https://doi.org/10.1080/00498250110043481.
Richard K, Hume R, Kaptein E, Stanley EL, Visser TJ, Coughtrie MW. Sulfation of thyroid hormone and dopamine during human development: ontogeny of phenol sulfotransferases and arylsulfatase in liver, lung, and brain. J Clin Endocrinol Metab. 2001;86(6):2734–42. https://doi.org/10.1210/jcem.86.6.7569.
Duanmu Z, Weckle A, Koukouritaki SB, Hines RN, Falany JL, Falany CN, et al. Developmental expression of aryl, estrogen, and hydroxysteroid sulfotransferases in pre- and postnatal human liver. J Pharmacol Exp Ther. 2006;316(3):1310–7. https://doi.org/10.1124/jpet.105.093633.
Cappiello M, Giuliani L, Rane A, Pacifici GM. Dopamine sulphotransferase is better developed than p-nitrophenol sulphotransferase in the human fetus. Dev Pharmacol Ther. 1991;16(2):83–8.
Ring JA, Ghabrial H, Ching MS, Smallwood RA, Morgan DJ. Fetal hepatic drug elimination. Pharmacol Ther. 1999;84(3):429–45.
Johnsrud EK, Koukouritaki SB, Divakaran K, Brunengraber LL, Hines RN, McCarver DG. Human hepatic CYP2E1 expression during development. J Pharmacol Exp Ther. 2003;307(1):402–7. https://doi.org/10.1124/jpet.102.053124.
University Hospital, Gasthuisberg. Transplacental transfer of drugs used in pregnant women [ClinicalTrials.gov identifier NCT02622802]. National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov. Accessed 14 Jan 2020.
Shintaku K, Arima Y, Dan Y, Takeda T, Kogushi K, Tsujimoto M, et al. Kinetic analysis of the transport of salicylic acid, a nonsteroidal anti-inflammatory drug, across human placenta. Drug Metab Dispos. 2007;35(5):772–8. https://doi.org/10.1124/dmd.106.013029.
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacomet Syst Pharmacol. 2017;6(2):87–109. https://doi.org/10.1002/psp4.12161.
Laitinen L, Takala E, Vuorela H, Vuorela P, Kaukonen AM, Marvola M. Anthranoid laxatives influence the absorption of poorly permeable drugs in human intestinal cell culture model (Caco-2). Eur J Pharm Biopharm. 2007;66(1):135–45. https://doi.org/10.1016/j.ejpb.2006.09.006.
Khan S, Elshaer A, Rahman AS, Hanson P, Perrie Y, Mohammed AR. Systems biology approach to study permeability of paracetamol and its solid dispersion. Int J Pharm. 2011;417(1–2):272–9. https://doi.org/10.1016/j.ijpharm.2010.12.029.
Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 2000;10(3):195–204.
Faassen F, Vogel G, Spanings H, Vromans H. Caco-2 permeability, P-glycoprotein transport ratios and brain penetration of heterocyclic drugs. Int J Pharm. 2003;263(1–2):113–22.
Tammela P, Laitinen L, Galkin A, Wennberg T, Heczko R, Vuorela H, et al. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch Biochem Biophys. 2004;425(2):193–9. https://doi.org/10.1016/j.abb.2004.03.023.
Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56(11):1303–30. https://doi.org/10.1007/s40262-017-0539-z.
Robertson RG, Van Cleave BL, Collins JJ Jr. Acetaminophen overdose in the second trimester of pregnancy. J Fam Pract. 1986;23(3):267–8.
Schalkwijk S, Buaben AO, Freriksen JJM, Colbers AP, Burger DM, Greupink R, et al. Prediction of fetal darunavir exposure by integrating human ex-vivo placental transfer and physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2018;57(6):705–16. https://doi.org/10.1007/s40262-017-0583-8.
Liu XI, Momper JD, Rakhmanina N, van den Anker JN, Green DJ, Burckart GJ, et al. Physiologically based pharmacokinetic models to predict maternal pharmacokinetics and fetal exposure to emtricitabine and acyclovir. J Clin Pharmacol. 2020;60(2):240–55. https://doi.org/10.1002/jcph.1515.
Tanaka S, Hori S, Satoh H, Sawada Y. Prediction of fetal ductus arteriosus constriction by systemic and local dermatological formulations of NSAIDs based on PK/PD analysis. Int J Clin Pharmacol Ther. 2016;54(10):782–94. https://doi.org/10.5414/cp202532.
Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003;123(5):369–75. https://doi.org/10.1248/yakushi.123.369.
Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch Biochem Biophys. 2005;436(1):50–61. https://doi.org/10.1016/j.abb.2005.02.001.
Vieira I, Sonnier M, Cresteil T. Developmental expression of CYP2E1 in the human liver. Hypermethylation control of gene expression during the neonatal period. Eur J Biochem. 1996;238(2):476–83.
Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43(8):487–514. https://doi.org/10.2165/00003088-200443080-00001.
Weigand UW, Chou RC, Maulik D, Levy G. Assessment of biotransformation during transfer of propoxyphene and acetaminophen across the isolated perfused human placenta. Pediatr Pharmacol (New York). 1984;4(3):145–53.
Acknowledgements
The authors would like to thank Dr Gilbert Koch for his valuable input related to MONOLIX.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
For this project, P. Mian was supported by the Sophia Stichting Wetenschappelijk Onderzoek (SSWO) (S16-08) and received a Short Term Minor (STM-2017) Grant from the Stichting Sophia Kinderziekenhuis to conduct this research, a travel grant from Eramus Trustfonds, and the Dr. Catharine van Tussenbroek, Mevr. Speleers Pharmacy grant.
Conflict of interest
Paola Mian, Karel Allegaert, Sigrid Conings, Pieter Annaert, Dick Tibboel, Marc Pfister, Kristel van Calsteren, and John van den Anker have no conflicts of interest directly related to this study. André Dallmann is an employee of Bayer AG, a company which is part of the Open Systems Pharmacology (OSP) member team and involved in OSP software development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40262_2020_861_MOESM1_ESM.docx
Ex vivoin vivoex vivoex vivoex vivoin vivoex vivoex vivoESM_1: Predicted maternal (panel A) and fetal (panel B) acetaminophen PK-profiles in venous umbilical cord plasma following administration of 1000 mg oral acetaminophen using the three different placental transfer approaches (ex vivo cotyledon perfusion experiments, scaling of placental transfer rate via Caco-2 cell permeability (according to Zhang et al.) and scaling of placental transfer rate via physicochemical properties (Mobi® default method) while fixing Kf,m to 1.0 in all three approaches). Predicted maternal and fetal plasma acetaminophen PK-profiles were compared with observed umbilical cord blood concentrations for the maternal dose of 1000 mg [19]. (DOCX 413 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holdestimates of the fitted exer.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mian, P., Allegaert, K., Conings, S. et al. Integration of Placental Transfer in a Fetal–Maternal Physiologically Based Pharmacokinetic Model to Characterize Acetaminophen Exposure and Metabolic Clearance in the Fetus. Clin Pharmacokinet 59, 911–925 (2020). https://doi.org/10.1007/s40262-020-00861-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00861-7