Abstract
Summary
This study assessed the lifetime cost-effectiveness of a fracture liaison service (FLS) compared to no-FLS in the Netherlands from a societal perspective and suggested that FLS was cost-effective in patients with a recent fracture aged 50 years and older. The implementation of FLS could lead to lifetime health-economic benefits.
Introduction
The objective of this study was to investigate the lifetime cost-effectiveness of a fracture liaison service (FLS) compared to no-FLS in the Netherlands from a societal perspective and using real-world data.
Methods
Annual fracture incidence, treatment scenarios as well as treatment initiation in the years 2017–2019 were collected from a large secondary care hospital in the Netherlands. An individual-level, state transition model was designed to simulate lifetime costs and quality-adjusted life years (QALYs). Treatment pathways were differentiated by gender, presence of osteoporosis and/or prevalent vertebral fracture, and treatment status. Results were presented as incremental cost-effectiveness ratios (ICER). Both one-way and probabilistic sensitivity analyses were conducted.
Results
For patients with a recent fracture aged 50 years and older, the presence of an FLS was associated with a lifetime €45 higher cost and 0.11 additional QALY gained leading to an ICER of €409 per QALY gained, indicating FLS was cost-effective compared to no-FLS at the Dutch threshold of €20,000/QALY. The FLS remained cost-effectiveness across different age categories. Our findings were robust in all one-way sensitivity analyses, the higher the treatment initiation rate in FLS, the greater the cost-effective of FLS. Probabilistic sensitivity analyses revealed that FLS was cost-effective in 90% of the simulations at the threshold of €20,000/QALY, with women 92% versus men 84% by gender.
Conclusion
This study provides the first health-economic analysis of FLS in the Netherlands, suggesting the implementation of FLS could lead to lifetime health-economic benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fractures are associated with pain, disability, loss of independence, reduced quality of life, increased subsequent fracture risk and excess mortality, resulting in a substantial and escalating healthcare and financial burden for the society. In the Netherlands, as reported by the SCOPE (Scorecard for Osteoporosis in Europe) 2021 study [1], the number of fragility fractures was estimated at 99,600 in 2019, corresponding to 273 fractures per day and 11 fractures per hour, accounting for approximately 1.8% of healthcare spending (i.e., €1.4 billion out of €75.0 billion in 2019). The projected number of fragility fractures in 2034 is 137,000, suggesting an increase of 37.4% over a 15-year interval. A prior fracture is a strong predictor of subsequent fracture as reported by a Dutch study [2] with the relative risk of subsequent fracture ranging from 5.3 within 1 year to 1.4 between 6 and 10 years after the first fracture in postmenopausal women older than 50 years compared to those without a recent fracture. Recurrent fractures are partly preventable by drug therapy and to a lesser extent by non-pharmacological interventions such as lifestyle changes. Although the high risk of subsequent fractures was acknowledged, the magnitude of drug treatment gap (defined as the percentage of persons who are eligible for treatment but not receiving a treatment) is reported to be highly variable throughout Europe, ranging between 25 and 95% [3], which was estimated to vary from 60 to 72% in the Dutch population [4]. In response to the treatment gap, post-fracture care programs such as fracture liaison services (FLS) were introduced, which is considered as the most effective organizational structure for secondary fracture prevention.
FLSs were first reported by McLellan et al. in 2003 [5] and internationally endorsed by the International Osteoporosis Foundation (IOF) [6], the European Alliance of Associations for Rheumatology (EULAR) [7], the multidisciplinary Fragility Fracture Network (FFN) [8], and the American Society of Bone and Mineral Research ASBMR) [9]. In the Netherlands, the guideline on osteoporosis and fracture prevention (2011) [10] recommends to evaluate all fracture patients of 50 years or older in preferentially a nurse-led structured program. The first FLS-related initiatives and outcomes were reported from Groningen in 2004 [11], and the FLS in VieCuri Medical Centre of Venlo was launched in 2008. To optimize FLS initiatives and facilitate the communication between healthcare professionals, a formal national network (Dutch Osteoporosis Nurses Association VF&O) [12] was launched in 2008, and a five-step approach has been proposed by van den Bergh et al. [13] in 2012 to strive for standardized FLS care. With emphasizing the importance of initiating FLS in hospitals by several Dutch scientific committees, there were 90 FLS and 95 osteoporosis nurses registered in the database of VF&O as reported by a study published in 2015 [14].
However, the intensity and quality of implementation of FLS vary between hospitals and countries [15, 16]; patient identification and selection differed markedly among FLS in terms of proportion of in- and outpatients with a fracture included, age, the inclusion of women and/or men, and fracture site (any fracture or only patients with a nonvertebral fracture) [17], potentially leading to different clinical and economic outcomes. Worldwide, with the increasing implementation of FLS, the effectiveness and efficacy of FLS was reported in many countries and summarized in several systematic reviews and meta-analyses [18,19,20], suggesting that FLS care is generally (cost-)effective for healthcare systems by improving patient care, reducing secondary fracture rates, and ultimately decreasing the burden on the healthcare system and society. However, we found most published economic evaluations used simulation model assessing the cost-effectiveness of FLS without use of real-world data. Considering most Dutch hospitals initiated an FLS, its cost-effectiveness remains unknown. The objective of this study was therefore to investigate the lifetime societal cost-effectiveness of an FLS compared to no-FLS in the Netherlands from a societal perspective using real-world data whenever possible.
Methods
We adapted a previously validated Markov microsimulation model [21] to estimate the cost-effectiveness of FLS compared to no-FLS for patients with a recent fracture from the Dutch societal perspective with a lifetime horizon. Treatment pathways in our model were based on Dutch guidelines on osteoporosis and fracture prevention [10, 22], recommending anti-osteoporosis drug treatment in those having osteoporosis (bone mineral density BMD) T-score ≤ − 2.5 standard deviations at the lumbar spine, femoral neck or total hip, and/or a clinical or prevalent vertebral fracture (VF) (> 25% reduction in vertebral body height at the anterior, mid, or posterior location) [23]) combined with a BMD T-score ≤ − 1.
The model was built up using TreeAge Pro 2022 software (TreeAge Pro Inc., Williamston, MA, USA) and adhered to the osteoporosis-specific guideline of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases and the US branch of the International Osteoporosis Foundation (IOF-ESCEO) for the design, conduct, and reporting of economic evaluations [24], and also to the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement [25]. Details of these two checklists can be found in Appendices I and II of the Supplementary information. A description of the source of real-world data, target population, model structure and input data is provided here below. Table 1 presents key model input parameters.
Real-world FLS setting
Our model structure was adapted to a real-world FLS setting (VieCuri Medical Centre, a large secondary care hospital in the Netherlands) where a nurse, specialized in osteoporosis, invites patients aged 50 years and older, who visited the emergency department because of a recent fracture, to the FLS. Details of this FLS care pathway were published previously [26]. In brief, patients attending the FLS were scheduled for an outpatient visit including dual X-ray absorptiometry (DXA) measurement to assess BMD, prevalent VF based on vertebral fracture assessment (VFA), and a blood test. Lifestyle advice and drug treatment (when applicable), based on presence of osteoporosis and/or prevalent VF according to Dutch guideline [10]. Patients attending the FLS who are diagnosed with osteoporosis or vertebral fractures will initiate anti-osteoporosis treatment and be scheduled for a follow-up consultation of the FLS and be advised to have a yearly extra general practitioner (GP) visit during their treatment period after the first year in FLS.
As part of the FLS care, real-world data in this study were collected in the years 2017–2019 including annual fracture incidence (by age, gender, and fracture type categories), treatment scenarios (pharmacy data) as well as treatment initiation after (2017–2019) the implementation of FLS from the VieCuri Medical Centre.
Population
Analyses were conducted in patients with an index hip, clinical vertebral (CV) or non-hip non-vertebral (NHNV) fracture aged 50 years and older. Both genders were included considering differences in various model input parameters. Based on 3-year (2017–2019) data from VieCuri Medical Centre, the population in our study entered the model with a distribution of starting age (i.e., 11%, 13%, 13%, 13%, 13%, 11%, 10%, 8%, and 5% patients had a starting age between 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85–89, and 90 + years, respectively), with women accounting for 67%.
Model structure and treatment pathways
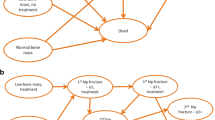
The model structure combines a decision tree with a Markov model. The decision tree (Fig. 1) distinguished groups by the presence of FLS, gender, presence of osteoporosis or VF, and treatment with anti-osteoporosis drugs in this with osteoporosis and/or VF (osteoporosis and/or VF + treatment, osteoporosis and/or VF + no treatment, no osteoporosis & no VF) that is in consistent with treatment indications suggested by Dutch guideline [10]. Patients entered both FLS and no-FLS branches have identical gender distribution and prevalence of osteoporosis and/or VF. A higher proportion of treatment initiation was modelled for FLS (40%) compared to no-FLS (5%) branch based on real-world data [27] and expert opinion, respectively.
Of note, all patients (the combination of attenders and non-attenders) were included in the FLS branch. To adjust for prognostic differences between attenders and non-attenders, we reduced the future fracture and mortality risk of the attenders who had a hip and vertebral fracture compared to the non-attenders. This is supported by a recent systematic review and meta-analysis [18] and a Dutch study (patients in VieCuri Medical Center as our study) [26], details can be found in the description of model input data section.
After allocating persons to sub-branches of the decision tree, patients entered the Markov model (Fig. 2) one-by-one to capture the long-term costs and health benefits (expressed as quality-adjusted life year, QALY). An individual-level state transition model was used to track individual trajectories (incorporating the impact of history on future events), and tracker variables were used to record the number and type of subsequent fractures. Each patient began in the “index fracture” state (a recent fracture) and had a probability of having a new (subsequent) hip fracture, clinical CV, or NHNV fracture or of dying. Patients in a subsequent fracture state can stay in the same fracture state if they re-fracture, change to another fracture state, die, or change in the next cycle to the post subsequent fracture state. Patients in a post subsequent fracture state might have another fracture at any site, move to “recent fracture,” or die. We used a lifetime horizon and a 6-month cycle as recommended by IOF-ESECO guideline [24]. Discount rate of 4% and 1.5% was used as recommended by the Dutch guideline for economic evaluations in healthcare [28] for costs and QALYs, respectively.
Model input data
Osteoporosis, clinical, or prevalent vertebral fracture, fracture risk
The prevalence of osteoporosis and/or clinical or prevalent VF was estimated at 49% based on a Dutch study [27] which included consecutive patients aged 50 years and older with a recent non-VF visiting the FLS of VieCuri Medical Centre.
When entering the Markov model, the annual incidence of hip, CV fracture, and NHNV fracture in the general Dutch population were obtained and estimated from fracture data in VieCuri Medical Centre in the years 2017–2019. Considering the presence of osteoporosis and/or previous fracture without treatment is associated with higher subsequent fracture risk, adjustment were made to reflect the increased fracture risk. Time-dependent relative risk (RR) of subsequent fracture was modelled as reported in Dutch studies [2, 29], i.e., the pooled RR for women was 2.1 (1.7–2.6), ranging from 5.3 (4.0–6.6) within 1 year to 1.4 (1.0–1.8) within 6–10 years; 1.5 times increased risk in men relative to women was modelled; no increased risk was assumed (RR = 1) after 10 years for both genders. In addition, to take into account the impact of osteoporosis, the increased risk of subsequent fracture for persons with osteoporosis relative to persons without osteoporosis was also modelled. Specifically, for patients with osteoporosis aged 50–59, 60–69, 70–79, and over 80 years, the RRs of having a hip fracture were estimated at 5.66, 3.39, 2.25, and 1.57, respectively. The RR for CV fracture ranged from 2.68 at 50 years to 1.51 at 100 years, which were slightly higher than the RRs for NHNV fracture [30]. As fractures could be non-osteoporosis related (especially non-hip non-vertebral fracture NHNV), an adjustment was made to only include osteoporotic fractures in the model. Specifically, the osteoporosis-related probability of future fracture was dependent on fracture type, age and gender. For female patients with an index hip fracture at the age of 50–64 years, 65–84 years, and 85 + years, the probability of 0.8, 0.9, and 0.95 were modelled, respectively. Compared to hip and vertebral fracture, the osteoporosis attribution probability for patients with NHNV fracture is much lower with 0.575, 0.60, and 0.70 for female patients at the age of 50–64 years, 65–84 years, and 85 + years, respectively [31].
Mortality
Baseline mortality data for the age- and gender-stratified Dutch population was obtained from the official registry (Centraal Bureau voor de Statistiek CBS) in the years 2017–2019 [32]. We further modelled lifetime increased mortality risk after hip and CV fracture in line with a meta-analysis [33] with the RR of 2.9 and 3.76 for women and men, respectively. Considering excess mortality may also be attributable to other factors such as comorbidities, we conservatively assumed that only 25% of the excess mortality following a hip or CV fracture could be attributable to the fractures themselves [34, 35].
Fracture cost
In line with Dutch guideline for economic evaluations in healthcare [28], a societal perspective for the cost estimation was used including both direct and indirect costs. The direct gender-stratified hip, CV, and NHNV fracture costs were estimated from a Dutch study based on claims data (all costs were expressed in €2020) [36]. Hip fractures are also associated with long-term nursing home costs, the yearly cost was estimated at €25,741 (Dutch standard daily nursing home cost*365), and an average 21% of patients in the years 2017–2019 were institutionalized following the hip fracture as reported by Dutch Hip Fracture Audit [37]. To estimate productivity costs of employed persons sustained a fracture, maximum 2-month work absence were assumed according to the friction cost method as suggested by the Dutch guideline for economic evaluations in healthcare [28]. Based on the work-related absence rate estimated for different fracture types (hip 0.99, CV 0.79, NHNV 0.64) in a previous study [38] and the average annual salary in the Netherlands in 2020 [39], the productivity costs for patients with a hip (CV, NHNV) fracture aged 50–54, 55–59, and 60–64 years were estimated at €7927 (€6325, €5124), €7717 (€6166, €4995), and €7319 (€5841, €4732), respectively. Productivity costs were not included for patients aged over 65 years.
Utility values
The age- and gender-stratified baseline utilities in patients with a recent fracture were obtained from a recently published Dutch study [40] which estimated age- and gender-specific health state utility values (HSUV) by the EuroQol 5-dimension (EQ-5D) questionnaire in patients visiting the FLS in VieCuri Medical Centre. The utility ranged from 0.813 (50 years) to 0.665 (90 years) in women, which was relatively higher in men, ranging from 0.855 to 0.743. The effects of hip and clinical vertebral fractures on utility for the first and subsequent years were derived from the large International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) study [41]. We obtained disutility multipliers for NHNV fracture from a previous cost-effectiveness analysis [42] since NHNV fractures were not included in the ICUROS study.
Drug treatment effects and costs
As we mentioned before, in our model, 40% and 5% of patients in FLS and no-FLS branch initiated drug therapy based on real-world data [27] and expert opinion, respectively. When relating the prevalence of osteoporosis and/or clinical or prevalent VF to these treatment initiation rates, it can be estimated that 80% (40%/49% = 80%) and 10% (5%/49% = 10%) of patients with osteoporosis and/or clinical or prevalent VF received drug therapies in FLS and no-FLS branches, respectively. These data were used in decision tree.
Treatment scenarios were obtained from pharmacy data in VieCuri Medical Centre, i.e. for patients initiated drug therapy, 70%, 13.0%, 14.3%, and 2.7% patients received oral bisphosphonates (alendronate, risedronate, ibandronate), zoledronic acid, denosumab, and teriparatide, respectively.
The pooled treatment efficacy data for oral bisphosphonates were obtained from a report by the National Institute for Clinical Health and Excellence (NICE) [43], suggesting a RR of 0.67 (95% CI: 0.48–0.96), 0.45 (95% CI: 0.31–0.65), 0.81 (95% CI: 0.46–1.44) for hip, CV, and NHNV fracture, respectively. Treatment efficacy data for zoledronic acid were extracted from HORIZON Pivotal Fracture Trial [44], reporting a RR of 0.59 (95% CI: 0.42–0.83), 0.23 (95% CI: 0.14–0.37), 0.75 (95% CI: 0.64–0.87) for hip, CV, and NHNV fracture, respectively. Aligned with a recent review of cost-effectiveness of denosumab [45], efficacy data from the FREEDOM study [46] were used, suggesting that denosumab resulted in a RR of 0.6 (95% CI: 0.37–0.97), 0.31 (95% CI: 0.26–0.41), 0.8 (95% CI: 0.67–0.95) for hip, CV, and NHNV fracture, respectively. Treatment efficacy for teriparatide were obtained from a systematic review, reporting 0.36 (95% CI: 0.15–0.81), 0.23 (95% CI: 0.17–0.32), 0.57 (95% CI: 0.54–0.74) for hip, CV, and NHNV fracture, respectively.
Treatment duration in our model was consistent with the recommendation of Dutch guidelines [10, 47], namely maximum 5-year therapy with oral bisphosphonates, 3-year treatment with zoledronic acid, 5-year treatment with denosumab, and 2-year treatment with teriparatide followed by 3-year oral bisphosphonates. For patients initiated treatment with oral bisphosphonates or zoledronic acid, after medication discontinuation, a linear decrease of the effects for 3 years (offset time) was assumed as suggested by clinicians. Considering the rebound effect, 1-year offset time after discontinuing denosumab was assumed. We assumed the effect of teriparatide remained once oral bisphosphonates initiated, a linear decrease of the effects for 3 years was assumed after discontinuing oral bisphosphonates.
Given treatment efficacy can be largely affected by persistence, we incorporated persistence rates in the study. The persistence rate of oral bisphosphonates was obtained from a Dutch study [48], reporting 75%, 61.3%, and 45.3% after 1, 3, and 5 years, respectively. The Kaplan–Meier curve in this study indicated an approximately linear decrease in persistence over time, we therefore estimated the persistence rates after the treatment of 6 months, 2 years, and 4 years manually. Persistence rates of zoledronic acid after 1, 2, and 3 years were obtained from VieCuri Medical Centre (in the year 2018) as 100%, 69%, and 48%, respectively. The long-term persistence rates of denosumab in the Netherlands is unknown; persistence rates up to 3 years was extracted from the same systematic review [49], suggesting 100%, 81%, 67%, 55%, 35%, and 26% after 6, 12, 18, 24, 30, and 36 months, respectively. We assumed the persistence remained unchanged after 36 months given the unavailability of relevant data. Two-year persistence of 75% with teriparatide was obtained from a Dutch study [50], the persistence with sequential oral bisphosphonates was assumed the same as the first 3-year monotherapy with oral bisphosphonates.
Treatment costs in our study refer to drug costs and related side effect costs. Annual drug costs for oral bisphosphonates, zoledronic acid, denosumab, and teriparatide were retrieved from Dutch official data [51], it was estimated at €20, €258, €400, and €3480 (in the year 2020), respectively. For side effect costs, it was assumed that patients initiated treatment requiring 0.041 extra GP consultations during the first cycle (6 months) and 0.021 GP consultations during the following cycles in line with a previous study [52]; the average standard consulting cost of the general practitioner was estimated at €34.74 (in 2020).
FLS-related model input data
Given all patients attending the FLS were registered in the diagnosis treatment combination (DBC) system (besides the fracture DBC, all FLS attenders have an osteoporosis DBC), we therefore used the mean DBC price (€450 in 2020) in the Netherlands for FLS visit [53] in our analysis. Related to FLS visit, the DBC price covers the cost of DXA, lab test, VFA, fall risk assessment etc., extra GP consultation in the follow-up was also included (once per year).
Compared to no-FLS branch, higher treatment initiation rate (40% vs. 5%) and greater treatment persistence (57% vs. 34.1%, deriving from a literature review and meta-analysis [19]) were modelled for FLS branch. In addition, we modelled a lower mortality (hazard ratio 0.43; 95% CI, 0.34–0.56) and subsequent fracture (subdistribution hazard ratio 0.80; 95% CI, 0.60–1.07) risk for FLS attenders with a hip or clinical vertebral fracture as reported by a Dutch study [26], no effect was assumed for patients with NHNV fracture.
Analyses and outcomes
A total of 1,000,000 trials (1st-order Monte-Carlo simulation) were run for both base-case and one-way sensitivity analyses. With regard to the base-case analysis, we applied point estimates of model input parameters to estimate total costs (including direct healthcare cost and indirect productivity cost), number of fractures prevented, and total QALYs for both FLS and no-FLS branches. In addition, incremental costs, incremental QALYs and the incremental cost/effectiveness ratio (ICER) were also estimated. The ICER were calculated as incremental cost (expressed in €2020) per QALY gained. Besides, multiple scenario analyses were conducted to assess the economic value of FLS in patients at different starting ages (50 − 80 years).
In the Netherlands, there is no single willingness-to-pay (WTP) threshold, ranging from €20,000 to €80,000 per QALY gained [54]. As suggested by Zorginstituut Nederland (ZIN), the selection of WTP threshold should base on burden of illness (BOI), the proportional shortfall (PS) method is recommended [55, 56]. PS is measured on a scale from 0 (no QALY loss) to 1 (complete loss of remaining QALY). If PS falls between 0.10 and 0.40, the WTP threshold of €20,000/QALY is recommended; the maximum reimbursement of €50,000/QALY refers to PS = 0.41–0.70; the endpoint of €80,000/QALY is in relation to the highest BOI with PS estimated at 0.71–1.00. In our study, we used a disease burden calculator released by Institute for Medical Technology Assessment (iMTA) [57], PS was estimated at 0.16, therefore the WTP threshold of €20,000/QALY is applied.
One-way sensitivity analyses were performed to test the robustness of the model results by varying a single parameter each time, including a healthcare perspective, a shorter time horizon (5 years), a different discount rate (3%, 5% for both costs and QALYs), and some other parameters including gender (100% female or male), FLS cost (± 50%), treatment initiation rate in FLS and no-FLS (± 50%), fracture costs (± 50%), drug costs (± 50%), nursing home costs (± 25%), probability of nursing home (± 50%), baseline utility (− 20%), excess mortality attribution probability (± 100%), osteoporosis attribution probability (− 25%), and relative risk of subsequent fracture associated with osteoporosis/a prior fracture (− 25%).
Probabilistic sensitivity analyses were also undertaken to examine the effect of the joint uncertainty surrounding the model variables (the uncertainty surrounding the point estimate used in the base-case analysis under a range of thresholds for willingness to pay). A specific distribution was attributed to each parameter around the point estimate used in the base-case analysis. Specifically, a beta distribution was used for fracture incidence (i.e., the distribution was estimated based on the number of fractures and the population in the age range of 70 − 74 years) and the effects of fracture on utility (based on 90% confidence interval). Besides, log-normal distributions were assumed for the relative risk of having subsequent fracture, excess mortality following a fracture, treatment efficacy, and osteoporosis attribution probability. In addition, normal distributions with a standard deviation (SD) assumed to be 20% of the mean (given the lack of standard error) were used for fracture cost, productivity cost, nursing home cost, probability of nursing home admission, excess mortality attribution probability, and treatment initiation rate. For each probabilistic sensitivity analysis, the model was run 200 times (2nd-order Monte-Carlo simulation) based on runs of 25,000 trials per pathway. Cost-effectiveness acceptability curves (CEAC) were done to show the probability of the FLS being cost-effective compared to no-FLS as a function of WTP thresholds.
Results
Base-case analysis
Table 2 presents the lifetime costs, accumulated QALYs, number of fractures, incremental cost and QALY, and the ICER (expressed in cost per QALY gained) of FLS compared to no-FLS in patients with a recent fracture at the age of 50 years and older. FLS was associated with a €45 higher cost and 0.11 additional QALY gained compared to no-FLS, the ICER was thus estimated at €409 per QALY gained, lower than the threshold of €20,000/QALY, indicating FLS was cost-effective compared to no-FLS. In 1,000,000 simulated patients with a recent fracture, FLS led to a reduction of total 53,090 lifetime subsequent fractures, namely the availability of an FLS would avoid 53 subsequent fractures over the lifetime of every 1000 patients.
Table 3 presents the ICERs of FLS compared to no-FLS in patients at different ages. The cost per QALY gained was estimated at €1812, €450, €627, and €421 in patients at the age of 50, 60, 70, and 80 years, respectively, suggesting the cost-effectiveness of FLS was remained in all age categories. Compared to younger groups, patients aged 80 years resulted in slightly greater QALY gained.
Sensitivity analysis
Table 4 reports the results of the one-way sensitivity analyses. Our results were robust in all one-way sensitivity analyses given the ICERs of FLS relative to no-FLS remained below €20,000 per QALY gained. When conducting the analysis from the healthcare perspective, FLS was associated with a €100 higher cost and 0.12 additional QALY gained compared to no-FLS, the ICER was thus estimated at €833 per QALY gained, suggesting FLS is still cost-effective. With a 5-year time horizon, FLS led to a reduction of 41 fractures per 1000 patients compared to no-FLS (i.e., 8.1% fracture prevention). FLS was dominant (more QALY for less total costs) in female patients, when decreasing the FLS cost (− 50%) and drug costs (− 50%), and when increasing treatment initiation rate in FLS (+ 25%) and fracture costs (+ 50%). The ICERs were shown to be markedly affected by the probability of treatment initiation in the FLS, suggesting the higher the treatment initiation rate, the greater the cost-effective of FLS. In addition, women in FLS incurred with higher cumulative lifetime costs (€14,360 vs. €9904 per patient) but also greater QALYs (10.39 vs. 10.21 per patient) compared to men in FLS; When compared to no-FLS, more favorable ICER was identified in female patients also; for every 1000 female patients with a recent fracture, the availability of an FLS would avoid 60 subsequent fractures over their lifetime, which was 37 subsequent fractures in male patients. Other analyses suggested that the ICERs of FLS were shown to greatly increase with the impact from high to low when decreasing relative risk of subsequent fracture by a prior fracture (− 25%), decreasing osteoporosis attribution probability (− 25%), decreasing fracture costs (− 50%), decreasing relative risk of subsequent fracture by osteoporosis (− 25%), and increasing FLS cost (+ 50%).
The results of the probabilistic sensitivity analyses are reported in Figs. 3 and 4. At the threshold of €20,000 per QALY gained, the cost-effectiveness acceptability curves suggest that FLS was cost-effective compared to no-FLS in 89.5% of the simulations (95.5% and 96.5% at the threshold of €50,000 and €80,000/QALY, respectively). The FLS in women was associated with a higher probability to be cost-effective compared to men (91.5% vs. 84.0%). In addition, FLS was shown to be cost-effective in 67.0%, 78.5%, 89.5%, and 98.5% of the simulations at the age of 50, 60, 70, and 80 years, respectively, at a threshold of €20,000 per QALY gained; which was 78.5%, 90.5%, 96.5%, and 100.0% correspondingly when the WTP threshold is €50,000 per QALY gained, and 81.0%, 93.0%, 97.5%, and 100.0% when the WTP threshold is €80,000 per QALY gained. The cost-effectiveness results of FLS for different age and gender categories were also displayed using cost-effectiveness plane, details can be found in Appendix III of the Supplementary information.
Discussion
In this study, a Markov microsimulation model from a Dutch societal perspective and a lifetime horizon was used to estimate the cost-effectiveness of the FLS in patients aged 50 years and older with a recent fracture. In all of the simulated populations, the ICERs of FLS were below the Dutch accepted thresholds of €20,000 per QALY gained, suggesting FLS is cost-effectives compared to no-FLS. The cost-effectiveness of FLS remained favorable in all age categories. Compared to younger groups, patients aged 80 years resulted in slightly higher QALY gained. With fracture trackers in the model, FLS was estimated to lead to a reduction of 41 subsequent fractures in per 1,000 simulated individuals with the time horizon of 5 years, which was comparable to a recent UK study [58] reporting FLS was associated with a reduction of 30 subsequent fractures in per 1000 individuals.
Our findings were robust in all one-way sensitivity analyses and probabilistic sensitivity analyses. For women, a FLS was associated with more favorable ICER and higher probability to be cost-effective compared to men. There are two potential reasons: first, men have a higher baseline mortality risk and the impact of fracture on mortality was also greater compared to women, leading to shorter life expectancy to gain benefits from the FLS, and thus less QALY gains (compared to women); second, women are associated with a higher risk of fracture recurrence than men, the presence of an FLS would thus lead to more subsequent fractures avoidance and more health benefits. Next to gender, treatment initiation in the FLS was found to be particularly influential when varied within the model; the higher treatment initiation rate was associated with greater cost-effective results. It is quite reasonable since more patients are identified and treated, more subsequent fractures are avoided, which is also the mission of post-fracture care programs.
To our knowledge, this study provides the first results about the cost-effectiveness of the FLS in the Netherlands. Our finding supports a recently issued Dutch report [59] suggesting FLS is associated with reduction in fragility fractures and offer clear cost-effectiveness compared to current practice with a time horizon of 5 years. Given the report related original study is not published yet, we cannot make detailed comparison regarding the modelling strategy, model input data as well as subsequent fracture risk estimation and relevant assumptions.
The main strength of our study is that real-world data from a Dutch hospital were obtained and used for several model input parameters. These data are reliable and valid since the FLS program in VieCuri Medical Centre had been implemented for 15 years, and the quality of their FLS was rated as “gold” according to Best Practice Framework (BPF) of IOF. Besides, the success of FLS implementation largely depends on the intensity of attendance and treatment; the performance of FLS in VieCuri Medical Centre has a relatively high FLS attendance rate (51%) and the initiation rate of anti-osteoporosis drugs (40% for attenders) [26], which are comparable to rates reported by several systematic reviews and meta-analysis [17, 19], attributing to the cost-effective results of FLS in our study. One systematic review [15] suggested that the more intensive the FLS model, the more effective of which in subsequent fracture prevention, the greater the economic benefits. Given we only assessed the cost-effectiveness of the FLS in one Dutch FLS clinic, the impact of improving the intensity and quality of FLS on cost-effectiveness was not revealed, it would be of interest for future research. To improve the quality of FLS, the BPF and eleven patient-level key performance indicator developed by the IOF could serve as guidelines in the design of adequate FLSs and improving the quality of existing FLSs. In addition to the real-world data, most recent estimates for utility values, mortality rate, FLS cost, drug costs as well as nursing home admission were obtained from Dutch publications or official website, assuring valid estimations in our analysis.
Although the absolute benefits of FLS in terms of QALY gained or fractures avoided could be consider as low, the results of our study are in line with a previous international systematic review [20] of 23 cost-effectiveness analyses of FLS, suggesting the FLS was a cost-effective secondary fracture prevention strategy although it was implemented in different ways and settings. And the QALY gained in these 23 articles were reported from 0.004 to 0.118 per patient, which is comparable to our study (on average 0.11 QALY gained per patient). As we mentioned before, the number of fracture prevented in our study is also comparable to a recent UK study [58]. Compared to previous studies, our study has several strengths. First, drug treatment indications in the Netherlands recommended by Dutch guidelines [10, 22] were reflected in our model, which is more consistent with real-life FLS setting when treating patients with a recent fracture. Second, real-world treatment scenarios including four types of medications (drug strategies as recommended by Dutch guideline) were modelled in our study, leading to real-world cost-effectiveness assessment of FLS rather than a hypothetical estimation. Third, age- and gender-stratified data were retrieved for most parameters to facilitate the investigation of differences in cost-effectiveness estimations for patients with different baseline characteristics. Fourth, most previous studies simply assumed 100% persistence to anti-osteoporosis medications; we took into account the impact of medication persistence on treatment effect by incorporating data from literature.
There are however some potential limitations in this study. First, given the lack of patient data before the implementation of FLS in VieCuri Medical Centre, we did not have an accurate treatment initiation rate for the no-FLS branch, the modelled rate of 5% was based on expert opinion. However, we explored the uncertainty of this parameter in sensitivity analysis, our results were remained. Second, for patients who suffered subsequent fractures in the simulation, the change in treatment strategy (extension or switch) and the corresponding (new) therapy efficacy and duration were not modelled in our study given the lack of relevant data and the complexity of modelling. Third, patients entered our model with a mixture of fracture type (hip, vertebrae, NHNV), the availability of input data was insufficient to calculate ICER separately by type of baseline, making it difficult to compared between groups (e.g., major vs. non-major fractures). Fourth, the analysis in this study was conducted based on the real-life data from a single hospital in the Netherlands, the generalizability of our results should be conducted with caution given the intensity and quality of implementation of FLS vary between hospitals and countries, potentially leading to different clinical and economic outcomes. Fifth, some assumptions such as consistent prevalence of osteoporosis and/or prevalent VF in both branches, consistence treatment efficacy, persistence, and utility multipliers in female and male patients were made given the lack of relevant data. Sixth, given the absence of productivity costs in the Netherlands, we conservatively assumed patients with a fracture had a 2-month work absence at most; however, more complicated scenarios could be found in the real life given not only disease aspects but also personal characteristics and job factors have an influence on work ability and further on productivity costs.
Conclusion
This study provides the first economic results of FLS in the Netherlands, suggesting that FLS is cost-effective compared to no-FLS in patients aged 50 years and older with a recent fracture. The cost-effectiveness of FLS remained in all age categories. The implementation of FLS could lead to reduced subsequent fracture risk and lifetime economic benefits.
Data availability
All data analyzed as part of this study are included in this published article (and its supplementary information files).
References
Kanis JA, Norton N, Harvey NC, et al (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos 16(1). https://doi.org/10.1007/s11657-020-00871-9
Van Geel TACM, Van Helden S, Geusens PP, Winkens B, Dinant GJ (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68(1):99–102. https://doi.org/10.1136/ard.2008.092775
Solomon DH, Johnston SS, Boytsov NN, Mcmorrow D, Lane JM, Krohn KD (2014) Osteoporosis Medication use after hip fracture in U . S . patients between 2002 and 2011. J Bone Miner Res 29(9):1929–1937. https://doi.org/10.1002/jbmr.2301
Dunnewind T, Dvortsin EP, Smeets HM et al (2017) Economic Consequences and potentially preventable costs related to osteoporosis in the Netherlands. Value Heal 20(6):762–768. https://doi.org/10.1016/j.jval.2017.02.006
McLellan AR, Gallacher SJ, Fraser M, McQuillian C (2003) The fracture liaison service: Success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int 14(12):1028–1034. https://doi.org/10.1007/s00198-003-1507-z
https://www.iofbonehealth.org/. Accessed 16 Oct 2022.
Lems WF, Dreinhöfer KE, Bischoff-Ferrari H et al (2017) EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 76(5):802–810. https://doi.org/10.1136/annrheumdis-2016-210289
Dreinhöfer KE, Mitchell PJ, Bégué T et al (2018) A global call to action to improve the care of people with fragility fractures. Injury 49(8):1393–1397. https://doi.org/10.1016/j.injury.2018.06.032
Eisman JA, Bogoch ER, Dell R et al (2012) Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 27(10):2039–2046. https://doi.org/10.1002/jbmr.1698
Dutch Institute for Healthcare Improvement CBO (2011) Richtlijn Osteoporose en Fractuurpreventie. www.diliguide.nl/document/1015/file/pdf/. Accessed 16 Oct 2022.
Hegeman JH, Oskam J, van der Palen J, ten Duis HJ, Vierhout PAM (2004) The distal radial fracture in elderly women and the bone mineral density of the lumbar spine and hip. J Hand Surg Am 29(5):473–476. https://doi.org/10.1016/j.jhsb.2004.05.002
https://osteoporoseverpleegkundige.nl/. Accessed 16 Oct 2022.
Van Den Bergh JP, Van Geel TA, Geusens PP (2012) Osteoporosis, frailty and fracture: Implications for case finding and therapy. Nat Rev Rheumatol 8(3):163–172. https://doi.org/10.1038/nrrheum.2011.217
van den Berg P, Schweitzer DH, van Haard PMM, van den Bergh JP, Geusens PP (2015) Meeting international standards of secondary fracture prevention: a survey on Fracture Liaison Services in the Netherlands. Osteoporos Int 26(9):2257–2263. https://doi.org/10.1007/s00198-015-3117-y
Ganda K, Puech M, Chen JS et al (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24(2):393–406. https://doi.org/10.1007/s00198-012-2090-y
Wu C, Chen C, Chen P et al (2018) Identifying characteristics of an effective fracture liaison service : systematic literature review. Published Online 29:1023–1047
Vranken L, Wyers CE, van den Bergh JPW, Geusens PPMM (2017) The Phenotype of patients with a recent fracture: a literature survey of the fracture liaison service. Calcif Tissue Int 101(3):248–258. https://doi.org/10.1007/s00223-017-0284-1
Li N, Hiligsmann M, Boonen A et al (2021) The impact of fracture liaison services on subsequent fractures and mortality: a systematic literature review and meta-analysis. Osteoporos Int Published online. https://doi.org/10.1007/s00198-021-05911-9
Wu CH, Te TuS, Chang YF et al (2018) Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: a systematic literature review and meta-analysis. Bone 111(138):92–100. https://doi.org/10.1016/j.bone.2018.03.018
Wu CH, Kao IJ, Hung WC et al (2018) Economic impact and cost-effectiveness of fracture liaison services: a systematic review of the literature. Osteoporos Int 29(6):1227–1242. https://doi.org/10.1007/s00198-018-4411-2
Hiligsmann M, Ethgen O, Bruyère O, Richy F, Gathon HJ, Reginster JY (2009) Development and validation of a markov microsimulation model for the economic evaluation of treatments in osteoporosis. Value Heal 12(5):687–696. https://doi.org/10.1111/j.1524-4733.2008.00497.x
Vereniging NI, Specialisten M (2023) Osteoporose en fractuurpreventie Inhoudsopgave. Published online.
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148. https://doi.org/10.1002/jbmr.5650080915
Hiligsmann M, Reginster J, Tosteson ANA et al (2019) Osteoporos Int ArticleTitle: Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos Int 30(1):45–57
Husereau D, Drummond M, Augustovski F et al (2022) Consolidated Health Economic Evaluation Reporting Standards 2022 ( CHEERS 2022) Statement : Updated Reporting Guidance for Health Economic Evaluations Consolidated Health Economic Evaluation Reporting Standards 2022 ( CHEERS 2022) Statement : Updated Re. J Med Econ 25(S1):1–7. https://doi.org/10.1080/13696998.2021.2014721
Vranken L, de Bruin IJA, Driessen AHM et al (2022) Decreased Mortality and subsequent fracture risk in patients with a major and hip fracture after the introduction of a fracture liaison service: a 3-year follow-up survey. J Bone Miner Res 37(10):2025–2032. https://doi.org/10.1002/jbmr.4674
van der Velde RY, Bours SPG, Wyers CE, Lems WF, Geusens PPMM, van den Bergh JPW (2017) Effect of implementation of guidelines on assessment and diagnosis of vertebral fractures in patients older than 50 years with a recent non-vertebral fracture. Osteoporos Int 28(10):3017–3022. https://doi.org/10.1007/s00198-017-4147-4
Versteegh M, Knies S, Brouwer W (2016) From good to better: New Dutch Guidelines for Economic Evaluations in Healthcare. Pharmacoeconomics 34(11):1071–1074. https://doi.org/10.1007/s40273-016-0431-y
Van Geel TACM, Huntjens KMB, Van Den Bergh JPW, Dinant GJ, Geusens PP (2010) Timing of subsequent fractures after an initial fracture. Curr Osteoporos Rep 8(3):118–122. https://doi.org/10.1007/s11914-010-0023-2
Hiligsmann M, Reginster JY (2011) Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics 29(10):895–911. https://doi.org/10.2165/11539980-000000000-00000
Melton LJ, Thamer M, Ray NF et al (1997) Fractures attributable to osteoporosis: report from the national osteoporosis foundation. J Bone Miner Res 12(1):16–23. https://doi.org/10.1359/jbmr.1997.12.1.16
mortality rate. https://opendata.cbs.nl/. Accessed 16 Oct 2022.
Haentjens P, Magaziner J, Colón-Emeric CS et al (2010) Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 152(6):380–390. https://doi.org/10.7326/0003-4819-152-6-201003160-00008
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15(2):108–112. https://doi.org/10.1007/s00198-003-1516-y
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32(5):468–473. https://doi.org/10.1016/S8756-3282(03)00061-9
Lötters FJB, van den Bergh JP, de Vries F, Rutten-van Mölken MPMH (2016) Current and future incidence and costs of osteoporosis-related fractures in the netherlands: combining claims data with BMD measurements. Calcif Tissue Int 98(3):235–243. https://doi.org/10.1007/s00223-015-0089-z
Dutch hip fracture audit. https://dica.nl/jaarrapportage-2019/dhfa. Accessed 16 Oct 2022.
Meerding WJ, Looman CWN, Essink-Bot ML, Toet H, Mulder S, Van Beeck EF (2004) Distribution and determinants of health and work status in a comprehensive population of injury patients. J Trauma - Inj Infect Crit Care 56(1):150–161. https://doi.org/10.1097/01.TA.0000062969.65847.8B
Average annual salary in the Netherlands in 2020. https://www.statista.com/statistics/538406/average-annual-salary-in-the-netherlands-by-age/. Accessed 16 Oct 2022.
Li N, van Oostwaard M, van den Bergh JP et al (2021) Health-related quality of life of patients with a recent fracture attending a fracture liaison service: a 3-year follow-up study. Osteoporos Int 33(3):577–588. https://doi.org/10.1007/s00198-021-06204-x
Svedbom A, Borgström F, Hernlund E et al (2018) Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures—results from the ICUROS. Osteoporos Int 29(3):557–566. https://doi.org/10.1007/s00198-017-4317-4
Söreskog E, Lindberg I, Kanis JA et al (2021) Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int 32(3):585–594. https://doi.org/10.1007/s00198-020-05780-8
National Institute for Health and Clinical Excellence (NICE). Systematic reviews of clinical effectiveness prepared for the guideline “Osteoporosis assessment of fracture risk and the prevention of osteoporotic fractures in individuals at high risk.
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Peter L, Huilin H, John C, Karen T, Theresa RJ, Joel K, Trisha FH, Deborah S, Erik F (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 356(18):1809–1822
Wan Y, Zeng F, Tan H, et al (2022) Cost-effectiveness analyses of denosumab for osteoporosis: a systematic review. 33. https://doi.org/10.1007/s00198-021-06268-9
Cummings SR, Martin JS, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. Obstet Gynecol Surv 64(12):805–807. https://doi.org/10.1097/01.ogx.0000363236.41902.96
Lems WF, Geusens P (2013) Osteoporose en fractuurpreventie. Leerb Reumatol en Klin Immunol. Published online 215–226. https://doi.org/10.1007/978-90-313-9894-2_20
Klop C, Welsing PMJ, Elders PJM et al (2015) Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos Int 26(6):1831–1840. https://doi.org/10.1007/s00198-015-3084-3
Koller G, Goetz V, Vandermeer B et al (2020) Persistence and adherence to parenteral osteoporosis therapies: a systematic review. Osteoporos Int 31(11):2093–2102. https://doi.org/10.1007/s00198-020-05507-9
van Maren MA, Wyers CE, Driessen JHM et al (2019) Two-year persistence with teriparatide improved significantly after introduction of an educational and motivational support program. Osteoporos Int 30(9):1837–1844. https://doi.org/10.1007/s00198-019-05052-0
https://www.medicijnkosten.nl/. Accessed 16 Oct 2022.
Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY (2019) Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum 49(2):184–196. https://doi.org/10.1016/j.semarthrit.2019.01.006
DBC-zorgproduct. https://www.opendisdata.nl/msz/zorgproduct/131999067. Accessed 16 Oct 2022.
Institute DNHC. Cost-effectiveness in practice. https://www.zorginstituutnederland.nl/. Accessed 16 Oct 2022.
Vijgen S, Heesch F van, Obradovic M. Ziektelast in de praktijk. Dutch Natl Heal Care Inst. Published online 2018:1–34. https://www.zorginstituutnederland.nl/publicaties/rapport/2018/05/07/ziektelast-in-de-praktijk. Accessed 16 Oct 2022.
Reckers-Droog VT, van Exel NJA, Brouwer WBF (2018) Looking back and moving forward: On the application of proportional shortfall in healthcare priority setting in the Netherlands. Health Policy (New York) 122(6):621–629. https://doi.org/10.1016/j.healthpol.2018.04.001
https://imtamodels.shinyapps.io/iDBCv2_1/. Accessed 16 Oct 2022.
Pinedo-Villanueva R, Burn E, Maronga C, Cooper C, Kassim JM (2023) Expected benefits and budget impact from a microsimulation model support the prioritisation and implementation of Fracture Liaison Services. J Bone Miner Res 00(00):1–13. https://doi.org/10.1002/jbmr.4775
https://www.osteoporosis.foundation/sites/iofbonehealth/files/202301/2022_country_profile_ned_25.01.pdf. Accessed 19 Feb 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nannan Li is funded by the China Scholarship Council (grant number 201909110080). Mickaël Hiligsmann has received research grants through institution from Amgen, Radius Health and ViiV, consulting fee from UCB and lecture fee from Mylan Pharmaceuticals; Joop P. van den Bergh has received research funding from Amgen, and UCB; Annelies Boonen, Caroline E. Wyers, and Sandrine P.G. Bours declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, N., van den Bergh, J.P., Boonen, A. et al. Cost-effectiveness analysis of fracture liaison services: a Markov model using Dutch real-world data. Osteoporos Int 35, 293–307 (2024). https://doi.org/10.1007/s00198-023-06924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06924-2