Abstract
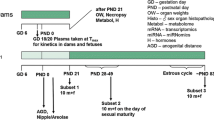
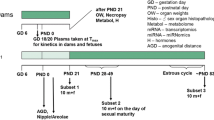
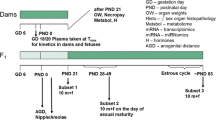
The main objective of this 28-day oral gavage toxicity study in the rat was to investigate which of the current and/or additional parameters of the OECD Test Guideline 407 would reliably and sensitively detect the endocrine-mediated effects of the nonsteroidal antiestrogen tamoxifen. In addition, as this study was performed using two subgroups of five animals of each sex run concurrently, it enabled an assessment of the intralaboratory reproducibility while also assessing the potential value of using ten animals of each sex per group instead of using the standard five animals of each sex per group stipulated by the current guideline. Tamoxifen was administered daily by gavage to groups of 7-week-old Wistar rats for at least 28 days at dose levels of 5, 30, or 200 µg/kg body weight. Additional parameters specified in the enhanced OECD Test Guideline 407 were spermatozoa enumeration and morphology of the cauda epididymis, hormonal analysis of the thyroid-stimulating hormone (TSH), triiodothyronine (T3) and thyroxine (T4) levels, monitoring of the estrous cycle during week 4 of treatment to ensure females were in diestrus on the day of terminal sacrifice, organ weight of ovary, uterus, thyroid gland, prostate gland (ventral and dorsolateral parts), seminal vesicles with coagulation glands and pituitary gland, and microscopic investigation of the pituitary gland, vagina, mammary gland, seminal vesicles with coagulating glands, epididymis, and prostate gland (ventral and dorsolateral parts). Overall, 200 µg/kg per day was considered to be the Maximum Tolerated Dose (MTD) in both sexes, resulting in a marked reduction of body weight gain, together with slight effects on clinical signs, hematology, plasma chemistry, and microscopic changes in some endocrine tissues. Five micrograms per kilogram per day represented the No Observed Adverse Effect Level (NOAEL) in males and the No Observed Effect Level (NOEL) in females. At the intermediate dose level (30 µg/kg per day), the current OECD Test Guideline 407 was appropriate to detect the specific endocrine-related changes induced by tamoxifen in females, based on the histopathology findings observed in the ovary and the uterus. The additional parameters which were found to be changed in females (thyroid hormone levels, ovary and uterus weights, and histopathology of vagina) provided supplementary information further confirming tamoxifen-mediated endocrine effects. In males, when data from the current Test Guideline 407 were considered at the intermediate dose level, specific endocrine effects were only indicated on the basis of the histopathology findings observed in the prostate gland. The additional parameters examined which were found to be changed (prostate gland and seminal vesicle weights, and histopathology of seminal vesicle and mammary gland) were necessary to confirm the specific tamoxifen-mediated endocrine effects. Hence, amongst the additional parameters contained in the enhanced OECD Test Guideline 407, organ weights and histopathological examination of endocrine-related organs were the most helpful in confirming the detection of tamoxifen-mediated endocrine effects. The reproducibility evaluation showed that a group size of five animals of each sex consistently allowed the detection of endocrine effects with the current Test Guideline in both sexes at the high dose level and in females at the intermediate dose level. Doubling the animal number from five to ten of each sex per dose level did not notably increase the sensitivity of detection of endocrine-mediated effects.
Similar content being viewed by others
References
Andrews P, Freyberger A, Hartmann E, Eiben R, Loof I, Schmidt U, Temerowski M, Becka M (2001) Feasibility and potential gains of enhancing the subacute rat study protocol (OECD test guideline no. 407) by additional parameters selected to determine endocrine modulation. A pre-validation study to determine endocrine-mediated effects of the antiandrogenic drug flutamide. Arch Toxicol 75:65–73
Andrews P, Freyberger A, Hartmann E, Eiben R, Loof I, Schmidt U, Temerowski M, Folkerts A, Stahl B, Kayser M (2002) Sensitive detection of the endocrine effects of the estrogen analogue ethinylestradiol using a modified enhanced subacute rat study protocol (OECD Test Guideline no. 407). Arch Toxicol 76:194–202
Bigsby RM, Young CM (1994) Estrogenic effects of the antiprogestin onapristone (ZK98.299) in the rodent uterus. Am J Obstet Gynecol 171:188–194
Furr BJA, Jordan VC (1984) The pharmacology and clinical uses of tamoxifen. Pharmacol Ther 25:127–205
Gill-Sharma MK, Gopalkrishnan K, Balasinor N, Parte P, Jayaraman S, Juneja HS (1993) Effects of tamoxifen on the fertility of male rats. J Reprod Fertil 99:395–402
Greaves P, Goonetilleke R, Nunn G, Topham J, Orton T (1993) Two-year carcinogenicity study of tamoxifen in Alderley Park Wistar-derived rats. Cancer Res 53:3919–3924
Hayes DF, Van Zyl JA, Hacking A, Goedhals L, Bezwada WR, Mailliard JA, Jones SE, Vogel CL, Berris RB, Shemano I, Schoenfelder J (1995) Randomized comparison of tamoxifen and two separate doses of toremifene in postmenopausal patients with metastatic breast cancer. J Clin Oncol 13:2556–2566
Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB (1997) A role for oestrogens in the male reproductive system. Nature 390:509–512.
Hess RA, Bunick D, Bahr J (2001) Oestrogen, its receptors and function in the male reproductive tract—a review. Mol Cell Endocrinol 178:29–38.
Jordan VC, Lababidi MK, Mirecki DM (1990) Anti-estrogenic and anti-tumor properties of prolonged tamoxifen therapy in C3H/OUJ mice. Eur J Cancer 26:718–721
Kallio S, Kangas L, Blanco G, Johansson R, Karjalainen A, Perila M, Pippo I, Sundquist H, Sodervall M, Toivola R (1986) A new triphenylethylene compound, Fc-1157a. I. Hormonal effects. Cancer Chemother Pharmacol 17:103–108
Knapp GG, Nayfield S, Parkinson DR, Prasad VK, Prorok PC, Sausville EA, Sigman CC (1994) Clinical development plans for cancer chemopreventive agents: tamoxifen. J Cell Biochem 20(Suppl):252–267
MacNab MW, Tallarida RJ, Joseph R (1984) An evaluation of tamoxifen as a partial agonist by classical receptor theory—an explanation of the dual action of tamoxifen. Eur J Pharmacol 103:321–326
Meyer OA, Tilson HA, Byrd WC, Riley MT (1979) A method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neurobehav Toxicol 1:233–236
Nayfield SG, Karp JE, Ford LG, Dorr A, Kramer BS (1991) Potential role of tamoxifen in prevention of breast cancer. J Natl Cancer Inst 83:1450–1459
Nephew KP, Osborne E, Lubet RA, Grubbs CJ, Khan SA (2000) Effects of oral administration of tamoxifen, toremifene, dehydroepiandrosterone, and vorozole on uterine histomorphology in the rat. Proc Soc Exp Biol Med, 223:288–294
OECD (1995) OECD Guideline no. 407. OECD test guidelines for testing of chemicals, Section 4, Health effects (updated 27 July, 1995). Organisation for Economic Co-operation and Development, Paris
OECD (1999) OECD protocol for investigating the efficacy of the Enhanced Test Guideline 407 (issued 4 August, 1999). Organisation for Economic Co-operation and Development, Paris
OECD (2000) Protocol for investigating the efficacy of the Enhanced TG 407 Test Guideline (phase 2). Rationale for the investigation, and description of the protocol (issued 4 May, 2000). Organisation for Economic Co-operation and Development, Paris
Okazaki K, Okazaki S, Nishimura S, Nakamura H, Kitamura Y, Hatayama K, Nakamura A, Tsuda T, Katsumata T, Nishikawa A, Hirose M (2001) A repeated 28-day oral dose toxicity study of methoxychlor in rats, based on the 'Enhanced OECD Test Guideline 407' for screening endocrine-disrupting chemicals. Arch Toxicol 75:513–521
Okazaki K, Imazawa T, Nakamura H, Furukawa F, Nishikawa A, Hirose M (2002a) A repeated 28-day oral dose toxicity study of 17α-methyltestosterone in rats, based on the 'Enhanced OECD Test Guideline 407' for screening the endocrine-disrupting chemicals. Arch Toxicol 75:635–642
Okazaki K, Okazaki S, Nakamura H, Kitamura Y, Hatayama K, Wakabayashi S, Tsuda T, Katsumata T, Nishikawa A, Hirose M (2002b) A repeated 28-day oral dose toxicity study of genistein in rats, based on the 'Enhanced OECD Test Guideline 407' for screening endocrine-disrupting chemicals. Arch Toxicol 76:553–559.
Padmalatha Rai S, Vijayalaxmi KK (2001) Tamoxifen citrate induced sperm shape abnormalities in the in vivo mouse. Mutat Res 492:1–6
Rumpel E, Kühnel W, Michna H (1993) Östrogene und antiöstrogene Effekte am Uterus intakter Ratten nach Langzeitbehandlung mit Tamoxifen. Ann Anat Suppl 175:45
Sharpe RM (1997) Do males rely on female hormones? Nature 390:447–448
Sibonga JD, Dobnig H, Harden RM, Turner RT (1998) Effect of the high-affinity estrogen receptor ligand ICI 182,780 on the rat tibia. Endocrinology 139:3736–3742
Stenbygaard LE, Herrstedt J, Thomsen J, Svendsen KR, Engelholm SA, Dombernowsky P (1993) Tomerifene and tamoxifen in advanced breast cancer: a double-blind, cross-over trial. Breast Cancer Res Treat 25:57–63
Toyoda K, Shibutani M, Tamura T, Koujitani T, Uneyama C, Hirose M (2000) Repeated dose (28 days) oral toxicity study of flutamide in rats, based on the draft protocol for the 'Enhanced OECD Test Guideline 407' for screening for endocrine-disrupting chemicals. Arch Toxicol 74:127–132
Wade GN, Powers JB (1993) Tamoxifen antagonizes the effects of estradiol on energy balance and estrous behavior in Syrian hamsters. Am J Physiol 265(3.2):R559-R562
Yamasaki K, Sawaki M, Noda S, Imatanaka N, Takatsuki M (2002a) Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the 'Enhanced OECD Test Guideline no. 407'. Arch Toxicol 76:65–74
Yamasaki K, Tago Y, Nagai K, Sawaki M, Noda S, Takatsuki M (2002b) Comparison of toxicity studies based on the draft protocol for the 'Enhanced OECD Test Guideline no. 407' and the research protocol of 'Pubertal Development and Thyroid Function in Immature Male Rats' with 6-n-propyl-2-thiouracil. Arch Toxicol 76:495–501
Acknowledgements
The authors wish to thank the Endocrine Modulator Study Group for substantial financial support of this investigation and the European Commission for the Marie Curie Fellowship held by G. Espuña. The authors certify that the study was performed in accordance with the French laws for animal experimentation
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kennel, P., Pallen, C., Barale-Thomas, E. et al. Tamoxifen: 28-day oral toxicity study in the rat based on the Enhanced OECD Test Guideline 407 to detect endocrine effects. Arch Toxicol 77, 487–499 (2003). https://doi.org/10.1007/s00204-003-0476-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0476-5