Abstract
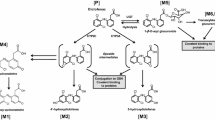
The carboxylic acid NSAID fenclozic acid exhibited an excellent preclinical safety profile and promising clinical efficacy, yet was withdrawn from clinical development in 1971 due to hepatotoxicity observed in clinical trials. A variety of modern in vitro approaches have been used to explore potential underlying mechanisms. Covalent binding studies were undertaken with [14C]-fenclozic acid to investigate the possible role of reactive metabolites. Time-dependent covalent binding to protein was observed in NADPH-supplemented liver microsomes, although no metabolites were detected in these incubations or in reactive metabolite trapping experiments. In human hepatocytes, covalent binding was observed at lower levels than in microsomes and a minor uncharacterizable metabolite was also observed. In addition, covalent binding was observed in incubations undertaken with dog and rat hepatocytes, where a taurine conjugate of the drug was detected. Although an acyl glucuronide metabolite was detected when liver microsomes from human, rat and dog were supplemented with UDPGA, there was no detectable UDPGA-dependent covalent binding. No effects were observed when fenclozic acid was assessed for P450-dependent and P450-independent cytotoxicity to THLE cell lines, time-dependent inhibition of five major human cytochrome P450 enzymes, inhibition of the biliary efflux transporters BSEP and MRP2 or mitochondrial toxicity to THLE or HepG2 cells. These data suggest that Phase 1 bioactivation plays a role in the hepatotoxicity of fenclozic acid and highlight the unique insight into mechanisms of human drug toxicity that can be provided by investigations of biotransformation and covalent binding to proteins.





Similar content being viewed by others
References
Alcock S (1970) An anti-inflammatory compound: non-toxic to animals but with an adverse action in man. Proc Eur Soc Study Drug Toxic 12:7
Argoti D, Liang L, Conteh A, Chen L, Bershas D, Yu CP, Vouros P, Yang E (2005) Cyanide trapping of iminium ion reactive intermediates followed by detection and structure identification using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Chem Res Toxicol 18(10):1537–1544
Bauman JN, Kelly JM, Tripathy S, Zhao SX, Lam WW, Kalgutkar AS, Obach RS (2009) Can in vitro metabolism-dependent covalent binding data distinguish hepatotoxic from nonhepatotoxic drugs? An analysis using human hepatocytes and liver s-9 fraction. Chem Res Toxicol 22(2):332–340
Boelsterli UA (2002) Xenobiotic acyl glucuronides and acyl CoA thioesters as protein-reactive metabolites with the potential to cause idiosyncratic drug reactions. Curr Drug Metab 3(4):439–450
Bradbury A, Powell GM, Curtis CG, Rhodes C (1981) The enhanced biliary secretion of a taurine conjugate in the rat after intraduodenal administration of high doses of fenclozic acid. Xenobiotica 11(10):665–674
Chalmers TM, Kellgren JH, Platt DS (1969a) Evaluation in man of fenclozic acid (I.C.I. 54,450: Myalex), a new anti-inflammatory agent. II. Clinical trial in patients with rheumatoid arthritis. Ann Rheum Dis 28(6):595–601
Chalmers TM, Pohl JE, Platt DS (1969b) Evaluation in man of fenclozic acid (I.C.I. 54,450: Myalex), a new anti-inflammatory agent. I. Serum concentration studies in healthy individuals and in patients with rheumatoid arthritis. Ann Rheum Dis 28(6):590–594
Dawson S, Stahl S, Paul N, Barber J, Kenna JG (2012) In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40(1):130–138
Evans DC, Watt AP, Nicoll-Griffith DA, Baillie TA (2003) Drug − protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem Res Toxicol 17(1):3–16
FDA (2008) FDA Guidance for Industry: safety testing of drug metabolites
Foulkes DM (1970) The metabolism of C14-ICI 54,450 (myalex) in various species–an in vivo NIH shift. J Pharmacol Exp Ther 172(1):115–121
Goldszer F, Tindell GL, Walle UK, Walle T (1981) Chemical trapping of labile aldehyde intermediates in the metabolism of propranolol and oxprenolol. Res Commun Chem Pathol Pharmacol 34(2):193–205
Hargreaves T (1965) Inhibition of conjugation by anti-inflammatory drugs. Nature 208(5015):1101–1102
Hart FD, Bain LS, Huskisson EC, Littler TR, Taylor RT (1970) Hepatic effects of fenzlozic acid. Ann Rheum Dis 29(6):684
Hepworth W, Newbould BB, Platt DS, Stacey GJ (1969) 2-(4-chlorophenyl)thiazol-4-ylacetic acid(‘Myalex’): a new compound with anti-inflammatory, analgesic and antipyretic activity. Nature 221(5180):582–583
Knights KM, Sykes MJ, Miners JO (2007) Amino acid conjugation: contribution to the metabolism and toxicity of xenobiotic carboxylic acids. Expert Opin Drug Metab Toxicol 3(2):159–168
Levi AJ, Gatmaitan Z, Arias IM (1969) Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest 48(11):2156–2167
Lu C, Li P, Gallegos R, Uttamsingh V, Xia CQ, Miwa GT, Balani SK, Gan LS (2006) Comparison of intrinsic clearance in liver microsomes and hepatocytes from rats and humans: evaluation of free fraction and uptake in hepatocytes. Drug Metab Dispos 34(9):1600–1605
Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y (2007) Circumventing the crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci 97(2):539–547
Mitchell JR, Jollow DJ, Gillette JR, Brodie BB (1973) Drug metabolism as a cause of drug toxicity. Drug Metab Dispos 1(1):418–423
Nakayama S, Atsumi R, Takakusa H, Kobayashi Y, Kurihara A, Nagai Y, Nakai D, Okazaki O (2009) A zone classification system for risk assessment of idiosyncratic drug toxicity using daily dose and covalent binding. Drug Metab Dispos 37(9):1970–1977
Newbould BB (1969) The pharmacology of fenclozic acid (2-(4-chlorophenyl)-thiazol-4-ylacetic acid; I.C.I. 54,450; ‘Myalex’); a new compound with anti-inflammatory, analgesic and antipyretic activity. Br J Pharmacol 35(3):487–497
Obach RS, Kalgutkar AS, Soglia JR, Zhao SX (2008) Can in vitro metabolism-dependent covalent binding data in liver microsomes distinguish hepatotoxic from nonhepatotoxic drugs? An analysis of 18 drugs with consideration of intrinsic clearance and daily dose. Chem Res Toxicol 21(9):1814–1822
Platt DS (1971) Pharmacokinetics of fenclozic acid in animals and man. J Pharm Sci 60(3):366–371
Shipkova M, Armstrong VW, Oellerich M, Wieland E (2003) Acyl glucuronide drug metabolites: toxicological and analytical implications. Ther Drug Monit 25(1):1–16
Soltanpour Y, Hilgendorf C, Ahlstrom MM, Foster AJ, Kenna JG, Petersen A, Ungell AL (2012) Characterization of THLE-cytochrome P450 (P450) cell lines: gene expression background and relationship to P450-enzyme activity. Drug Metab Dispos 40(11):2054–2058
Thompson RA, Isin EM, Li Y, Weaver R, Weidolf L, Wilson I, Claesson A, Page K, Dolgos H, Kenna JG (2011) Risk assessment and mitigation strategies for reactive metabolites in drug discovery and development. Chem Biol Interact 192(1–2):65–71
Thompson RA, Isin EM, Li Y, Weidolf L, Page K, Wilson I, Swallow S, Middleton B, Stahl S, Foster AJ, Dolgos H, Weaver R, Kenna JG (2012) In vitro approach to assess the potential for risk of idiosyncratic adverse reactions caused by candidate drugs. Chem Res Toxicol
Usui T, Mise M, Hashizume T, Yabuki M, Komuro S (2009) Evaluation of the potential for drug-induced liver injury based on in vitro covalent binding to human liver proteins. Drug Metab Dispos 37(12):2383–2392
Acknowledgments
We would like to thank Prof. Dr. Bruno Stieger for provision of hBSEP expressing baculovirus stocks; Johan E. Palm, DMPK iMED, AstraZeneca R&D Mölndal, for provision of the hMRP2 encoding plasmid; and the Reagents and Assay Development team, Discovery Sciences, AstraZeneca R&D Alderley Park, for preparation of membrane vesicles. Pavandeep Rai is acknowledged for help with the assessment of mitochondrial toxicity. Gillian Smith is acknowledged for completion of the CYP time-dependent inhibition assay.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodrigues, A.V.M., Rollison, H.E., Martin, S. et al. In vitro exploration of potential mechanisms of toxicity of the human hepatotoxic drug fenclozic acid. Arch Toxicol 87, 1569–1579 (2013). https://doi.org/10.1007/s00204-013-1056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1056-y