Abstract
Annual mortality rates due to infectious diarrhea are about 2.2 million; children are the most vulnerable age group to severe gastroenteritis, representing group A rotaviruses as the main cause of disease. One of the main factors of rotavirus pathogenesis is the NSP4 protein, which has been characterized as a viral toxin involved in triggering several cellular responses leading to diarrhea. Furthermore, the rotavirus protein NSP1 has been associated with interferon production inhibition by inducing the degradation of interferon regulatory factors IRF3, IRF5, and IRF7. On the other hand, probiotics such as Bifidobacterium and Lactobacillus species in combination with prebiotics such as inulin, HMO, scGOS, lcFOS have been associated with improved generalized antiviral response and anti-rotavirus effect by the reduction of rotavirus infectivity and viral shedding, decreased expression of NSP4 and increased levels of specific anti-rotavirus IgAs. Moreover, these probiotics and prebiotics have been related to shorter duration and severity of rotavirus diarrhea, to the prevention of infection and reduced incidence of reinfections. In this review we will discuss in detail about the rotavirus pathogenesis and immunity, and how probiotics such as Lactobacillus and Bifidobacterium species in combination with prebiotics have been associated with the prevention or modulation of rotavirus severe gastroenteritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe diarrhea in the acute gastroenteritis is the primary cause of dehydration, which can lead to medical complications or death if left untreated (Hostetler et al. 2004). Annual mortality rates due to infectious diarrhea are about 2.2 million, and infants and very young children are the age group most vulnerable to severe gastroenteritis (Boschi-Pinto et al. 2008); children mortality were 578,000 worldwide (Liu et al. 2015). Viruses are the major agents of acute gastroenteritis in children up to 5-years-old (Chhabra et al. 2013). The most reported viruses associated with gastrointestinal infections are rotavirus (RV), norovirus, sapovirus, enteric adenovirus, and astrovirus (Elliott 2007). RV is the main cause of gastroenteritis in children; this virus is responsible for 453,000 deaths of children worldwide (Tate et al. 2012). The second place in the list of agents of acute viral gastroenteritis in children is for norovirus, which is related to 218,000 children deaths worldwide (Koo et al. 2010). Enteric adenovirus, sapovirus, and astrovirus have been detected in children up to 5-years-old with severe and mild gastroenteritis (Finkbeiner et al. 2009; Rezaei et al. 2012; Sdiri-Loulizi et al. 2011). Other viruses such as aichi virus, parechovirus, and bocavirus have been related to cases of acute diarrhea. Nevertheless, their participation as gastrointestinal pathogens remains unclear (Chhabra et al. 2013).
Rotavirus
RV is a member of the genus Rotavirus within the family Reoviridae; mature viral particles are about 70–100 nm in diameter and possess a triple-layered icosahedral protein capsid composed of an outer layer, an intermediated layer, and an inner core layer. The RV genome contains 11 segments of double-stranded RNA (dsRNA), segments which encode six structural proteins (VP1–VP4, VP6, and VP7) and six non-structural proteins (NSP1–NSP5/NSP6) (Estes and Greenberg 2013). RV is classified in eight distinct groups (A to H), RVs A, B, and C are found in both humans and animals, whereas D, E, F, G, and H have been only found in animals (Matthijnssens et al. 2012).
RV causes significant diarrheal disease in infants and young of various mammalian and avian species (Estes and Greenberg 2013). Within RV, viruses are classified into serotypes and genotypes. The binary classification for RV is based on distinct types of the structural proteins in the external capsid VP7 (genotype G) and VP4 (genotype P). In 2008, a complete genome classification system was developed to RVA that assigns a specific genotype to each of the 11 genomic segments according to established nucleotide percent cutoff values (Matthijnssens et al. 2008). Most of the human RV associated to diarrheic disease worldwide are G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] with emerging genotypes such as G9 and G12 (Rahman et al. 2007; Santos and Hoshino 2005). These common human RVs may co-circulate within a single season which would be favorable for the formation of reassortant viruses and thereby to the genetic diversity of RV (Jain et al. 2014).
Rotavirus pathogenesis
RVs infection and replication are primarily in the nondividing, mature enterocytes near the tips of the small intestinal villi (Estes and Greenberg 2013). Nevertheless, RV infection may not be limited to the gut; recently, several cases of antigenemia and viremia have been reported, although the impact of systemic RV on disease burden remains to be determined (Blutt and Conner 2007; Estes and Greenberg 2013). The human RV pathogenesis is still unclear, some studies in volunteers, with animal models and recently in a novel in vitro human intestinal enteroids model (Saxena et al. 2016) point that the viral pathogenesis may be multifactorial and associated with several factors such as: (a) the viral infection to mature enterocytes in the lining of the gastrointestinal tract is related to enterocyte vacuolization and loss, crypt hyperplasia and villous blunting, which is associated with malabsorption by intestine; although, the presence of symptoms of such diarrhea has been reported before the epithelial damage is detected (Jourdan et al. 1997), (b) the activity of the RV non-structural protein NSP4 (Fig. 1), which has been characterized as a viral toxin inducing Ca2+-dependent Cl− secretion associated with the inhibition of the Na+/glucose-cotransporter SGLT1, and alterations in cytoskeletal structure, in the integrity of the tight junctions and the regulation of Na+/K pump (Ball et al. 1996; Lundgren and Svensson 2001; Ousingsawat et al. 2011). This intracellular dysregulation in the enterocyte, together with the decreased expression of digestive enzymes, glucose malabsorption and activation of cystic fibrosis conductance regulator (CFTR)-independent Cl− secretion, may be the cause of diarrhea (Ousingsawat et al. 2011), (c) the enteric nervous system is associated with RV secretory diarrhea and increased intestinal motility, the evidence of this association is the modulation effect of drugs that block this pathway in RV-induced diarrhea (Lundgren et al. 2000), (d) other factor in viral pathogenesis is the ability of RV to infect enterochromaffin cells (EC), as consequence serotonin (5-hydroxytryptamine) is released from EC and acts through the enteric nervous system inducing activation of vagal afferent nerves to brain structures associated with nausea and vomiting (Hagbom et al. 2011).
Model of RV infection and pathogenesis: virus entry, formation of viroplasms and replication, and release of virions and viral proteins such as NSP4; this protein mobilizes intracellular calcium from endoplasmic reticulum (ER). Released NSP4 affects uninfected cells, resulting in mobilization of intracellular calcium by a PLC-dependent pathway that activates chloride secretion. Tight junctions can also be disrupted by RV infection and by the NSP4 activity; the disruption of epithelial cell structure may lead to cell death and alterations of the paracellular pathway of fluid movement. RV infection activates epithelial cell signaling resulting in the activation of the enteric nervous system, intestinal secretion, and immune responses (Estes and Greenberg 2013; Ramig 2004)
Rotavirus immunity
The mechanisms responsible for generating protective immunity to RV infections and illness following natural infection are not completely understood, particularly in humans where it is difficult to study the acquired cellular immune response in young children due to limitations with timely and sufficient specimens (Estes and Greenberg 2013). Most of the knowledge about the immune response to RV has been studied in several animal models, but the most used are mice and pigs (Estes and Greenberg 2013). B or T cells knockout mice were observed to be chronically infected with RV; the same effect has been described in children with B or T cells immunodeficiency (Chhabra et al. 2013; Williams et al. 1998). CD4+ cells are critical for the establishment of protective long-term memory responses and important for the development of 90% of the RV-specific IgA (Kuklin et al. 2001). On the other hand, CD8+ T cells are associated with short-term protection against RV reinfection and with timely resolution of primary RV infection (Jiang et al. 2008). In the same animal model, intestinal tract homing of both B and T cells plays a major role in promoting RV immunity mediated by the integrin α4β7 and CCR9 (Jiang et al. 2008; Kuklin et al. 2001; Williams et al. 1998).
Innate immune response and evasive strategies of rotavirus
In the absence of T cell help, a protective B cell response is present; nevertheless, this response is reduced compared with wild-type mice, and T cells can mediate their effect against RV infection in the absence of perforin, Fas, and interferon γ (Franco et al. 1997, 2006; Gilger et al. 1992). Apparently, T cells can clear infection more quickly and efficiently than B cells. CD8+ T cells can mediate primary RV infection and almost complete or partial protection from reinfection (Estes and Greenberg 2013).
On the other hand, RV has developed multiple mechanisms to evade the innate immune response, particularly the interferon response (INF). The protein NSP1 has been characterized as an inhibitor of interferon (INF) production by inducing the degradation of interferon regulatory factor IRF 3, IRF5 and IRF7 in a host cell-dependent process (Fig. 2) (Arnold and Patton 2011). Due to the loss of IRF3, the expression of IFN-β is suppressed, the degradation of IRF5 is associated with the down-regulation of the activation of genes producing proinflammatory cytokines. Finally, the degradation of IRF7 is related to the decreased expression of type I IFN and to an altered activation of IFN-α genes (Barro and Patton 2007). NSP1 also mediate degradation of β-TrCP and inhibition of NFκB activation (Morelli et al. 2015). All these effects depend of the RV strain, and cell type, NSP1 from some animal RV degrade IRF3, IRF5, and IRF7; nevertheless, human RV NSP1 only degrades IRF5 and IRF7, which may result in less efficient inhibition of IFN response (Arnold and Patton 2011). NSP1 has also been associated with the degradation of other proteins such as the pattern recognition receptor (cytosolic receptor) known as retinoic acid-inducible gene I (RIG-I); TNF receptor-associated factor 2 (TRAF2), and the mitochondrial antiviral signaling protein (MAVS, also known as IPS-1, VISA, and Cardif). These data indicate that NSP1 can block innate immune signaling at both the transcriptional (IRF, NF-κB) and at pattern recognition receptor (PRR) level, but not signaling through the TLR3/TRIF pathway or PKR (Broquet et al. 2011). On the other hand, RV activates the PI3K/Akt pathway to prevent premature apoptosis, and it is also related to the post-transcriptional depletion of p53, possibly through the NSP1 activity; as a result, early cell apoptosis is prevented (Bagchi et al. 2010; Bhowmick et al. 2013).
Rotavirus interactions with the host innate system: viral entry into cells and viral double strand RNA (dsRNA) induce the generation of pathogen-associated molecular pathways (PAMPs). As a result, cytosolic pathogen recognition receptors (PRRs), such as RIG-I and MDA-5 are activated, leading to mitochondrial-associated adaptor protein MAVS-dependent activation of transcription factor IRF3/IRF7. Activated IRF3/IRF7 translocates to nucleus, where it induces the transcription of several genes resulting in the transcription and expression of IFN-α/β. IFN secretion from rotavirus infected cells results in the establishment of antiviral state in bystander cells, mediated by signaling through the transcription factors STAT1, STAT2, and IRF9. The viral protein NSP1 induces proteosomal degradation of RIG-I, MAVS, IRF3, and IRF7. On the other hand, the induction of IFN in rotavirus infected cells also requires nuclear factor κβ (NF-κβ), following the proteosomal degradation of its inhibitory partner IκB-α; NSP1 can block this pathway by inducing the proteosomal degradation of β-TrCP, which is an essential co-factor for IκB-α degradation. As a result, NSP1 affects the quality and intensity of the interferon response (Arnold and Patton 2011; Estes and Greenberg 2013)
Probiotics and prebiotics vs rotavirus gastroenteritis
Current treatment of RV gastroenteritis consists of oral rehydration (oral rehydration solutions, ORS) to replace fluids and electrolytes lost by vomiting and diarrhea. Zinc supplementation improves the oral rehydration, and it is recommended by the WHO for children with acute gastroenteritis. Several other additives to the ORS formulation are currently under investigation; these include lactoferrin and lysozyme and various amino acids including glycine, alanine, and glutamine (Estes and Greenberg 2013). Additionally, the RV vaccines (Rotarix and RotaTeq) have shown to be safe and effective in the prevention of RV severe gastroenteritis. Nevertheless, they are not globally implemented due to their cost, storage and transport requirements (at 2–8 °C) and because of the lower protection offered in developing countries (Bines and Kirkwood 2015). Moreover, RV gastroenteritis seems to be modulated by nutritional interventions such as bioactive components of breast milk, probiotics or prebiotics (Rigo-Adrover et al. 2016).
Probiotics such as Lactobacillus and Bifidobacterium species, and Saccharomyces boulardii have been associated with the prevention of RV infection, to shorter duration and severity of RV diarrhea, to reduced incidence of reinfections and to the modulation of the immune response and viral shedding (Das et al. 2016; Lee et al. 2015; Maragkoudakis et al. 2010; Rigo-Adrover et al. 2017; Varyukhina et al. 2012).
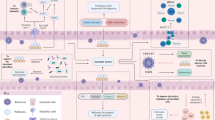
Out of the reported probiotics showing potential as gut pathogens antagonists, some species of Lactobacillus and Bifidobacterium are commonly reported worldwide (Servin 2004). Focusing against RV, an in vivo evaluation on mouse demonstrated that oral administration of Bifidobacterium breve strongly protected against RV-induced diarrhea, thus observing an anti-RV IgA level increase in feces, mammary gland and intestine of treated mouse (Yasui et al. 1995). In other murine models, pathogen-free rats infected with SA11 RV strain and orally treated with L. casei, small intestine lesions, and RV infection level were reduced in all intestine sections, as well as diarrhea (Guérin-Danan et al. 2001). In vitro and in vivo studies revealed that some of the mechanisms of probiotics against RV infection are the production of antimicrobial substances (lactic acid, nitric oxide, H2O2 and bacteriocins), stimulation of antimicrobial peptides, mucin production by epithelial cells, stimulation of local adaptive (specific IgA response), and innate immune responses (Fig. 3) (Gänzle et al. 2000; Kaila et al. 1995). Moreover, Lactobacillus and Bifidobacterium species have been associated to the stimulation of production of cytokines IL25, IL33, TGF by intestinal cells; IL22, by innate immune cells; IL12, IL25, IL10 and TGF, by antigen-presenting cells; resulting in improved intestinal barrier function, reduced effector and increased regulatory immune responses (Vlasova et al. 2016).
Prebiotics, probiotics, and gut immunity: interaction of prebiotics and probiotics such as Lactobacillus and Bifidobacterium species and the immune system, described from in vitro and in vivo assays with mice and gnotobiotic pigs. Prebiotics such as HMO, scGOS or lcFOS together with probiotics Lactobacillus and Bifidobacterium may improve the immune response against enteric pathogens. These probiotics inhibit some viruses by producing lactic acid, H2O2, NO, short-chain fatty acids (SCFA), bacteriocins, promotes and preserve the integrity of the epithelium, and compete with pathogens for intestinal epithelial cell (Vlasova et al. 2016)
On the other hand, prebiotics such as the sialic acid containing human milk oligosaccharides (HMO) has been associated to in vitro reduced RV infectivity and replication (Hester et al. 2013). HMO have also been associated with the reduction of the duration of RV diarrhea in piglets by modulating colonic microbiota and immune response to RV infection (Li et al. 2014). Moreover, a mixture of short-chain galactooligosaccharides (scGOS), long-chain fructooligosaccharides (lcFOS) and Bifidobacterium breve showed protection against RV infection in suckling rats (Rigo-Adrover et al. 2016). In children with acute RV gastroenteritis, the oral administration of a mixture of Bifidobacterium lactis B94 and inulin as prebiotic showed a shorter duration of RV acute watery diarrhea (İşlek et al. 2014). On the other hand, a mixture of prebiotics such scGOS, lcFOS and pectin-derived acidic oligosaccharides mixture and heat-treated probiotics in fermented milk components in RV-induced diarrhea in suckling rats was associated with a decreased viral shedding and reduced clinical signs (Rigo-Adrover et al. 2017).
Although the probiotics and prebiotics mechanisms against RV are not well defined yet, there is some recent evidence about the beneficial effect of them in the viral pathogenesis and immune response modulation (Table 1). The activity of probiotics and prebiotics against RV pathogenesis may be attributable to decreased viral shedding possibly due to the interaction of probiotics (or their metabolites) and prebiotics with the viral particles avoiding the entry into enterocytes and as a consequence reducing the RV replication (Rigo-Adrover et al. 2017). Moreover, the in vitro effect of metabolites of Lactobacillus casei, and Bifidobacterium adolescentis was associated with a reduced expression of the RV enterotoxin NSP4 and reduced levels of Ca2+ liberation suggesting that cell will not reach the electrolyte imbalance caused by this pathway (Olaya Galán et al. 2016). On the other hand, the modulation of RV immune response by probiotics and prebiotics has been associated with a generalized antiviral response via pattern recognition receptor signaling and through promoting type I IFNs, which are key regulators of IFN signaling pathway (Ishizuka et al. 2016; Kang et al. 2015). Bifidobacterium infantis MCC12 and Bifidobacterium breve MCC1274 have been associated with a significant reduction of RVs titers in infected porcine intestinal epithelial cells (PIE); the beneficial effects of both bifidobacteria were associated with the reduction of A20 expression and improvements of IRF-3 activation, IFN-ß production, and MxA and RNase L expressions. The reduction of A20 is associated with the IFN stimulation response and IFN promoter dependent transcription by physically interacting with NF-κB-activating kinase/Traf family member-associated NFκB activator-binding kinase 1 and IKK-i/IKKe, and inhibiting dimerization of IRF-3 following engagement of TLR3 by dsRNA. In this regard, the up-regulation of MxA inhibits viruses by sequestering the newly synthesized viral proteins, and RNase L would be related to the lower RVs replication (Ishizuka et al. 2016). Thus, probiotics and prebiotics would be associated with generalized antiviral effect and to specific anti-RV activity.
Conclusion
RV is the main cause of severe gastroenteritis in children up to 5-years-old worldwide. The current progress described in this review is the description of the strains of probiotics with the best effect against the RVs gastroenteritis, and how their effect may be improved by the presence of prebiotics such as inulin, HMO, scGOS, lcFOS, pectin-derived acidic oligosaccharides mixture and heat-treated probiotics in fermented milk components. Although more evidence is needed to support the beneficial effects and the mechanisms of prebiotics and probiotics against RV gastroenteritis severity; it is possible that the beneficial activity of probiotics and prebiotics are associated to: (a) the improvement in the intestinal microenvironment and the healthy intestinal microbiota balance strengthen the intestinal epithelial barrier, (b) the interaction of both probiotic (metabolites) or prebiotic with viral particles avoiding the RV cell entry, (c) increased generalized antiviral response, (d) decreased expression of the viral enterotoxin NSP4 and possibly of NSP1 and (e) the increased levels of specific anti-RVs IgAs. Together, all these factors would be associated to decreased RV infectivity, viral shedding, to shorter duration and severity of RV diarrhea, to the prevention of RV infection and reduced incidence of reinfections. Moreover, further studies are needed for the elucidation of the mechanisms of action of probiotics/prebiotics mixtures against RV severe gastroenteritis and the implementation of the effective and safe use of probiotic/prebiotics as preventive and therapeutic strategies in the management of RV gastroenteritis.
References
Arnold MM, Patton JT (2011) Diversity of interferon antagonist activities mediated by NSP1 proteins of different rotavirus strains. J Virol 85:1970–1979
Bagchi P et al (2010) Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate viral growth by activating the cell survival pathways during early stages of infection. J Virol 84:6834–6845
Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK (1996) Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101
Barro M, Patton JT (2007) Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J Virol 81:4473–4481
Bhowmick R, Halder UC, Chattopadhyay S, Nayak MK, Chawla-Sarkar M (2013) Rotavirus-encoded nonstructural protein 1 modulates cellular apoptotic machinery by targeting tumor suppressor protein p53. J Virol 87:6840–6850
Bines JE, Kirkwood CD (2015) Conquering rotavirus: from discovery to global vaccine implementation. J Paediatr Child Health 51:34–39
Blutt SE, Conner ME (2007) Rotavirus: to the gut and beyond! Curr Opin Gastroenterol 23:39–43
Boschi-Pinto C, Velebit L, Shibuya K (2008) Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ 86:710–717
Broquet AH, Hirata Y, McAllister CS, Kagnoff MF (2011) RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol 186:1618–1626
Chhabra P et al (2013) Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J Infect Dis 208:790–800
Das S, Gupta PK, Das RR (2016) Efficacy and safety of Saccharomyces boulardii in acute rotavirus diarrhea: double blind randomized controlled trial from a developing country. J Trop Pediatr 62:464–470
Elliott EJ (2007) Acute gastroenteritis in children. BMJ Br Med J 334:35–40
Estes M, Greenberg H (2013) Rotaviruses. In: Knipe DM, Howley PM (eds) Fields virology. Lippincott Williams & Wilkins, Philadelphia
Finkbeiner SR et al (2009) Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J 6:161
Franco MA, Tin C, Rott LS, VanCott JL, McGhee JR, Greenberg HB (1997) Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon. J Virol 71:479–486
Franco MA, Angel J, Greenberg HB (2006) Immunity and correlates of protection for rotavirus vaccines. Vaccine 24:2718–2731
Gänzle MG, Höltzel A, Walter J, Jung G, Hammes WP (2000) Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl Environ Microbiol 66:4325–4333
Gilger M, Matson D, Conner M, Rosenblatt H, Finegold M, Estes M (1992) Extraintestinal rotavirus infections in children with immunodeficiency. J Pediatr 120:912–917
Guérin-Danan C et al (2001) Food supplementation with milk fermented by Lactobacillus casei DN-114 001 protects suckling rats from rotavirus-associated diarrhea. J Nutr 131:111–117
Hagbom M et al (2011) Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 7:e1002115
Hester SN et al (2013) Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br J Nutr 110:1233–1242
Hostetler MA, Nakanishi AK, Whiteman PJ (2004) Gastroenteritis: an evidence-based approach to typical vomiting, diarrhea, and dehydration. Pediatr Emerg Med Pract 1:1–19
Ishizuka T et al (2016) Immunobiotic bifidobacteria strains modulate rotavirus immune response in porcine intestinal epitheliocytes via pattern recognition receptor signaling. PLoS One 11:e0152416
İşlek A, Sayar E, Yılmaz A, Baysan BÖ, Mutlu D, Artan R (2014) The role of Bifidobacterium lactis B94 plus inulin in the treatment of acute infectious diarrhea in children. Turk J Gastroenterol 25:628–633
Jain S, Vashistt J, Changotra H (2014) Rotaviruses: is their surveillance needed? Vaccine 32:3367–3378
Jiang JQ, He X-S, Feng N, Greenberg HB (2008) Qualitative and quantitative characteristics of rotavirus-specific CD8 T cells vary depending on the route of infection. J Virol 82:6812–6819
Jourdan N, Maurice M, Delautier D, Quero AM, Servin AL, Trugnan G (1997) Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J Virol 71:8268–8278
Kaila M, Isolauri E, Saxelin M, Arvilommi H, Vesikari T (1995) Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Arch Dis Child 72:51–53
Kang JY, Lee DK, Ha NJ, Shin HS (2015) Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J Microbiol 53:796–803
Koo HL, Ajami N, Atmar RL, DuPont HL (2010) Noroviruses: the leading cause of gastroenteritis worldwide. Discov Med 10:61–70
Kuklin NA, Rott L, Feng N, Conner ME, Wagner N, Müller W, Greenberg HB (2001) Protective intestinal anti-rotavirus B cell immunity is dependent on α4β7 integrin expression but does not require IgA antibody production. J Immunol 166:1894–1902
Lee DK, Park JE, Kim MJ, Seo JG, Lee JH, Ha NJ (2015) Probiotic bacteria, B. longum and L. acidophilus inhibit infection by rotavirus in vitro and decrease the duration of diarrhea in pediatric patients. Clin Res Hepatol Gastroenterol 39:237–244
Li M et al (2014) Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J 8:1609–1620
Liu L et al (2015) Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385:430–440
Lundgren O, Svensson L (2001) Pathogenesis of rotavirus diarrhea. Microbes Infect 3:1145–1156
Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L (2000) Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287:491–495
Maragkoudakis PA, Chingwaru W, Gradisnik L, Tsakalidou E, Cencic A (2010) Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol 141:S91–S97
Matthijnssens J et al (2008) Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol 82:3204–3219
Matthijnssens J, Otto PH, Ciarlet M, Desselberger U, Van Ranst M, Johne R (2012) VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch Virol 157:1177–1182
Morelli M, Dennis AF, Patton JT (2015) Putative E3 ubiquitin ligase of human rotavirus inhibits NF-κB activation by using molecular mimicry to target β-TrCP. MBio 6:e02490–e02514
Olaya Galán NN, Ulloa Rubiano JC, Velez Reyes FA, Duarte F, Karem P, Salas Cárdenas SP, Gutierrez Fernandez MF (2016) In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP4 protein production. J Appl Microbiol 120:1041–1051
Ousingsawat J, Mirza M, Tian Y, Roussa E, Schreiber R, Cook DI, Kunzelmann K (2011) Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflugers Arch 461:579–589
Rahman M et al (2007) Evolutionary history and global spread of the emerging G12 human rotaviruses. J Virol 81:2382–2390
Ramig RF (2004) Pathogenesis of intestinal and systemic rotavirus infection. J Virol 78:10213–10220
Rezaei M, Sohrabi A, Edalat R, Siadat SD, Gomari H, Rezaei M, Gilani SM (2012) Molecular epidemiology of acute gastroenteritis caused by subgenus F (40, 41) enteric adenoviruses in inpatient children. Lab Med 43:10–15
Rigo-Adrover M et al (2016) A combination of scGOS/lcFOS with Bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur J Nutr 56:1657–1670
Rigo-Adrover M et al (2017) A fermented milk concentrate and a combination of short-chain galacto-oligosaccharides/long-chain fructo-oligosaccharides/pectin-derived acidic oligosaccharides protect suckling rats from rotavirus gastroenteritis. Br J Nutr 117:209–217
Santos N, Hoshino Y (2005) Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 15:29–56
Saxena K et al (2016) Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 90:43–56
Sdiri-Loulizi K et al (2011) Molecular detection of genogroup I sapovirus in Tunisian children suffering from acute gastroenteritis. Virus Genes 43:6–12
Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440
Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD (2012) 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 12:136–141
Varyukhina S et al (2012) Glycan-modifying bacteria-derived soluble factors from Bacteroides thetaiotaomicron and Lactobacillus casei inhibit rotavirus infection in human intestinal cells. Microbes Infect 14:273–278
Vlasova AN, Kandasamy S, Chattha KS, Rajashekara G, Saif LJ (2016) Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol 172:72–84
Williams M, Rosé J, Rott L, Franco M, Greenberg H, Butcher E (1998) The memory B cell subset that is responsible for the mucosal IgA response and humoral immunity to rotavirus expresses the mucosal homing receptor, α 4 β 7. J Immunol 161:4227–4235
Yasui H, Kiyoshima J, Ushijima H (1995) Passive protection against rotavirus-induced diarrhea of mouse pups born to and nursed by dams fed Bifidobacterium breve YIT4064. J Infect Dis 172:403–409
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gonzalez-Ochoa, G., Flores-Mendoza, L.K., Icedo-Garcia, R. et al. Modulation of rotavirus severe gastroenteritis by the combination of probiotics and prebiotics. Arch Microbiol 199, 953–961 (2017). https://doi.org/10.1007/s00203-017-1400-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1400-3