Abstract
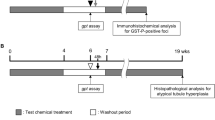
This study examined the carcinogenic potential of di-isodecyl phthalate (DIDP) in rasH2 mice. DIDP was administered to 15 rasH2 mice/gender/group at dietary levels of 0, 0.1, 0.33, or 1% and 15 wild-type mice/gender/group at dietary levels of 0 and 1% for 26 weeks. Non-neoplastic changes were observed in the liver (parenchymal inflammation, fatty changes, diffuse hepatocyte hypertrophy with eosinophilic granules and focal necrosis) and kidneys (tubular basophilia and tubular hyperplasia) after administration of DIDP in the rasH2 and wild-type mice. In the neoplastic lesions, there were a higher number of hepatocellular adenomas in the male rasH2 mice receiving 1% DIDP, compared with the findings in the liver of control rasH2 mice or wild-type mice. The incidence of hepatocellular adenomas in the 0.1, 0.33, and 1% DIDP exposed rasH2 mice was 7% (1/15), 7% (1/15), and 33% (5/15), respectively. This study adds a set of results for an additional test chemical for the performance of the rasH2 short-term transgenic model to the existing database of 3 compounds (WY-14643, DEHP, and clofibrate) tested in the ILSI/HESI ACT project.


Similar content being viewed by others
Abbreviations
- DIDP:
-
Di-isodecyl phthalate
- MNU:
-
N-Methyl-N-nitrosourea
- DEHP:
-
Di(2-ethylhexyl)phthalate
- DINP:
-
Di-isononyl phthalate
- CIEA:
-
Central Institute for Experimental Animals
- ILSI-HESI:
-
International Life Sciences Institute/Health and Environmental Science Institute
- Tg:
-
Transgenic
- WT:
-
Wild-type
- ICH:
-
International Conference on Harmonization
- MTD:
-
Maximum Tolerated Dose
References
Alden C, Smith P, Morton D (2002) Application of genetically altered models as replacement for the lifetime mouse bioassay in pharmaceutical development. Toxicol Pathol 30:135–138
Cho WS, Han BS, Ahn B, Nam KT, Choi M, Oh SY, Kim SH, Jeong J, Jang DD (2008) Peroxisome proliferator di-isodecyl phthalate has no carcinogenic potential in Fischer 344 rats. Toxicol Lett 178:110–116
Cohen SM, Robinson D, MacDonald J (2001) Alternative models for carcinogenicity testing. Toxicol Sci 64:14–19
David RM, Moore MR, Cifone MA, Finney DC, Guest D (1999) Chronic peroxisome proliferation and hepatomegaly associated with the hepatocellular tumorigenesis of di(2-ethylhexyl)phthalate and the effects of recovery. Toxicol Sci 50:195–205
Giam CS, Atlas E, Powers MA Jr, Leonard JE (1984) Phthalic acid esters. In: Hutzinger O (ed) Handbook of environmental chemistry — Anthropogenic compounds vol. 3. Springer, Berlin, pp 67–142
Graham PR (1973) Phthalate ester plasticizers–why and how they are used. Environ Health Perspect 3:3–12
ICH (1997) ICH harmonized tripartite guideline Testing for carcinogenicity of pharmaceuticals
Ito Y, Yamanoshita O, Kurata Y, Kamijima M, Aoyama T, Nakajima T (2007) Induction of peroxisome proliferator-activated receptor alpha (PPARalpha)-related enzymes by di(2-ethylhexyl) phthalate (DEHP) treatment in mice and rats, but not marmosets. Arch Toxicol 81(3):219–226
James NH, Soames AR, Roberts RA (1998) Suppression of hepatocyte apoptosis and induction of DNA synthesis by the rat and mouse hepatocarcinogen diethylhexylphlathate (DEHP) and the mouse hepatocarcinogen 1, 4-dichlorobenzene (DCB). Arch Toxicol 172(12):784–790
Kaufmann W, Deckardt K, McKee RH, Butala JH, Bahnemann R (2002) Tumor induction in mouse liver: di-isononyl phthalate acts via peroxisome proliferation. Regul Toxicol Pharmacol 36:175–183
Kim CK, Han BS, Oh JH, Cho W-S, Park KD, Choi M, Cho MJ, Oh SY, Kim SJ, Jeong JY, Kim SH, Jang DD (2006) Four-week repeated-dose toxicity study of di-isodecyl phthalate (DIDP) in rasH2 wild type mice. Lab Animal Res 22:289–293
Kim HS, Ishizaka M, Kazusaka A, Fujita S (2007) Di-(2-ethylhexyl) phthalate suppresses tamoxifen-induced apoptosis in GH3 pituitary cells. Arch Toxicol 81(1):27–33
Kluwe WM, Haseman JK, Douglas JF, Huff JE (1982) The carcinogenicity of dietary di(2-ethylhexyl) phthalate (DEHP) in Fischer 344 rats and B6C3F1 mice. J Toxicol Environ Health 10:797–815
Lington AW, Bird MG, Plutnick RT, Stubblefield WA, Scala RA (1997) Chronic toxicity and carcinogenic evaluation of diisononyl phthalate in rats. Fundam Appl Toxicol 36:79–89
Marsman DS, Cattley RC, Conway JG, Popp JA (1988) Relationship of hepatic peroxisome proliferation and replicative DNA synthesis to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)phthalate and [4-chloro-6-(2, 3-xylidino)-2-pyrimidinylthio]acetic acid (Wy-14, 643) in rats. Cancer Res 48:6739–6744
Mitsumori K, Loizumi H, Nomura T, Yamamoto S (1998) Pathological features of spontaneous and induced tumors in transgenic mice carrying a human prototype c-Ha-ras gene used for six-month carcinogenicity studies. Toxicol Pathol 26:520–531
Morton D, Alden C, Roth AJ, Usui T (2002) The Tg rasH2 mouse in cancer hazard identification. Toxicol Pathol 30:139–146
Nesfield SR, Clarke CJ, Hoivik DJ, Miller RT, Allen JS, Selinger K, Santostefano MJ (2005) Evaluation of the carcinogenic potential of clofibrate in the rasH2 mouse. Int J Toxicol 24:301–311
Peters JM, Cattley RC, Gonzalez FJ (1997) Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14, 643. Carcinogenesis 18:2029–2033
Saitoh A, Kimura M, Takahashi R, Yokoyama M, Nomura T, Izawa M, Sekiya T, Nishimura S, Katsuki M (1990) Most tumors in transgenic mice with human c-Ha-ras gene contained somatically activated transgenes. Oncogene 5:1195–11200
Takaoka M, Sehata S, Maejima T, Imai T, Torii M, Satoh H, Toyosawa K, Tanakamaru Z-Y, Adachi T, Hisada S, Ueda M, Ogasawara H, Matsumoto M, Kobayashi K, Mutai M, Usui T (2003) Interlaboratory comparison of short-term carcinogenicity studies using CB6F1-rasH2 transgenic mice. Toxicol Pathol 31:191–199
Tamaoki N (2001) The rasH2 transgenic mouse: nature of the model and mechanistic studies on tumorigenesis. Toxicol Pathol 29 Suppl: 81–89
Toyosawa K, Okimoto K, Kobayashi I, Kijima K, Kikawa E, Kohchi M, Koujitani T, Tanaka K, Matsuoka N (2001) Di(2-ethylhexyl)phthalate induces hepatocellular adenoma in transgenic mice carrying a human prototype c-Ha-ras gene in a 26-week carcinogenicity study. Toxicol Pathol 29:458–466
Usui T, Mutai M, Hisada S, Takoaka M, Soper K, McCullough B, Alden C (2001) CB6F1-rasH2 mouse: overview of available data. Toxicol Pathol 29(Suppl):90–108
Xu Y, Agrawal S, Cook TJ, Knipp GT (2007) Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch Toxicol 81(1):57–62
Acknowledgments
The authors wish to thank Professor Byeongwoo Ahn (Veterinary Pathologist, Chungbuk National University) for performing the pathology peer review. This study was supported by a grant from the Korea National Toxicology Program from the National Institute of Toxicological Research of Korea Food and Drug Administration.
Author information
Authors and Affiliations
Corresponding authors
Additional information
W.-S. Cho and J. Jeong contributed equally.
Rights and permissions
About this article
Cite this article
Cho, WS., Jeong, J., Choi, M. et al. 26-Week carcinogenicity study of di-isodecyl phthalate by dietary administration to CB6F1-rasH2 transgenic mice. Arch Toxicol 85, 59–66 (2011). https://doi.org/10.1007/s00204-010-0536-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-010-0536-6