Abstract
Helicobacter pylori (H. pylori) is a well-known pathogen that infects approximately half of the world’s population. It is a pathogenic agent with potential health hazards related to diverse diseases, especially digestive diseases, such as chronic gastritis, peptic ulcer, and gastric carcinoma. In clinical, antibiotics are commonly applied in eradication therapy of H. pylori. However, the increase in antibiotic resistance and side effects has induced the failure of eradication therapy. Recent studies have shown that probiotic supplementation has promising application prospects. It can restore the gastrointestinal microbiota balance and prevent dysbacteriosis caused by antibiotics. Furthermore, it has been reported to have direct or indirect inhibitory effects on H. pylori. Probiotics may have a beneficial effect on H. pylori eradication. However, the strain, dosages, duration times, and safety of probiotic supplementation need further study before clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
H. pylori is a bacterium that infects approximately half of the world’s population (Hooi et al. 2017). Although most H. pylori-positive individuals remain asymptomatic, it is known that H. pylori is related to the development of various clinical conditions, such as peptic ulcers, gastric adenocarcinomas, and mucosa-associated lymphoid tissue lymphomas (Brito et al. 2019; Ford et al. 2020). It was first explicitly formulated in the Kyoto Global Consensus Report that H. pylori gastritis should be considered an infectious disease, whether the affected individual had any symptoms, complications, or consequent illnesses (Sugano et al. 2015). Following the European Maastricht V/Florence consensus statement, anyone with H. pylori infection should undergo the eradication treatment (Malfertheiner et al. 2017). Since the 1997 Maastricht consensus, standard triple therapy has been employed in most countries as the first-line regimen of H. pylori eradication. The quadruple therapy was exercised later by adding bismuth, which was also used as the first-line regimen (Malfertheiner et al. 1997). However, the increase in antibiotic resistance and the reduction of compliance with therapeutic regimens have induced eradication therapy failure and changes in the therapeutic regimen. Therefore, alternative treatments have been proposed to eradicate H. pylori, including novel antibiotics or classical ones in different combinations, which have been used in regular clinical practice as novel and more effective treatments.
Probiotics are considered as living microorganisms. Adequate amounts of probiotics are beneficial to health (Hill et al. 2014). Studies on human and animal models have revealed the mechanisms of probiotics, including the production of antibacterial substances, competitive inhibition of adherence to intestinal epithelium of pathogens and toxins, immunological regulation, maintenance of intestinal epithelial homeostasis, etc. (Yan and Polk 2020; Quigley 2019). Besides, studies have shown that probiotics could suppress drug-resistant bacteria growth and drug-resistant gene transmission (Kunishima et al. 2019). Meanwhile, fecal microbial transplantation decolonized some pathogenic antibiotic-resistant organisms (Wieers et al. 2019; Millan et al. 2016). Many clinical trials have reported that using specific probiotics alone could diminish the H. pylori bacterial load. The specific probiotics supplementation in the H. pylori standard eradication treatment protocol may improve the eradication rate and reduce the side effects caused by antibiotics (Homan and Orel 2015). A meta-analysis of 19 randomized controlled trials found that adjunctive therapy of multiple strain probiotics could improve eradication rates of H. pylori and prevent the occurrence of adverse events and antibiotic-associated diarrhea, but not all the mixtures were effective (McFarland et al. 2016).
This review was performed to summarize recent studies on the status of H. pylori infection, diagnosis, therapeutic regimen, the effects of probiotics on H. pylori, mechanism, and application recommendations. Unlike the systematic review which has focused on one aspect to analysis, multiple aspects of H. pylori were mentioned and a number of literatures, which include reviews, guidelines, meta-analyses, cell and animal studies, and clinical trials, were cited in our review.
Status, Pathogenesis, and treatment of H. pylori infection
Status of H. pylori infection
H. pylori is a gram-negative bacterium that infected approximately 4.4 billion individuals worldwide in 2015, based on regional prevalence estimates (Hooi et al. 2017). Reports of infection prevalence rates range widely among geographic regions, reaching the highest levels in developing countries and showing a well-established relationship with socioeconomic status and hygiene conditions (Roberts et al. 2016; Alzahrani et al. 2014). H. pylori infection prevalence in the United States was estimated to be around 35% by Hooi et al. while the infection rate was higher in Africa, Central America, Central Asia, and Eastern Europe (Hooi et al. 2017). A cross-sectional study conducted in the United Arab Emirates found a prevalence of 41% in healthy children and adults (Melese et al. 2019). The most significant studies performed in Korea involved 24,471 subjects and had a seroprevalence of 41.5% (Lim et al. 2018). A 12-year retrospective study in a tertiary center study from East China on 3252 subjects showed a prevalence of 27.5% (Tang et al. 2019). In Malaysia, the H. pylori infection rate had a specific ethnic structure—H. pylori prevalence was the highest among Indians (> 50%), followed by Chinese (40–50%), whereas Malays had a relatively low prevalence of infection, approximately 10–20%(Goh 2018). Conversely, Bolivia had up to 80%, despite the inclusion of children, suggesting an early acquisition in childhood in the country. A study from Brazil reported that the prevalence rates of the infection reached 70–90% in adults and children aged 5–10 years old (Coelho et al. 2018; Sivapalasingam et al. 2014). Although reports have shown a continuous decline in H. pylori prevalence in many regions worldwide, including Korea, China, Iran, and Austria (Leja et al. 2019), management of this infection remains a formidable challenge due to antibiotic-resistant increases.
Pathogenesis of H. pylori infection
Virulence factors
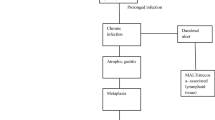
H. pylori can activate the immune response, which would induce gastritis, peptic ulcers, and gastric cancer. It may also contribute to immune thrombocytopenic purpura and increase the risk of acute coronary syndrome, cerebrovascular disease, and neurodegenerative disease (Tsay and Hsu 2018). However, various virulence factors and virulence genes of H. pylori are involved in the pathological process of gastrointestinal disorders caused by H. pylori (Brito et al. 2019).
Urease is a critical factor that enables bacterial colonization in the gastric mucosa, protects the bacterium from gastric acidity, promotes bacterial nutrition, and generates the proton motive force during the hydrolysis of urea (Ansari and Yamaoka 2019). Adding to urease's impact on acid neutralization, its catalysis has pathogenicity. Ammonia impairs cell junctions, breaches cellular integrity, and damages gastric epithelium. CO2 protects the bacterium from the bactericidal activity of metabolic products like nitric oxide and intracellular killing by phagocytes (Ansari and Yamaoka 2019; Debowski et al. 2017).
The cag pathogenicity island contains genes encoding a secreted effector protein CagA and components of the type IV secretion system Cag T4SS (Cover et al. 2020). CagA injects the cell via the pilus formed by T4SS and induces cellular alterations (Ansari and Yamaoka 2020). Meanwhile, CagA can facilitate carcinogenesis by modulating apoptosis, disrupting cell polarity, and promoting genetic instability (Sterbenc et al. 2019). Moreover, CagA is highly associated with the induction and further progression of EMT in gastric mucosa, which is related to greater invasiveness properties. However, consensus recommends considering CagA as an effector protein rather than a toxin because CagA could not act without the bacterial cell, elicit acute damage to host cells, and counteract the activities of the established H. pylori toxin VacA (Knorr et al. 2019).
VacA is a cytotoxin involved in pore formation, and its gene expression can be observed in all H. pylori strains, but toxin activity differed depending on exposure time to the host cells. VacA promotes autophagy pathways in cells in acute exposure. However, VacA improves impaired autophagosome appearance and induces intracellular vacuole formation, which enables H. pylori survival in the host cells (Baj et al. 2020). H. pylori has been proven to downregulate autophagic protein expression and inhibit autophagy, while the VacA toxin plays a vital role in it (Ricci 2016). Except for autophagy, VacA is involved in many processes of damaging gastric epithelial cells, such as alterations in mitochondrial functioning, apoptosis, and necrosis (Foegeding et al. 2016). Abdullah et al. revealed that a synergistic effect of VacA and CagA on accumulating CagA in VacA induced impaired autophagosomes (Abdullah et al. 2019).
DupA is a H. pylori virulence factor with multifunctional biological activities, and it can be considered an essential biomarker in duodenal ulcer (DU) (Alam et al. 2020). The prevalence of the DupA gene in strains was higher in patients with duodenal ulcers than in patients with gastritis or gastric cancer (Ansari and Yamaoka 2017). However, some studies have shown that DupA is a protective factor that prevents gastric carcinogenesis (GC) and the proliferation and growth of GC cells by over-activating the mitochondria-mediated apoptotic pathway and the high tolerance of DupA-positive strains to the acidic gastric microenvironment (Talebi et al. 2012; Queiroz et al. 2011).
In addition, H. pylori protease, PqqE, could disrupt the structure and function of tight junctions of epithelium and damage gastric epithelial integrity by cleaving junctional adhesion molecule A (Marques et al. 2021). The expression of outer membrane proteins (OMPs) helps H. pylori attach to gastric epithelial cells in the primary stage and increases H. pylori virulence (Xu et al. 2020). Encoded by the hopH gene, OipA is associated with bacterial adherence, colonization, induction, and the progression of gastrointestinal disorders. Further, it may regulate CagA and VacA syntheses (Al-Maleki et al. 2017).
Immunological aspects
H.pylori infection always results in intense and complex host immune responses, but it hardly induces clearance of the infection. H. pylori is thought to downregulate inflammation and control the host's immune response via various virulence factors involved in provoking and maintaining a pro-inflammatory immune response (Kusters et al. 2006). H. pylori infection could activate the innate immunity. Various H. pylori antigens and products, such as lipoteichoic acid, lipoproteins, lipopolysaccharide, HSP-60, NapA, DNA, and RNA, bind to gastric cell receptors located on epithelial cell membranes. H. pylori LPS is a primary activator of the innate immune response in epithelial cells, and it could activate NF-κB via TLR2 and TLR4-mediated recognition (Smith 2014; Nagashima et al. 2015).
Many pathogenic effects of H. pylori infection are related to chronic active inflammation, which is controlled and maintained by complex interplay of pro-inflammatory and anti-inflammatory mediators. In H. pylori-positive patients, studies found a Th1-polarized response, characterized by lack of IL-4 and growth of gamma interferon, tumor necrosis factor, IL-1β, IL-6, IL-7, IL-8, and IL-10 (Brito et al. 2019). IL-1β is a potent pro-inflammatory cytokine and the most potent inhibitor of acid secretion up to now. It induces gastric secretion reduction, which is associated with corpus-predominant colonization by H. pylori, causing gastritis, atrophic gastritis, and even gastric cancer (El-Omar et al. 2000).
Treatment of H. pylori infection
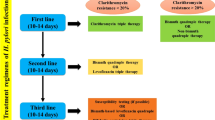
Most commonly, antibiotic therapy is recommended for eradication therapy. Triple therapy, which is based on combining two antibiotics plus one proton pump inhibitor (PPI) with a duration of 7–14 days, remains the standard treatment protocol (Malfertheiner et al. 2017; Talebi 2017). However, the increase in antibiotic application worldwide has induced antibiotic resistance among the bacterium, including H. pylori, inducing a reduction in the success rate of first-line anti-H. pylori therapies(Siddique et al. 2018). A study showed that, in most WHO regions, the pooled prevalence of both primary and secondary resistance of H. pylori to clarithromycin, metronidazole, and levofloxacin is > 15%—this is the common threshold for choosing alternative empiric regimens (Savoldi et al. 2018). It was reported that H. pylori resistance rate to metronidazole in China increased by approximately 50% between 2000 and 2014, and clarithromycin resistance increased from 14.8% in 2000 to 52.6% in 2014 (Thung et al. 2016). The eradication rate of traditional triple therapy based on metronidazole and clarithromycin was less than 80% (Chey et al. 2017). A network meta-analysis on the first-line treatment of H. pylori infection showed that the eradication rate of standard triple therapy with clarithromycin and amoxicillin or metronidazole for seven days was only 73% rather than the required quality criterion of 80% (Li et al. 2015). Therefore, addressing antibiotic resistance has become a major factor in H. pylori eradication success.
A systematic review and meta-analysis of 45 randomized controlled trials (RCTs), including a total of 7722 patients, provided evidence that 14 days was the optimum duration for clarithromycin-containing triple therapy (PPI, clarithromycin and amoxicillin or metronidazole/tinidazole) (Farup et al. 2002). The pooled eradication rate of the 14-day regimen significantly exceeded that of the 7-day regimen (81.9% vs. 72.9%). All the international guidelines agree that clarithromycin-containing triple therapy remains useful in the first-line treatment of H. pylori infection, with extending to 14 days (Malfertheiner et al. 2017; Chey et al. 2017; Fallone et al. 2016). Bismuth quadruple therapy and concomitant therapy are the best first-line empirical treatments in areas with high clarithromycin resistance and individuals with previous application of macrolides, or the 14-day clarithromycin-containing triple therapy is also a proper regimen (Zagari et al. 2021). However, a study comparing the standard 7-day triple therapy and the 14-day triple therapy with the 7-day regimen with bismuth revealed that the eradication rate of 14-day triple therapy (89%) exceeded that of 7-day standard triple therapy (79%). Nevertheless, 14-day triple therapy did not achieve the targeted 90% eradication rate in the intention-to-treat (ITT) analysis. The supplement of bismuth in the 7-day standard triple therapy did not improve the eradication rate(Leow et al. 2018). The eradication rates of different regimens varied in subpopulations with resistant infections (Graham and Dore 2016).
The eradication rate of different regimens (14-day triple therapies, sequential therapies, concomitant therapies, bismuth quadruple therapies, levofloxacin triple therapies, or 7-day vonoprazan triple therapies) exceeds 95% in subpopulations with susceptible infections (Hu et al. 2017a). More so, the H. pylori infection eradication rate with first-line treatment decreased below 80% in many countries, even less than 70% in some regions (Hu et al. 2017b). Recently, some new treatment regimens have been proposed, such as triplet therapy with vonoprazan, five-combination therapy, high-dose dual therapy, and standard triple therapy with probiotics (Hu et al. 2017b). More studies are needed to prove the safety and effectiveness of these emerging treatments.
Roles of probiotics in H. pylori eradication therapy
Effects on eradication rate and side effects
Recently, many studies have shown that probiotic supplementation could improve the eradication rate and reduce the side effects caused by antibiotics. The changes in the microbiome induced by antibiotics could induce diarrhea and other side effects, which could be avoided by probiotic supplementation and prevent antibiotic-related adverse events (Ianiro et al. 2014, 2016). Moreover, compared with placebos, probiotics have the therapeutic effect of H. pylori eradication (Goderska et al. 2018). It was reported that taking probiotics alone can diminish bacterial load, whereas applying probiotics with antibiotics can improve the eradication rate and alleviate side effects (Homan and Orel 2015; Song et al. 2019). However, other studies found that probiotic or sulforaphane with triple therapy for H. pylori infection neither increased the eradication rate nor decreased the incidence of adverse events (Chang et al. 2020; Mukai et al. 2020).
Probiotic monotherapy
Zhang et al. performed a study that enrolled 150 subjects infected with H. pylori to assess the efficacy and safety of monotherapy in eradication treatment with Clostridium butyricum, Bacillus coagulans, or C. butyricum plus B. coagulans for eight weeks. ITT analysis revealed that the three groups achieved similar eradication rates, comparable compliance rates, and adverse events during treatment (Zhang et al. 2020). Lee et al. also suggested no significant inhibitive effects of the three probiotic strains (L. acidophilus, L. rhamnosus, and L. sporogenes) on H. pylori (Lee et al. 2017). However, following the research by Yoon's team, reduction in H. pylori density and histologic inflammation improvement could be observed in patients treated with fermented milk containing L. paracasei HP7 and G. glabra (Yoon et al. 2019). In a randomized double-blind placebo-controlled clinical trial, the decrease in the mean HpSA and HpSA titer exhibited a significant difference between Saccharomyces boulardii and the control group, thereby indicating that Saccharomyces boulardii could positively reduce the colonization of H. pylori in the human gastrointestinal system, but it is incapable of eradication as monotherapy (Namkin et al. 2016). A meta-analysis reported that taking probiotics alone could reduce the bacterial load and eradicate H. pylori with a 14% eradication rate, which was far from satisfactory clinically (Losurdo et al. 2018). Therefore, probiotic monotherapy cannot be used in H. pylori eradication therapy, though it could inhibit bacterium growth.
Probiotics with PPI
An open-label single-center study performed on L. reuteri plus pantoprazole twice a day for eight weeks cured 13.6% of patients with H. pylori infection by ITT analysis and 14.2% by per-protocol (PP) analysis. The overall urease activity assessed before and after 4–6 weeks of therapy showed a significant reduction with a mean of 38.8 vs. 25.4 by a one-tailed test (P = 0.002) (Dore et al. 2014). Another similar controlled study found that L. reuteri DSMZ 17,648 plus pantoprazole for eight weeks achieved a similar efficacy as standard triple therapy for 14 days. The symptoms of satiety, bloating, pain, and anxiety score changes in the two groups had no statistical significance. L. reuteri may be a good alternative to antibiotics, and it can be used to eradicate H. pylori infection in patients with chronic dyspepsia (Muresan et al. 2019). However, Dore et al. studied that the cure rate of taking PPI with Lactobacillus reuterii instead of antibiotics or bismuth was 12% (Dore et al. 2019a). Two studies evaluating whether the treatment of PPI plus probiotics rather than antibiotics would eradicate H. pylori infections were halted because the cure rates were far below the acceptable. Besides, it was also found that the therapy of PPI plus probiotics could not provide a clinically meaningful rate of H. pylori eradication (Opekun et al. 2018; Dore et al. 2019b).
Probiotics with standard eradication treatment
Many studies have attempted to elucidate probiotics' role as supplements in H. pylori eradication therapy, but the results are various. In a Japanese retrospective study, 468 patients with H. pylori infection were divided into three groups, PPI/amoxicillin (AMX)/clarithromycin (CLR), vonoprazan (VPZ)/AMX/CLR, and PPI/AMX/CLR/probiotics, respectively. The study showed a significant difference in the eradication rate between the PPI plus probiotics group and the PPI group in ITT analysis. The main side effects were diarrhea, eruption, and stomatitis, and no significant differences were observed between the three groups (Mukai et al. 2020). A study from China using traditional triple therapy plus probiotics or placebo among patients with H. pylori-positive peptic ulcers shared a similar conclusion that triple therapy with probiotics in treating H. pylori-positive peptic ulcer could significantly improve the H. pylori eradication rate with less adverse reaction (Ma et al. 2015). A study performed by Goran Hauser et al. drew a similar conclusion (Hauser et al. 2015). Probiotics with sequential treatment could also enhance the eradication rate and reduce side effects, such as diarrhea (Cekin et al. 2017; Seddik et al. 2019). The cure rates of the quadruple therapy containing bismuth and the treatment of Gastrus® plus two antibiotics and PPI were 79.6% and 88% by ITT analysis. By PP analysis, the rates were 84.8% and 95.7%, respectively. Moreover, the patient’s compliance was exceptionally nice, and side effects were mild in both regimens (Dore et al. 2019b). Bismuth-containing quadruple therapy (BCQT) with probiotic supplementation could achieve excellent eradication rates and produce fewer side effects (Zhu et al. 2018, 2017), even in patients who experienced failing treatment before (Liu et al. 2020). A meta-analysis showed that, compared with the control group (only BCQT), the probiotic group (BCQT with probiotic supplementation) had a higher eradication rate with statistical significance (P = 0.000), and fewer adverse reactions were reported in the probiotic group than in the control group (P = 0.000) (Si et al. 2017).
Regarding the efficacy of standard eradication treatment with probiotics, the results remain contradictory. In a study from Spain, 209 patients were prescribed eradication therapy (10-day triple or non-bismuth quadruple concomitant therapy) and randomly received probiotics (Lactobacillus plantarum and Pediococcus acidilactici) or placebo. The eradication rates were similar in the two groups (placebo 95% vs. probiotic 97%), and no differences were observed in compliance or side effects (McNicholl et al. 2018). Among the patients with H. pylori-positive chronic gastritis or peptic ulcer but without clarithromycin resistance, the eradication rates and the frequencies of adverse events were similar in the triple therapy group and the triple therapy with probiotics group by ITT analysis and PP analysis (Chang et al. 2020). McNicholl et al. also revealed no significant differences were observed between the control and probiotic groups regarding efficacy and side effects (McNicholl et al. 2018). Some studies have shown that triple therapy with probiotics only improved the cure rate but had no impact on reducing side effects (Grgov et al. 2016; Francavilla et al. 2014; Efrati et al. 2012). However, other studies showed the reverse in reducing side effect incidence but not improving the eradication rate (Zhang et al. 2015; Chotivitayatarakorn et al. 2017).
A meta-analysis that included 45 RCTs and 6997 participants reported that the overall eradication rates of the control and probiotic groups were 72.08% and 82.31%, thus reviewing that standard therapy application with probiotics was associated with the increased eradication rate by per-protocol set analysis or intention-to-treat analysis. Besides, adverse event incidence was 36.27% in the control group and 21.44% in the probiotic group. The specific reduction in adverse events ranged from 30 to 59% and was statistically significant (Zhang et al. 2015). The results of clinical studies and meta-analyses had a discrepancy. Several studies have shown that probiotic supplementation during anti-H. pylori treatment effectively improved H. pylori eradication rate and minimized the incidence of therapy-related adverse events related to specific strains of the bacterium (McFarland et al. 2016; Lu et al. 2016a; Konorev et al. 2016; Feng et al. 2017; Shi et al. 2019). Lau et al. reported that probiotic supplementation significantly increased the eradication rate by 12.2%. They decreased the risk of diarrhea, nausea, vomiting, and epigastric pain, but no significant differences were observed in efficacy between the various types of probiotics (Lau et al. 2016). However, in a study by Lu et al. compared with the placebo group, probiotics combined with standard therapy did improve the adverse effects of diarrhea and nausea but did not increase the H. pylori eradication rate (Lu et al. 2016b).
The application of probiotics in children with H. pylori infection
Some studies have applied probiotics as supplementation in H. pylori eradication therapy in children. Studies have shown that probiotics with triple therapy for H. pylori eradication in children could significantly enhance the H. pylori eradication rate and reduce side effect incidence (Ahmad et al. 2013; Wang and Huang 2014). Tolone et al. reported there were significant differences in the side effects of the control and probiotic groups, while no statistical differences were found in the eradication rate in both groups (Tolone et al. 2012). However, in a meta-analysis that included five studies, 484 pediatric patients concluded that Lactobacillus supplementation with standard triple therapy could increase the H. pylori eradication rate and reduce the incidence of therapy-related diarrhea in children (Fang et al. 2019). Probiotics supplementation in triple therapy for children with H. pylori-positive may have beneficial effects on eradication treatment and therapy-related side effects, particularly diarrhea (Li et al. 2014).
Effects on gut microbiota
The human gut microbiota theory showed gut microbes could modulate human physiological activities in dynamic balance, such as nutrient absorption, energy metabolism, immune function, and neurological landscapes (Adak and Khan 2019). H. pylori infection changes the microflora of gastric and intestinal (He et al. 2016; Shin et al. 2020), and eradication therapy induces gut microbiota disorders (Olekhnovich et al. 1902; Ma et al. 2021), but probiotic supplementation may protect and recover the gut microbiota (Ji and Yang 2020). Recently, animal tests and clinical trials have shown that probiotics positively affect the host's gut microbiota by maintaining the gastric microbiota balance during H. pylori infection and treatment (Zhu and Liu 2017; Chen et al. 2019). Liou JM et al. showed that α-diversity reduced significantly and β-diversity markedly altered at the end of triple therapy, concomitant therapy, and bismuth quadruple therapy, compared with baseline (Liou et al. 2019). Wu et al. found that the diversity of gut microbiota decreased remarkably in patients with H. pylori who only underwent triple therapy. Concurrently, probiotics supplementation of Bacillus subtilis and E. faecalis inhibited the reduction (Wu et al. 2019). According to Oh et al., the proportional change of functional gene families in the control group exceeded that in the probiotic group after H. pylori eradication treatment. The functional alterations of gut microbiota may be linked to the reduction in intestinal irritation and maintenance of bacterial diversity (Oh et al. 2016). Wang et al. found that prominent quantitative and qualitative alterations in the gut microbiota were observed after eradication treatment, whether standard therapy or probiotic supplementation, but most of the changes reverted on day 71 (Wang et al. 2017). Although a meta-analysis reported that microbial diversity decreased in the short-term follow-up, no research data could confirm subsequent alterations (Ye et al. 2020).
Mechanisms of probiotics in H. pylori eradication therapy
Probiotics have been proven effective and beneficial for several gastrointestinal diseases, including H. pylori infection, diarrhea caused by antibiotics, or clostridium difficile and pouchitis, while the precise mechanism requires exploration (Sebastian 2017). Many vitro and vivo experiments have shown that various probiotics have bacteriostatic and bactericidal activity against H. pylori through bacterial cells or metabolites (Chen et al. 2019; Asgari et al. 2020, 2018; Saracino et al. 2020; Urrutia-Baca et al. 2018). The antagonism between probiotics and H. pylori is achieved via immunological and non-immunological regulation mechanisms, including production of antimicrobial substances, mucosal barrier, and adhesion competition (Goderska et al. 2018; Qureshi et al. 2019; Eslami et al. 2019).
Immunological mechanism
H. pylori infection-induced inflammatory diseases were associated with the sustained expression of inflammatory factors, which had no effect on H. pylori eradication but continued inflammatory response (Brito et al. 2019). Probiotics modified the immunological response by modulating anti-inflammatory cytokine secretion, thereby inducing inflammation activity reduction (Wiese et al. 2012; Garcia-Castillo et al. 2018). The IL-8 release was the initial manifestation of the cytokine response, which induced the migration of neutrophils and monocytes to the mucosa. Numerous studies have found that probiotic strains, such as L. acidophilus, L. bulgaricus, L. gasseri, and L. rhamnosus, could strengthen the expression of the anti-inflammatory cytokine IL-10 (Zhao et al. 2018) and reduce the expression of IL-8 in H. pylori-infected cells via modulating the TLR4/IκBα/NF-κB pathway (Song et al. 2019; Chen et al. 2019; Yarmohammadi et al. 2021; Whiteside et al. 2021). A study showed that lactic acid-producing bacteria (LAB) treatments reduced the H. pylori loads, vacA gene expression, H. pylori specific IgA, and IgM levels in the stomach, alongside the serum levels of IFN-γ and IL-1β. The multi-LAB treatment recovered and increased the levels of some serum fatty acids and amino acids, which were important in immune functions modulation (Lin et al. 2020). Animal experiments revealed that Lactobacillus significantly reduced IL-6 levels but increased the IL-10 level and repaired mucosal damage (Zhou et al. 2021; Park et al. 2020). Moreover, 16S rRNA gene sequencing revealed that H. pylori relative abundance could be significantly decreased by L. plantarum ZJ316 administration (Zhou et al. 2021). Therefore, probiotics have a preventive and mitigating effect on the inflammation caused by H. pylori infection.
Non-immunological mechanism
Antimicrobial substances
Antimicrobial production is an essential function of probiotics. Some probiotic strains could produce various antimicrobial compounds, such as short-chain fatty acids (SCFAs), hydrogen peroxide, nitric oxide, and bacteriocins, which would inhibit H. pylori growth via antibacterial substances (Homan and Orel 2015). During carbohydrate metabolism, probiotics could produce short-chain fatty acids, such as acetic, propionic, and lactic acids, which would lower gastric pH (Zhou et al. 2021). Organic acid could inhibit H. pylor growth and suppress urease activity (Rezaee et al. 2019). Furthermore, catalase induced oxygen radical production, which interfered with H. pylori enzyme activity and produced oxidative damage in H. pylori-infected cells (Ji and Yang 2020; Song et al. 2018). Moreover, bacteriocins production is the primary factor in H. pylori inhibition by probiotics. Boyanova et al. found that Lactobacillus produced heat-stable bacteriocin-like inhibitory substances (BLISs), which inhibited the development of antibiotic-sensitive and antibiotic-resistant strains (Boyanova et al. 2017). Reuterin compounded by L. reuteri inhibited the growth of H. pylori and downregulated the expression of the virulence genes vacA and flaA (Urrutia-Baca et al. 2018).
Mucosal barrier
The mucins and large complex glycoproteins defend the gastrointestinal mucosa epithelium against toxic substances and pathogens. Probiotics protect the mucosal barrier from damage by modifying the expression of mucus and epithelial junction proteins, and releasing bioactive molecules to stabilize the barrier (Qureshi et al. 2019). IgA produced by probiotic strains could help strengthen the mucosal barrier against pathogen invasion. Vitro studies showed probiotics could upregulate tight-junction proteins and promote mucous secretion to stabilize the mucous layer by increasing the expressions of muc1, muc2, muc3, and muc5 (Goderska et al. 2018; Hanisch et al. 2014; Suez et al. 2019; Zhang et al. 2014; Dhar and McAuley 2019). Therefore, probiotics could restore the mucosal permeability of gastric mucosa and prevent H. pylori colonization.
Competition for adhesion
Adhesion to the host tissue was a vital process of H. pylori colonization of the gastric mucosa. Studies have shown that probiotics, such as LAB, Lactobacillus, and Streptococcus thermophilus, reduced adhesion to H. pylori epithelial cells (Chen et al. 2019; Rezaee et al. 2019; Marcial et al. 2017). Probiotics hinder H. pylori from binding to epithelial cells in different ways, such as antimicrobial substance secretion, competition of adhesion sites, or nutrients(Qureshi et al. 2019). Takeda et al. found the level of the CagA virulence protein of H. pylori in MKN45 cells, and some viable H. pylori adhering to MKN45 cells decreased with Lactobacillus paracasei 06TCa19 supplementation(Takeda et al. 2017). Moreover, the 06TCa19 strain notably increased some lactic acids in the supernatant of MKN45 cells. Lactic acid, which was released from the 06TCa19 strain, inhibited the adhesion of H. pylori to MKN45 cells, and prevented the H. pylori CagA from inserting into the cells. Saccharomyces boulardii contained selective neuraminidase activity, which could remove the α(2–3)-linked sialic acid and suppress the H. pylori adherence to duodenal epithelial cells, because sialic acid could hold the ligands of H. pylori adhesion (Sakarya and Gunay 2014).
Guidelines for the clinical use of probiotics
Many systematic reviews and meta-analyses indicated that standard H. pylori eradication therapy with probiotic supplementation was adequate for the growth of eradication rates and prevention of adverse effects. The present meta-analyses are inconsistent because different eradication regimens were applied in the included random control trials, and diverse probiotics were used, making it impossible to assess strain specificity. Recent guidelines revealed that probiotic supplementation was evaluated to manage H. pylori infection (Malfertheiner et al. 2017; Fallone et al. 2016; Zagari et al. 2015). In the Italian guideline for managing H. pylori infection, “Some probiotics reduce adverse effects during H. pylori eradication therapy” is stated (Evidence level 3a; Grade of recommendation B) (Zagari et al. 2015). The Tronto Consensus recommends routinely adding probiotics to eradication therapy to reduce adverse events or increasing eradication rates in patients with H. pylori infection (Fallone et al. 2016). While in Maastricht V/Florence Consensus Report, only certain probiotics could effectively minimize gastrointestinal side effects caused by H. pylori eradication therapies and may advantageously affect H. pylori eradication. The specific strains should be chosen only upon the basis of a revealed clinical efficacy (Malfertheiner et al. 2017).
Outlook
Because of the high prevalence and severe hazard of H. pylori infection, it is necessary to eradicate H. pylori. However, the effect of anti-H. pylori therapy is far from satisfactory. Probiotics supplementation with standard eradication therapy has been proven to improve cure rates, decrease side effects, and maintain the host gut microflora balance, but the results are contradictory in clinical trials and meta-analyses for the diversity of antibiotic regimens, probiotic strains, host susceptibility, and other factors. Therefore, to improve the H. pylori eradication effect, more relevant experiments are needed to explore the optimal selection and appropriate dosage of probiotics and antibiotics.
Data availability statement
Not available.
Availability of data and materials
Not available.
References
Abdullah M, Greenfield LK, Bronte-Tinkew D et al (2019) VacA promotes CagA accumulation in gastric epithelial cells during Helicobacter pylori infection[J]. Sci Rep 9(1):38
Adak A, Khan MR (2019) An insight into gut microbiota and its functionalities[J]. Cell Mol Life Sci 76(3):473–493
Ahmad K, Fatemeh F, Mehri N et al (2013) Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial[J]. Iran J Pediatr 23(1):79–84
Alam J, Sarkar A, Karmakar BC et al (2020) Novel virulence factor dupA of Helicobacter pylori as an important risk determinant for disease manifestation: an overview[J]. World J Gastroenterol 26(32):4739–4752
Al-Maleki AR, Loke MF, Lui SY et al (2017) Helicobacter pylori outer inflammatory protein A (OipA) suppresses apoptosis of AGS gastric cells in vitro[J]. Cell Microbiol 19(12):e12771
Alzahrani S, Lina TT, Gonzalez J et al (2014) Effect of Helicobacter pylori on gastric epithelial cells[J]. World J Gastroenterol 20(36):12767–12780
Ansari S, Yamaoka Y (2017) Survival of Helicobacter pylori in gastric acidic territory[J]. Helicobacter. https://doi.org/10.1111/hel.12386
Ansari S, Yamaoka Y (2019) Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity[J]. Toxins 11(11):677
Ansari S, Yamaoka Y (2020) Helicobacter pylori Virulence Factor Cytotoxin-Associated Gene A (CagA)-Mediated Gastric Pathogenicity[J]. Int J Mol Sci 21(19):7430
Asgari B, Kermanian F, Derakhshan N et al (2018) Honey-derived lactobacillus rhamnosus alleviates helicobacter pylori-induced gastro-intestinal infection and gastric inflammation in c57bl/6 mice: an immuno-histologic study[J]. Arq Gastroenterol 55(3):279–282
Asgari B, Kermanian F, Hedayat YM et al (2020) The Anti-Helicobacter pylori Effects of Lactobacillus acidophilus, L. plantarum, and L. rhamnosus in Stomach Tissue of C57BL/6 Mice[J]. Visc Med 36(2):137–143
Baj J, Forma A, Sitarz M et al (2020) Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment[J]. Cells 10(1):27
Boyanova L, Gergova G, Markovska R et al (2017) Bacteriocin-like inhibitory activities of seven Lactobacillus delbrueckii subsp. bulgaricus strains against antibiotic susceptible and resistant Helicobacter pylori strains[J]. Lett Appl Microbiol 65(6):469–474
Cekin AH, Sahinturk Y, Akbay HF et al (2017) Use of probiotics as an adjuvant to sequential H. pylori eradication therapy: impact on eradication rates, treatment resistance, treatment-related side effects, and patient compliance[J]. Turk J Gastroenterol 28(1):3–11
Chang YW, Park YM, Oh CH et al (2020) Effects of probiotics or broccoli supplementation on Helicobacter pylori eradication with standard clarithromycin-based triple therapy[J]. Korean J Intern Med 35(3):574–581
Chen YH, Tsai WH, Wu HY et al (2019) Probiotic Lactobacillus spp. act Against Helicobacter pylori-induced Inflammation[J]. J Clin Med 8(1):90
Chey WD, Leontiadis GI, Howden CW et al (2017) ACG Clinical Guideline: Treatment of Helicobacter pylori Infection[J]. Am J Gastroenterol 112(2):212–239
Chotivitayatarakorn P, Mahachai V, Vilaichone RK (2017) Effectiveness of 7-Day and 14-Day Moxifloxacin-Dexlansoprazole Based Triple Therapy and Probiotic Supplement for Helicobacter Pylori Eradication in Thai Patients with Non-Ulcer Dyspepsia: A Double-Blind Randomized Placebo-Controlled Study[J]. Asian Pac J Cancer Prev 18(10):2839–2843
Coelho L, Marinho JR, Genta R et al (2018) IVth Brazilian Consensus Conference On Helicobacter Pylori Infection[J]. Arq Gastroenterol 55(2):97–121
Cover TL, Lacy DB, Ohi MD (2020) The Helicobacter pylori Cag Type IV Secretion System[J]. Trends Microbiol 28(8):682–695
de Brito BB, Da SF, Soares AS et al (2019) Pathogenesis and clinical management of Helicobacter pylori gastric infection[J]. World J Gastroenterol 25(37):5578–5589
Debowski AW, Walton SM, Chua EG et al (2017) Helicobacter pylori gene silencing in vivo demonstrates urease is essential for chronic infection[J]. PLoS Pathog 13(6):e1006464
Dhar P, McAuley J (2019) The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation[J]. Front Cell Infect Microbiol 9:117
Dore MP, Cuccu M, Pes GM et al (2014) Lactobacillus reuteri in the treatment of Helicobacter pylori infection[J]. Intern Emerg Med 9(6):649–654
Dore MP, Bibbo S, Pes GM et al (2019a) Role of probiotics in helicobacter pylori eradication: lessons from a Study of Lactobacillus reuteri Strains DSM 17938 and ATCC PTA 6475 (Gastrus(R)) and a Proton-Pump Inhibitor[J]. Can J Infect Dis Med Microbiol 2019:3409820
Dore MP, Bibbo S, Loria M et al (2019) Twice-a-day PPI, tetracycline, metronidazole quadruple therapy with Pylera(R) or Lactobacillus reuteri for treatment naive or for retreatment of Helicobacter pylori. Two randomized pilot studies[J]. Helicobacter 24(6):e12659
Efrati C, Nicolini G, Cannaviello C et al (2012) Helicobacter pylori eradication: sequential therapy and Lactobacillus reuteri supplementation[J]. World J Gastroenterol 18(43):6250–6254
El-Omar EM, Carrington M, Chow WH et al (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer[J]. Nature 404(6776):398–402
Eslami M, Yousefi B, Kokhaei P et al (2019) Are probiotics useful for therapy of Helicobacter pylori diseases?[J]. Comp Immunol Microbiol Infect Dis 64:99–108
Fallone CA, Chiba N, van Zanten SV et al (2016) The toronto consensus for the treatment of helicobacter pylori infection in adults[J]. Gastroenterology 151(1):51–69
Fang HR, Zhang GQ, Cheng JY et al (2019) Efficacy of Lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: a meta-analysis of randomized controlled trials[J]. Eur J Pediatr 178(1):7–16
Farup PG, Lange OJ, Tholfsen J et al (2002) The effect of Helicobacter pylori retreatment with ranitidine bismuth citrate, clarithromycin, and metronidazole depends on the first-line therapy[J]. J Clin Gastroenterol 35(5):379–382
Feng JR, Wang F, Qiu X et al (2017) Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis[J]. Eur J Clin Pharmacol 73(10):1199–1208
Foegeding NJ, Caston RR, McClain MS et al (2016) An overview of helicobacter pylori VacA toxin biology[J]. Toxins 8(6):173
Ford AC, Yuan Y, Forman D et al (2020) Helicobacter pylori eradication for the prevention of gastric neoplasia[J]. Cochrane Database Syst Rev 7:D5583
Francavilla R, Polimeno L, Demichina A et al (2014) Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study[J]. J Clin Gastroenterol 48(5):407–413
Garcia-Castillo V, Zelaya H, Ilabaca A et al (2018) Lactobacillus fermentum UCO-979C beneficially modulates the innate immune response triggered by Helicobacter pylori infection in vitro[J]. Benef Microbes 9(5):829–841
Goderska K, Agudo PS, Alarcon T (2018) Helicobacter pylori treatment: antibiotics or probiotics[J]. Appl Microbiol Biotechnol 102(1):1–7
Goh KL (2018) Lessons learnt from the epidemiology of Helicobacter pylori infection in Malaysia: JGHF Marshall and Warren Lecture 2017[J]. J Gastroenterol Hepatol 33(6):1177–1184
Graham DY, Dore MP (2016) Helicobacter pylori therapy: a paradigm shift[J]. Expert Rev Anti Infect Ther 14(6):577–585
Grgov S, Tasic T, Radovanovic-Dinic B et al (2016) Can probiotics improve efficiency and safety profile of triple Helicobacter pylori eradication therapy? A prospective randomized study[J]. Vojnosanit Pregl 73(11):1044–1049
Hanisch FG, Bonar D, Schloerer N et al (2014) Human trefoil factor 2 is a lectin that binds alpha-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori[J]. J Biol Chem 289(40):27363–27375
Hauser G, Salkic N, Vukelic K et al (2015) Probiotics for standard triple Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial[J]. Medicine 94(17):e685
He C, Yang Z, Lu N (2016) Imbalance of Gastrointestinal Microbiota in the Pathogenesis of Helicobacter pylori-Associated Diseases[J]. Helicobacter 21(5):337–348
Hill C, Guarner F, Reid G et al (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic[J]. Nat Rev Gastroenterol Hepatol 11(8):506–514
Homan M, Orel R (2015) Are probiotics useful in Helicobacter pylori eradication?[J]. World J Gastroenterol 21(37):10644–10653
Hooi J, Lai WY, Ng WK et al (2017) Global Prevalence of Helicobacter pylori Infection: systematic review and meta-analysis[J]. Gastroenterology 153(2):420–429
Hu Y, Zhu Y, Lu NH (2017a) Primary Antibiotic Resistance of Helicobacter pylori in China[J]. Dig Dis Sci 62(5):1146–1154
Hu Y, Zhu Y, Lu NH (2017b) Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance[J]. Front Cell Infect Microbiol 7:168
Ianiro G, Bibbo S, Gasbarrini A et al (2014) Therapeutic modulation of gut microbiota: current clinical applications and future perspectives[J]. Curr Drug Targets 15(8):762–770
Ianiro G, Tilg H, Gasbarrini A (2016) Antibiotics as deep modulators of gut microbiota: between good and evil[J]. Gut 65(11):1906–1915
Ji J, Yang H (2020) Using probiotics as supplementation for helicobacter pylori antibiotic therapy[J]. Int J Mol Sci 21(3):1136
Knorr J, Ricci V, Hatakeyama M et al (2019) Classification of Helicobacter pylori Virulence Factors: Is CagA a Toxin or Not?[J]. Trends Microbiol 27(9):731–738
Konorev MR, Andronova TM, Matveenko ME (2016) Use of probiotics and probiotic-based immunomodulators as adjuvant therapy for Helicobacter pylori eradication[J]. Ter Arkh 88(12):140–148
Kunishima H, Ishibashi N, Wada K et al (2019) The effect of gut microbiota and probiotic organisms on the properties of extended spectrum beta-lactamase producing and carbapenem resistant Enterobacteriaceae including growth, beta-lactamase activity and gene transmissibility[J]. J Infect Chemother 25(11):894–900
Kusters JG, van Vliet AH, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection[J]. Clin Microbiol Rev 19(3):449–490
Lau CS, Ward A, Chamberlain RS (2016) Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta-analysis[J]. Infect Drug Resist 9:275–289
Lee CY, Shih HC, Yu MC et al (2017) Evaluation of the potential inhibitory activity of a combination of L. acidophilus, L. rhamnosus and L. sporogenes on Helicobacter pylori: a randomized double-blind placebo-controlled clinical trial[J]. Chin J Integr Med 23(3):176–182
Leja M, Grinberga-Derica I, Bilgilier C et al (2019) Review: Epidemiology of Helicobacter pylori infection[J]. Helicobacter 24(Suppl 1):e12635
Leow AH, Azmi AN, Loke MF et al (2018) Optimizing first line 7-day standard triple therapy for Helicobacter pylori eradication: Prolonging treatment or adding bismuth: which is better?[J]. J Dig Dis 19(11):674–677
Li S, Huang XL, Sui JZ et al (2014) Meta-analysis of randomized controlled trials on the efficacy of probiotics in Helicobacter pylori eradication therapy in children[J]. Eur J Pediatr 173(2):153–161
Li BZ, Threapleton DE, Wang JY et al (2015) Comparative effectiveness and tolerance of treatments for Helicobacter pylori: systematic review and network meta-analysis[J]. BMJ 351:h4052
Lim SH, Kim N, Kwon JW et al (2018) Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: a cross-sectional nationwide multicenter study[J]. PLoS One 13(10):e204762
Lin CC, Huang WC, Su CH et al (2020) Effects of Multi-Strain Probiotics on Immune Responses and Metabolic Balance in Helicobacter pylori-Infected Mice[J]. Nutrients 12(8):2476
Liou JM, Chen CC, Chang CM et al (2019) Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial[J]. Lancet Infect Dis 19(10):1109–1120
Liu A, Wang Y, Song Y et al (2020) Treatment with compound Lactobacillus acidophilus followed by a tetracycline- and furazolidone-containing quadruple regimen as a rescue therapy for Helicobacter pylori infection[J]. Saudi J Gastroenterol 26(2):78–83
Losurdo G, Cubisino R, Barone M et al (2018) Probiotic monotherapy and Helicobacter pylori eradication: A systematic review with pooled-data analysis[J]. World J Gastroenterol 24(1):139–149
Lu M, Yu S, Deng J et al (2016a) Efficacy of probiotic supplementation therapy for helicobacter pylori eradication: a meta-analysis of randomized controlled trials[J]. PLoS One 11(10):e163743
Lu C, Sang J, He H et al (2016b) Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: a meta-analysis[J]. Sci Rep 6:23522
Ma F, Zhou C, Wang J et al (2015) Probiotics in the treatment of peptic ulcer infected by helicobacter pylory and its safety[J]. Pak J Pharm Sci 28(3 Suppl):1087–1090
Ma X, Yang Z, Xu T et al (2021) Chlortetracycline alters microbiota of gut or faeces in pigs and leads to accumulation and migration of antibiotic resistance genes[J]. Sci Total Environ 796:148976
Malfertheiner P, Megraud F, O’Morain C et al (1997) Current European concepts in the management of Helicobacter pylori infection–the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG)[J]. Eur J Gastroenterol Hepatol 9(1):1–2
Malfertheiner P, Megraud F, O’Morain CA et al (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report[J]. Gut 66(1):6–30
Marcial G, Villena J, Faller G et al (2017) Exopolysaccharide-producing Streptococcus thermophilus CRL1190 reduces the inflammatory response caused by Helicobacter pylori[J]. Benef Microbes 8(3):451–461
Marques MS, Costa AC, Osorio H et al (2021) Helicobacter pylori PqqE is a new virulence factor that cleaves junctional adhesion molecule A and disrupts gastric epithelial integrity[J]. Gut Microbes 13(1):1–21
McFarland LV, Huang Y, Wang L et al (2016) Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events[J]. United European Gastroenterol J 4(4):546–561
McNicholl AG, Molina-Infante J, Lucendo AJ et al (2018) Probiotic supplementation with Lactobacillus plantarum and Pediococcus acidilactici for Helicobacter pylori therapy: A randomized, double-blind, placebo-controlled trial[J]. Helicobacter 23(5):e12529
Melese A, Genet C, Zeleke B et al (2019) Helicobacter pylori infections in Ethiopia; prevalence and associated factors: a systematic review and meta-analysis[J]. BMC Gastroenterol 19(1):8
Millan B, Park H, Hotte N et al (2016) Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent clostridium difficile infection[J]. Clin Infect Dis 62(12):1479–1486
Mukai R, Handa O, Suyama Y et al (2020) Effectiveness of including probiotics to Helicobacter pylori eradication therapies[J]. J Clin Biochem Nutr 67(1):102–104
Muresan I, Pop LL, Dumitrascu DL (2019) Lactobacillus reuteri versus triple therapy for the eradication of Helicobacter pylori in functional dyspepsia[J]. Med Pharm Rep 92(4):352–355
Nagashima H, Iwatani S, Cruz M et al (2015) Toll-like Receptor 10 in Helicobacter pylori Infection[J]. J Infect Dis 212(10):1666–1676
Namkin K, Zardast M, Basirinejad F (2016) Saccharomyces Boulardii in Helicobacter Pylori Eradication in Children: A Randomized Trial From Iran[J]. Iran J Pediatr 26(1):e3768
Oh B, Kim JW, Kim BS (2016) Changes in the functional potential of the gut microbiome following probiotic supplementation during helicobacter pylori treatment[J]. Helicobacter 21(6):493–503
Olekhnovich EI, Manolov AI, Samoilov AE et al (1902) Shifts in the human gut microbiota structure caused by quadruple helicobacter pylori eradication therapy[J]. Front Microbiol 2019:10
Opekun AR, Gonzales SA, Al-Saadi MA et al (2018) Brief report: Lactobacillus bulgaricus GLB44 (Proviotic()) plus esomeprazole for Helicobacter pylori eradication: a pilot study[J]. Helicobacter 23(2):e12476
Park H, Cho D, Huang E et al (2020) Amelioration of Alcohol Induced Gastric Ulcers Through the Administration of Lactobacillus plantarum APSulloc 331261 Isolated From Green Tea[J]. Front Microbiol 11:420
Queiroz DM, Rocha GA, Rocha AM et al (2011) dupA polymorphisms and risk of Helicobacter pylori-associated diseases[J]. Int J Med Microbiol 301(3):225–228
Quigley E (2019) Prebiotics and Probiotics in Digestive Health[J]. Clin Gastroenterol Hepatol 17(2):333–344
Qureshi N, Li P, Gu Q (2019) Probiotic therapy in Helicobacter pylori infection: a potential strategy against a serious pathogen?[J]. Appl Microbiol Biotechnol 103(4):1573–1588
Rezaee P, Kermanshahi RK, Falsafi T (2019) Antibacterial activity of lactobacilli probiotics on clinical strains of Helicobacter pylori[J]. Iran J Basic Med Sci 22(10):1118–1124
Ricci V (2016) Relationship between VacA toxin and host cell autophagy in helicobacter pylori infection of the human stomach: a few answers, many questions[J]. Toxins 8(7):203
Roberts SE, Morrison-Rees S, Samuel DG et al (2016) Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe[J]. Aliment Pharmacol Ther 43(3):334–345
Sakarya S, Gunay N (2014) Saccharomyces boulardii expresses neuraminidase activity selective for alpha2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells[J]. APMIS 122(10):941–950
Saracino IM, Pavoni M, Saccomanno L et al (2020) Antimicrobial efficacy of five probiotic strains against helicobacter pylori[J]. Antibiotics 9(5):244
Savoldi A, Carrara E, Graham DY et al (2018) Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions[J]. Gastroenterology 155(5):1372–1382
Sebastian DJ (2017) Review of the role of probiotics in gastrointestinal diseases in adults[J]. Gastroenterol Hepatol 40(6):417–429
Seddik H, Boutallaka H, Elkoti I et al (2019) Saccharomyces boulardii CNCM I-745 plus sequential therapy for Helicobacter pylori infections: a randomized, open-label trial[J]. Eur J Clin Pharmacol 75(5):639–645
Shi X, Zhang J, Mo L et al (2019) Efficacy and safety of probiotics in eradicating Helicobacter pylori: A network meta-analysis[J]. Medicine 98(15):e15180
Shin CM, Kim N, Park JH et al (2020) Changes in Gastric Corpus Microbiota With Age and After Helicobacter pylori Eradication: A Long-Term Follow-Up Study[J]. Front Microbiol 11:621879
Si XB, Lan Y, Qiao L (2017) A meta-analysis of randomized controlled trials of bismuth-containing quadruple therapy combined with probiotic supplement for eradication of Helicobacter pylori][J. Zhonghua Nei Ke Za Zhi 56(10):752–759
Siddique O, Ovalle A, Siddique AS et al (2018) Helicobacter pylori Infection: An Update for the Internist in the Age of Increasing Global Antibiotic Resistance[J]. Am J Med 131(5):473–479
Sivapalasingam S, Rajasingham A, Macy JT et al (2014) Recurrence of Helicobacter pylori infection in Bolivian children and adults after a population-based “screen and treat” strategy[J]. Helicobacter 19(5):343–348
Smith SM (2014) Role of Toll-like receptors in Helicobacter pylori infection and immunity[J]. World J Gastrointest Pathophysiol 5(3):133–146
Song HY, Zhou L, Liu DY et al (2018) What roles do probiotics play in the eradication of helicobacter pylori? Current knowledge and ongoing research[J]. Gastroenterol Res Pract 2018:9379480
Song H, Zhou L, Liu D et al (2019) Probiotic effect on Helicobacter pylori attachment and inhibition of inflammation in human gastric epithelial cells[J]. Exp Ther Med 18(3):1551–1562
Sterbenc A, Jarc E, Poljak M et al (2019) Helicobacter pylori virulence genes[J]. World J Gastroenterol 25(33):4870–4884
Suez J, Zmora N, Segal E et al (2019) The pros, cons, and many unknowns of probiotics[J]. Nat Med 25(5):716–729
Sugano K, Tack J, Kuipers EJ et al (2015) Kyoto global consensus report on Helicobacter pylori gastritis[J]. Gut 64(9):1353–1367
Takeda S, Igoshi K, Tsend-Ayush C et al (2017) Lactobacillus paracasei strain 06TCa19 suppresses inflammatory chemokine induced by Helicobacter pylori in human gastric epithelial cells[J]. Hum Cell 30(4):258–266
Talebi BAA (2017) Helicobacter pylori treatment: New perspectives using current experience[J]. J Glob Antimicrob Resist 8:123–130
Talebi BAA, Taghvaei T, Wolfram L et al (2012) Infection with Helicobacter pylori strains lacking dupA is associated with an increased risk of gastric ulcer and gastric cancer development[J]. J Med Microbiol 61(Pt 1):23–30
Tang M, Chung P, Chan HY et al (2019) Recent trends in the prevalence of Helicobacter Pylori in symptomatic children: A 12-year retrospective study in a tertiary centre[J]. J Pediatr Surg 54(2):255–257
Thung I, Aramin H, Vavinskaya V et al (2016) Review article: the global emergence of Helicobacter pylori antibiotic resistance[J]. Aliment Pharmacol Ther 43(4):514–533
Tolone S, Pellino V, Vitaliti G et al (2012) Evaluation of Helicobacter Pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone[J]. Ital J Pediatr 38:63
Tsay FW, Hsu PIH (2018) pylori infection and extra-gastroduodenal diseases[J]. J Biomed Sci 25(1):65
Urrutia-Baca VH, Escamilla-Garcia E, de la Garza-Ramos MA et al (2018) In Vitro Antimicrobial Activity and Downregulation of Virulence Gene Expression on Helicobacter pylori by Reuterin[J]. Probiotics Antimicrob Proteins 10(2):168–175
Wang YH, Huang Y (2014) Effect of Lactobacillus acidophilus and Bifidobacterium bifidum supplementation to standard triple therapy on Helicobacter pylori eradication and dynamic changes in intestinal flora[J]. World J Microbiol Biotechnol 30(3):847–853
Wang ZJ, Chen XF, Zhang ZX et al (2017) Effects of anti-Helicobacter pylori concomitant therapy and probiotic supplementation on the throat and gut microbiota in humans[J]. Microb Pathog 109:156–161
Whiteside SA, Mohiuddin MM, Shlimon S et al (2021) In vitro framework to assess the anti-helicobacter pylori potential of lactic acid bacteria secretions as alternatives to antibiotics[J]. Int J Mol Sci 22(11):5650
Wieers G, Belkhir L, Enaud R et al (2019) How probiotics affect the microbiota[J]. Front Cell Infect Microbiol 9:454
Wiese M, Eljaszewicz A, Andryszczyk M et al (2012) Immunomodulatory effects of Lactobacillous plantarum and Helicobacter pylori CagA(+) on the expression of selected superficial molecules on monocyte and lymphocyte and the synthesis of cytokines in whole blood culture[J]. J Physiol Pharmacol 63(3):217–224
Wu L, Wang Z, Sun G et al (2019) Effects of anti-H. pylori triple therapy and a probiotic complex on intestinal microbiota in duodenal ulcer[J]. Sci Rep 9(1):12874
Xu C, Soyfoo DM, Wu Y et al (2020) Virulence of Helicobacter pylori outer membrane proteins: an updated review[J]. Eur J Clin Microbiol Infect Dis 39(10):1821–1830
Yan F, Polk DB (2020) Probiotics and Probiotic-Derived Functional Factors-Mechanistic Insights Into Applications for Intestinal Homeostasis[J]. Front Immunol 11:1428
Yarmohammadi M, Yadegar A, Ebrahimi MT et al (2021) Effects of a Potential Probiotic Strain Lactobacillus gasseri ATCC 33323 on Helicobacter pylori-Induced Inflammatory Response and Gene Expression in Coinfected Gastric Epithelial Cells[J]. Probiotics Antimicrob Proteins 13(3):751–764
Ye Q, Shao X, Shen R et al (2020) Changes in the human gut microbiota composition caused by Helicobacter pylori eradication therapy: a systematic review and meta-analysis[J]. Helicobacter 25(4):e12713
Yoon JY, Cha JM, Hong SS et al (2019) Fermented milk containing Lactobacillus paracasei and Glycyrrhiza glabra has a beneficial effect in patients with Helicobacter pylori infection: A randomized, double-blind, placebo-controlled study[J]. Medicine (baltimore) 98(35):e16601
Zagari RM, Romano M, Ojetti V et al (2015) Guidelines for the management of Helicobacter pylori infection in Italy: the III Working Group Consensus Report 2015[J]. Dig Liver Dis 47(11):903–912
Zagari RM, Frazzoni L, Marasco G et al (2021) Treatment of Helicobacter pylori infection: a clinical practice update[J]. Minerva Med 112(2):281–287
Zhang C, Zhang H, Yu L et al (2014) Helicobacter pylori dwelling on the apical surface of gastrointestinal epithelium damages the mucosal barrier through direct contact[J]. Helicobacter 19(5):330–342
Zhang MM, Qian W, Qin YY et al (2015) Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis[J]. World J Gastroenterol 21(14):4345–4357
Zhang J, Guo J, Li D et al (2020) The efficacy and safety of Clostridium butyricum and Bacillus coagulans in Helicobacter pylori eradication treatment: An open-label, single-arm pilot study[J]. Medicine 99(45):e22976
Zhao K, Xie Q, Xu D et al (2018) Antagonistics of Lactobacillus plantarum ZDY2013 against Helicobacter pylori SS1 and its infection in vitro in human gastric epithelial AGS cells[J]. J Biosci Bioeng 126(4):458–463
Zhou Q, Xue B, Gu R et al (2021) Lactobacillus plantarum ZJ316 Attenuates Helicobacter pylori-Induced Gastritis in C57BL/6 Mice[J]. J Agric Food Chem 69(23):6510–6523
Zhu XY, Liu F (2017) Probiotics as an adjuvant treatment in Helicobacter pylori eradication therapy[J]. J Dig Dis 18(4):195–202
Zhu XY, Du J, Wu J et al (2017) Influence of Saccharomyces boulardii Sachets combined with bismuth quadruple therapy for initial Helicobacter pylori eradication][J. Zhonghua Yi Xue Za Zhi 97(30):2353–2356
Zhu XY, Du J, Zhao WJ et al (2018) Influence of two kinds of probiotics combined with bismuth quadruple therapy for Helicobacter pylori eradication[J]. Zhonghua Yi Xue Za Zhi 98(28):2246–2249
Funding
This review was funded by the Guangzhou Clinical Major Technical Project; No. 2019ZD19.
Author information
Authors and Affiliations
Contributions
XB contributed to conceptualization; DT contributed to investigation; TW provided resources; XB and MZ contributed to writing—original draft preparation; JS and YH contributed to writing—review and editing; JS and YH contributed to visualization; YH contributed to supervision; JS administrated the project; JS acquired funding.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not available.
consent to participate
Not available.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bai, X., Zhu, M., He, Y. et al. The impacts of probiotics in eradication therapy of Helicobacter pylori. Arch Microbiol 204, 692 (2022). https://doi.org/10.1007/s00203-022-03314-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03314-w