Abstract
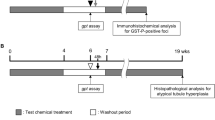
The carcinogenic potential of 3-monochloro-1,2-propanediol (3-MCPD) was evaluated in a short-term carcinogenicity testing study using CB6F1 rasH2-Tg (rasH2-Tg) mice. 3-MCPD is found in many foods and food ingredients as a result of storage or processing and is regarded as a carcinogen since it is known to induce Leydig cell and kidney tumors in rats. Male and female rasH2-Tg mice were administered 3-MCPD once daily by oral gavage at doses of 0, 10, 20, and 40 mg/kg body weight (bw) per day for 26 weeks. As a positive control, N-methyl-N-nitrosourea (MNU) was administered as a single intraperitoneal injection (75 mg/kg). In 3-MCPD-treated mice, there was no increase in the incidence of neoplastic lesions compared to the incidence in vehicle control mice. However, 3-MCPD treatment resulted in an increased incidence of tubular basophilia in the kidneys and germ cell degeneration in the testes, with degenerative germ cell debris in the epididymides of males at 20 and 40 mg/kg bw per day. In 3-MCPD-treated females, vacuolation of the brain and spinal cord was observed at 40 mg/kg bw per day; however, only one incidence of vacuolation was observed in males. Forestomach and cutaneous papilloma and/or carcinoma and lymphoma were observed in most rasH2 mice receiving MNU treatment. We concluded that 3-MCPD did not show carcinogenic potential in the present study using rasH2-Tg mice. The findings of this study suggest that the carcinogenic potential of 3-MCPD is species specific.





Similar content being viewed by others
References
Alden C, Smith P, Morton D (2002) Application of genetically altered models as replacement for the lifetime mouse bioassay in pharmaceutical development. Toxicol Pathol 30:135–138
Bakhiya N, Abraham K, Gurtler R, Appel KE, Lampen A (2011) Toxicological assessment of 3-chloropropane-1,2-diol and glycidol fatty acid esters in food. Mol Nutr Food Res 55:509–521
Boorman GA, Maronpot RR, Eustis SL (1994) Rodent carcinogenicity bioassay: past, present, and future. Toxicol Pathol 22:105–111
Bos JL (1989) Ras oncogenes in human cancer: a review. Cancer Res 49:4682–4689
Cavanagh JB, Nolan CC, Seville MP (1993) The neurotoxicity of alphachlorohydrin in rats and mice: I. Evolution of the cellular changes. Neuropathol Appl Neurobiol 19:240–252
Chen YY, Liu SL, Hu DP, Xing YQ, Shen Y (2014) N-methyl-N-nitrosourea-induced retinal degeneration in mice. Exp Eye Res 121:102–113
Cho WS, Han BS, Nam KT, Park K, Choi M, Kim SH, Jeong J, Jang DD (2008) Carcinogenicity study of 3-monochloropropane-1,2-diol in Sprague-Dawley rats. Food Chem Toxicol 46:3172–3177
Davis TS, Monro A (1995) Marked human pharmaceuticals reported to be tumorigenic in rodents. J Am Coll Toxicol 14:90–107
Ford WC, Waites GM (1982) Activities of various 6-chloro-6-deoxy sugars and (S)-alpha-chlorohydrin in producing spermatocoeles in rats and paralysis in mice and in inhibiting glucose metabolism in bull spermatozoa in vitro. J Reprod Fertil 65:177–183
Guerroro I, Pellicer A (1987) Mutational activation of oncogenes in animal model systems of carcinogenesis. Mutat Res 185:293–308
International Committee on Harmonization (1998) Guidance on testing for carcinogenicity of pharmaceuticals. Fed Reg 63:8983–8986
International Conference on Harmonization (1997) ICH harmonized tripartite guideline: testing for carcinogenicity of pharmaceuticals, Step 4
Jeong J, Han BS, Cho WS, Choi M, Ha CS, Lee BS, Kim YB, Son WC, Kim CY (2010) Carcinogenicity study of 3-monochloropropane-1, 2-diol (3-MCPD) administered by drinking water to B6C3F1 mice showed no carcinogenic potential. Arch Toxicol 84:719–729
Jones AR, Gadiel P, Stevenson D (1981) The fate of oxalic acid in the Wistar rat. Xenobiotica 11:385–390
Kim K, Song C, Park Y, Kim S, Kim J, Kim S, Kim Y, Kim SU, Jung H (2004) 3-monochloropropane-1,2-diol does not cause neurotoxicity in vitro or neurobehavioral deficits in rats. Neurotoxicology 25:377–385
Lee BS, Park SJ, Kim YB, Han JS, Jeong EJ, Moon KS, Son HY (2015) A 28-day oral gavage toxicity study of 3-monochloropropane-1,2-diol (3-MCPD) in CB6F1-non-Tg rasH2 mice. Food Chem Toxicol 86:95–103
Long GG, Goodman DG, Credille KM, Mann PC, Wilson JM, Cardy R (2010a) Hematopoietic proliferative lesions in the spleen of rasH2 transgenic mice treated with MNU. Toxicol Pathol 38:1026–1036
Long GG, Morton D, Peters T, Short B, Skydsgaard M (2010b) Alternative mouse models for carcinogenicity assessment: industry use and issues with pathology interpretation. Toxicol Pathol 38:43–50
Lynch BS, Bryant DW, Hook GJ, Nestmann ER, Munro IC (1998) Carcinogenicity of monochloro-1,2-propanediol (alpha-chlorohydrin, 3-MCPD). Int J Toxicol 17:47–76
MacDonald J, French JE, Gerson RJ, Goodman J, Inoue T, Jacobs A, Kasper P, Keller D, Lavin A, Long G, McCullough B, Sistare FD, Storer R, van der Laan JW (2004) The utility of genetically modified mouse assays for identifying human carcinogens: a basic understanding and path forward. The alternatives to carcinogenicity testing committee ILSI HESI. Toxicol Sci 77:188–194
Mitsumori K, Koizumi H, Nomura T, Yamamoto S (1998) Pathological features of spontaneous induced tumors in transgenic mice carrying a human prototype c-Ha-ras gene used for 6-month carcinogenicity studies. Toxicol Pathol 26:520–531
Nambiar PR, Morton D (2013) The rasH2 mouse model for assessing carcinogenic potential of pharmaceuticals. Toxicol Pathol 41:1058–1067
Nambiar PR, Turnquist SE, Morton D (2012) Spontaneous tumor incidence in rasH2 mice: review of internal data and published literature. Toxicol Pathol 40:614–623
OEHHA (2010) 3-monochloropropane-1,2-diol (3-MCPD; α-chlorohydrin). California Environmental Protection Agency. http://www.oehha.ca.gov/Prop65/hazard_ident/pdf_zip/123mcpd.pdf. Accessed May 2015
Paranjpe MG, Shah SA, Denton MD, Elbekai RH (2013) Incidence of spontaneous non-neoplastic lesions in transgenic CBYB6F1-Tg(HRAS) 2Jic mice. Toxicol Pathol 41:1137–1145
Robinson DE, MacDonald JS (2001) Background and framework for ILSI’s collaborative evaluation program on alternative models for carcinogenicity assessment. International Life Sciences Institute. Toxicol Pathol 29:13–19
Sehata S, Maejima T, Watanabe M, Ogata S, Makino T, Tanaka K, Manabe S, Takaoka M (2002) Twenty-six-week carcinogenicity study of chloroform in CB6F1 rasH2-transgenic mice. Toxicol Pathol 30:328–338
Sunahara G, Perrin I, Marchesini M (1993) Carcinogenicity study on 3-monochloropropane-1,2-diol (3-MCPD) administered in drinking water to Fischer 344 rats. Unpublished report No. RE-SR93003, submitted to WHO by Nestec Ltd., Research & Development, Switzerland, quoted by Bakhiya et al., 2011
Takaoka M, Sehata S, Maejima T, Imai T, Torii M, Satoh H, Toyosawa K, Tanakamaru Z-Y, Adachi T, Hisada S, Ueda M, Ogasawara H, Matsumoto M, Kobayashi K, Mutai M, Usui T (2003) Interlaboratory comparison of short-term carcinogenicity studies using CB6F1-rasH2 transgenic mice. Toxicol Pathol 31:191–199
Tamaoki N (2001) The rasH2 transgenic mouse: nature of the model and mechanistic studies on tumorigenesis. Toxicol Pathol 29(Suppl):81–89
Tennant RW, French JE, Spalding JW (1995) Identifying chemical carcinogens and assessing potential risk in short-term bioassays using transgenic mouse models. Environ Health Perspect 103:942–950
Tsuda H, Fukamachi K, Ohshima Y, Ueda S, Matsuoka Y, Hamaguchi T, Ohnishi T, Takasuka N, Naito A (2005) High susceptibility of human c-Ha-ras proto-oncogene transgenic rats to carcinogenesis: a cancer-prone animal model. Cancer Sci 96:309–316
Usui T, Mutai M, Hisada S, Takoaka M, Soper KA, Mccullough B, Alden C (2001) CB6F1-rasH2 mouse: overview of available data. Toxicol Pathol 29(Suppl):90–108
Van Duuren BL, Goldschmidt BM, Katz C, Seidman I, Paul JS (1974) Carcinogenic activity of alkylating agents. J Natl Cancer Inst 53:695–700
Weisburger EK, Ulland BM, Nam J, Gart JJ, Weisburger JH (1981) Carcinogenicity tests of certain environmental and industrial chemicals. J Natl Cancer Inst 67:75–88
Xu J, Futakuchi M, Alexander DB, Fukamachi K, Numano T, Suzui M, Shimizu H, Omori T, Kanno J, Hirose A, Tsuda H (2014) Nanosized zinc oxide particles do not promote DHPN induced lung carcinogenesis but cause reversible epithelial hyperplasia of terminal Bronchioles. Arch Toxicol 88:65–75
Yamamoto S, Urano K, Koizumi H, Wakana S, Hioki K, Mitsumori K, Kurokawa Y, Hayashi Y, Nomura T (1998) Validation of transgenic mice carrying the human prototype c-Ha-ras gene as a bioassay model for rapid carcinogenicity testing. Environ Health Perspect 106(Suppl 4):57–69
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Byoung-Seok Lee and Sang-Jin Park have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, BS., Park, SJ., Kim, YB. et al. Twenty-six-week oral carcinogenicity study of 3-monochloropropane-1,2-diol in CB6F1-rasH2 transgenic mice. Arch Toxicol 91, 453–464 (2017). https://doi.org/10.1007/s00204-016-1696-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1696-9