Abstract
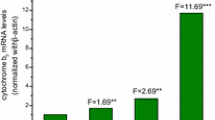
The effect of acute and chronic dioxane administration on hepatic, renal, pulmonary and nasal mucosa P450 enzymes and liver toxicity were investigated in male rats. The acute treatment consisted of two doses (2 g/kg) of dioxane given for 2 days by gavage, whereas the chronic treatment consisted of 1.5% of dioxane in drinking water for 10 days. Both the acute and chronic dioxane treatments induced cytochrome P450 2B1/2- and P450 2E1-dependent microsomal monooxygenase activities (pentoxyresorufin O-depentylase and p-nitrophenol hydroxylase) in the liver, whereas in the kidney and nasal mucosa, only the 2E1 marker activities were enhanced. In addition in the liver, an induction of 2α-testosterone hydroxylase (associated with the constitutive and hormone-dependent P450 2C11) was also revealed, whereas the hepatic P450 4A-dependent ω-lauric acid hydroxylase was not enhanced by any dioxane treatment. These inductions were mostly confirmed by western blot analysis of liver, kidney and nasal mucosa microsomes. In the lung, no alteration of P450 activities was observed. To assess the mechanism of 2E1 induction, the hepatic, renal and nasal mucosa 2E1 mRNA levels were also examined. Following two kinds of dioxane administration, in the liver the 2E1 induction was not accompanied by a significant alteration of 2E1 mRNA levels, while both in the kidney and nasal mucosa the 2E1 mRNA increased about 2- to 3-fold, indicating an organ-specific regulation of this P450 isoform. Furthermore, dioxane was unable to alter the plasma alanine aminotransferase activity and hepatic glutathione (GSH) content, examined as an index of toxicity, when it was administered into rats with P450 2B1/2 and 2E1 preinduced by phenobarbital or fasting pretreatment. These results support the lack of or a poor formation of reactive and toxic intermediates during the biotrasformation of this solvent, even when its metabolism was enhanced by P450 inducers. The chronic administration of dioxane was also unable to induce the palmitoyl CoA oxidase, a marker of peroxisome proliferation, excluding this as a way to explain its toxicity. Thus, although the mechanism of dioxane carcinogenicity remains unclear, the present results suggest that the induction of 2E1 following a prolonged administration of dioxane might provide oxygen radical species, and thereby contribute to its organ-specific toxicity.







Similar content being viewed by others
References
Agrawal AK, Shapiro BH (2000) Differential expression of gender-dependent hepatic isoforms of Cytochrome P450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat J. Pharm Exp Ther 292:228–237
Amato G, Longo V, Mazzaccaro A, Gervasi PG (1996) Microsomal oxidation of N,N-diethylformamide and its effect on P450-dependent monooxygenases in rat liver. Chem Res Toxicol 9:882–890
Amet Y, Berthou F, Goasduff T, Salaun JP, Le Breton L, Menez (1994) J.F. Evidence that cytochrome P450 2E1 is involved in the (ω−1)-hydroxylation of lauric acid in rat liver microsomes. Biochem Biophys Res Comm 203:1168–1174
Arlotto MP, Sonderfan AJ, Klassen CD, Parkinson A (1987) Studies on the pregnenolone-16 α-carbonitrile-inducible of rat liver microsomal cytochrome P-450 and UDP-glucuronosyltransferase. Biochem Pharmacol 36:3859–3866
Brady JF, Lee MJ, Li M, Ishizaki H, Yang CS (1988) Diethyl ether as a substrate for acetone/ethanol-inducible cytochrome P-450 and as an inducer for cytochrome(s) P-450. Mol Pharmacol 33:148–154
Braun WH, Young JD (1977) Identification of β-hydroxyethoxyacetic acid as the major urinary metabolite of 1,4-dioxane in the rat. Toxicol Appl Pharmacol 39:33–38
Bronfman M, Inestrosa NC, Leighton F (1979) Fatty acid oxidation by human liver peroxisomes. Biochem Biophys Res Comm 88:1030–1036
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
De Rosa CT, Wilbur S, Holler J, Richter P, Stevens YW (1996) Health evaluation of 1,4-dioxane. Toxicol Ind Health 12:1–43
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Fujita S, Morimoto R, Chiba M, Kitani K, Suzuki T (1989) Evaluation of the involvement of a male specific cytochrome P-450 isozyme in senescence-associated decline of hepatic drug metabolism in male rats. Biochem Pharmacol 38:3225–3231
Gemzik B, Greenway D, Nevins C, Parkinson A (1992) Regulation of two electrophoretically distinct proteins recognized by antibody against rat liver cytochrome P450 3A1. J Biochem Toxicol 7:43–52
Goldsworthy TL, Monticello TM, Morgan KT, Bermudez E, Wilson DM, Jackh R, Butterworh BE (1991) Examination of potential mechanisms of carcinogenicity of 1,4-dioxane in rat nasal epithelial cells and hepatocytes. Arch Toxicol 65:1–9
Guengerich FP, Kim DH, Iwasaki M (1991) Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol 4:168–179
Hawkins JM, Jones WE, Bonner FW, Gibson GG (1987) The effect of peroxisome proliferators on microsomal, peroxisomal and mitochondrial enzyme activities in the liver and kidney. Drug Metab Rev 18:441–551
IARC (International Agency for Research on Cancer) (1999) Monographs on the evaluation of cancinogenic risks to humans, vol 71. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. IARC Press, Lyon, pp 589–602
Imaoka S, Terano Y, Funae Y (1990) Changes in the amount of cytochrome P450s in rat hepatic microsomes with starvation. Arch Biochem Biophys 278:168–178
Ingelman-Sundberg M, Johansson I (1984) Mechanism of hydroxyl radical formation and ethanol oxidation by ethanol inducible and other forms of rabbit liver microsomal cytochrome P450, J Biol Chem 259:6447–6458
Johansson I, Ekstrom G, Scholte B, Puzycki D, Jornvall H, Ingelman-Sundberg M (1988) Ethanol-, fasting-, and acetone-inducible cytochromes P-450 in rat liver: regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry 27:1925–1934
Johnson MK (1966) Studies on glutathione S-alkyltransferase of the rat. Biochem J. 98:44–56
Kim H, Kim SG, Lee MY, Novak RF (1992) Evidence for elevation of cytochrome P4502E1 (alcohol-inducible form) mRNA levels in rat kidney following pyridine administration. Biochem Biophys Res Commun 186:846–853
Ko IY, Park SS, Song BJ, Patten C, Tan Y, Ha YC, Yang CS, Gelboin HV (1987) Monoclonal antibodies to ethanol-induced rat liver cytochrome P-450 that metabolizes aniline and nitrosamines. Cancer Res 47:3101–3109
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Longo V, Ingelman-Sundberg M (1993) Acetone-dependent regulation of cytochrome P4502E1 and P4502B1 in rat nasal mucosa. Biochem Pharmacol 46:1945–1951
Longo V, Mazzaccaro A, Naldi, Gervasi PG (1991) Drug-metabolizing enzymes in liver, olfactory and respiratory epithelium of cattle. J Biochem Toxicol 6:123–128
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lubet RA, Mayer RT, Cameron JW, Nims RW, Burke MD, Wolff T, Guengerich FP(1985) Dealkylation of pentoxyresorufin: a rapid and sensitive assay for measuring induction of cytochrome(s) P-450 by phenobarbital and other xenobiotics in the rat. Arch Biochem Biophys 238:43–48
Mathews JM, Parker MK, Matthews HB (1991) Metabolism and disposition of diethylene glycol in rat and dog. Drug Metab. Disp 19:1066–1070
Menicagli S, Longo V, Mazzaccaro A, Gervasi PG (1994) Microsomal metabolism of N,N-diethylacetamide and N,N-dimethylacetamide and their effects on drug-metabolizing enzymes of rat liver. Biochem Pharmacol 48:717–726
Okita JR, Castle PJ, Okita RT (1993) Characterization of cytochromes P450 in liver and kidney if rats treated with di-(2-ethyhexyl)phthalate. J Biochem Toxicol 8:135–144
Omura T, Sato R (1964) The carbon-monoxide binding protein of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 293:2370–2378
Pessayre D, Doider A, Artigou JY, Wandscheer JK, Descatoire V, Degott C, Benhamou JP (1979) Effect of fasting on metabolite-mediated hepatoxicity in the rat. Gastroenterology 77:264–271
Platt KL, Molitor E, Dohmer J, Dogra S, Oesch F (1989) Genetically engineered V79 Chinese hamster cell expression of purified cytochrome P-450IIB1 monoxygenase activity. J Biochem Toxicol 4:1–6
Reinke L.A, Moyer M.J (1985) p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos 13:548–552
Reinke LA, Sexter SH, Rikans LE (1985) Comparison of ethanol and imidazole pretreatments on hepatic monooxygenase activities in the rat. Res Commun Chem Pathol Pharmacol 47:97–106
Roberts RA (1999) Peroxisome proliferators: mechanism of adverse effects in rodents and molecular basis for species differences. Arch Toxicol 73:413–418
Sambrook J, Fritsch EF, Maniatis T (1989) Electrophoresis of RNA through gels containing formaldehyde. In: Ford N, Nolan C, Ferguson M (eds). Molecular cloning: a laboratory manual, vol 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor NY, pp 743–748
Song BJ (1995) Gene structure and multiple regulations of the ethanol inducible cytochrome P450 2E1. In: Watson RR (ed). Drug and alcohol abuse reviews, alcohol and hormones, vol 6. Humana press, Totowa NJ, pp 177–192
Towbin HT, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Tu Y, Yang CS (1983) High-affinity nitrosamine dealkylase system in rat liver microsomes and its induction by fasting. Cancer Res 43:623–629
Waxman DJ, Chang TKH (1995) Hormonal regulation of liver cytochrome P450 enzymes in cytochrome P450. In: Ortiz de Montellano PR (ed). Cytochrome P450: structure, function and mechanism. Plenum Press, New York, pp 391–418
Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH (1991) Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci USA 88:6868–6872
Woo YT, Arcos JC, Argus MF, Griffin GW, Nishiyama K (1977a) Metabolism in vivo of dioxane: identification of p-dioxane-2-one as the major urinary metabolite. Biochem Pharmacol 26:1535–1538
Woo YT, Argus MF, Arcos JC (1977b) Metabolism in vivo of dioxane: effect of inducers and inhibitors of hepatic mixed-function oxidases. Biochem Pharmacol 25:1539–1542
Young JD, Braun WH, Gehring PJ (1978) The dose-dependent fate of 1,4-dioxane in rats. J Environ Pathol Toxicol 2:263–282
Zanelli U, Puccini P, Acerbi D, Ventura P, Gervasi PG (1996) Induction of peroxisomal β-oxidation and P450 4A-dependent.activities by pivalic and tricloroacetic acid in rat liver and kidney. Arch Toxicol 70:145–149
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nannelli, A., De Rubertis, A., Longo, V. et al. Effects of dioxane on cytochrome P450 enzymes in liver, kidney, lung and nasal mucosa of rat. Arch Toxicol 79, 74–82 (2005). https://doi.org/10.1007/s00204-004-0590-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-004-0590-z