Abstract
Summary
FRAX® calculates the 10-year probability of major osteoporotic fractures (MOF), which are considered to have a greater clinical impact than other fractures. Our results suggest that, in postmenopausal women with severe osteoporosis, those treated with teriparatide had a 60% lower risk of FRAX®-defined MOF compared with those treated with risedronate.
Introduction
The VERO trial was an active-controlled fracture endpoint clinical trial that enrolled postmenopausal women with severe osteoporosis. After 24 months, a 52% reduction in the hazard ratio (HR) of clinical fractures was reported in patients randomized to teriparatide compared with risedronate. We examined fracture results restricted to FRAX®-defined major osteoporotic fractures (MOF), which include clinical vertebral, hip, humerus, and forearm fractures.
Methods
In total, 1360 postmenopausal women (mean age 72.1 years) were randomized to receive subcutaneous daily teriparatide (20 μg) or oral weekly risedronate (35 mg). Patient cumulative incidence of ≥ 1 FRAX®-defined MOF and of all clinical fractures were estimated by Kaplan-Meier analyses, and the comparison between treatments was based on the stratified log-rank test. Additionally, an extended Cox model was used to estimate HRs at different time points. Incidence fracture rates were estimated at each 6-month interval.
Results
After 24 months, 16 (2.6%) patients in the teriparatide group had ≥ 1 low trauma FRAX®-defined MOF compared with 40 patients (6.4%) in the risedronate group (HR 0.40; 95% CI 0.23–0.68; p = 0.001). Clinical vertebral and radius fractures were the most frequent FRAX®-defined MOF sites. The largest difference in incidence rates of both FRAX®-defined MOF and all clinical fractures between treatments occurred during the 6- to 12-month period. There was a statistically significant reduction in fractures between groups as early as 7 months for both categories of clinical fractures analyzed.
Conclusion
In postmenopausal women with severe osteoporosis, treatment with teriparatide was more efficacious than risedronate, with a 60% lower risk of FRAX®-defined MOF during the 24-month treatment period. Fracture risk was statistically significantly reduced at 7 months of treatment.
Clinical trial information
ClinicalTrials.gov Identifier: NCT01709110
EudraCT Number: 2012-000123-41
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic fractures are associated with substantial social, economic, and healthcare burden. The VERtebral fracture treatment comparison in Osteoporotic women (VERO) trial was the first active-controlled, fracture endpoint study in postmenopausal women with low bone mass and prevalent vertebral fractures. VERO compared the effectiveness of the bone-forming drug teriparatide with that of the antiresorptive risedronate, with fractures as the primary outcome [1]. Previously, we reported a 52% reduction in the risk of clinical fractures (a composite of clinical vertebral and non-vertebral fragility fractures; p < 0.001), a 42% reduction in the risk of major, fragility non-vertebral fractures (p = 0.06), and a 71% reduction in the risk of clinical vertebral fractures in patients randomized to teriparatide compared with risedronate after 24 months of treatment (p = 0.002) [1].
The development of the fracture risk assessment tool or FRAX® algorithm has led to an improvement in identifying patients at risk of fracture and provides an opportunity to identify those most likely to benefit from osteoporosis treatment using easily obtained clinical risk factors for fracture [2]. FRAX® computes the 10-year probability of hip fracture or of major osteoporotic fractures (MOF), which includes clinical spine, hip, forearm, and proximal humerus fractures [2]. These types of fractures are considered to be more related to osteoporosis, have greater clinical impact, and are associated with higher health-related costs than fractures at other sites [2]. Consequently, FRAX®-defined MOF have become an increasingly reported efficacy endpoint in osteoporosis trials and pharmacoeconomic evaluations [2,3,4,5].
In the pre-specified statistical analysis plan of the VERO trial [1], the list of non-vertebral fractures was defined according to the European Guidelines for the Evaluation of Drugs for the Treatment of Osteoporosis [6]. Here, we present the results of a post hoc analysis of the fracture results from the VERO trial, restricted to the MOF included in the FRAX® model. Moreover, given the importance of early antifracture efficacy of osteoporosis drugs for patients with severe osteoporosis at high imminent fracture risk, we also analyzed the time pattern of the FRAX®-defined MOF antifracture efficacy, and how it compares with the predefined list of all clinical fractures.
Methods
Study design and participants
The VERO study design has previously been described in detail [1]. In summary, VERO was an international, multicenter, randomized, double-blind, active-controlled, parallel-group, 24-month trial. In total, 1360 postmenopausal women (mean age 72.1 years) with severe osteoporosis were randomized following a 1:1 scheme to receive either subcutaneous teriparatide (20 μg daily) plus an oral weekly placebo or oral risedronate (35 mg weekly) plus a subcutaneous daily placebo for up to 24 months. Patients were enrolled at 116 centers in 14 countries across Europe, South America, and North America.
Eligible participants were ambulatory postmenopausal women aged > 45 years with a baseline bone mineral density (BMD) T-score less than or equal to − 1.50 standard deviations (SD) at the femoral neck, total hip, or lumbar spine. Patients had to have radiographic evidence of at least two moderate (between a 26% and 40% reduction in vertebral body height) or one severe (more than 40% reduction in vertebral body height) prevalent vertebral fragility fractures according to the classification of Genant et al. [7]. Patients were excluded if they had (a) low serum 25-hydroxy-vitamin D levels (< 9.2 ng/mL or 23 nmol/L), (b) abnormally elevated serum intact parathyroid hormone (PTH [1-84]) at baseline (> 72 pg/mL or > 7.6 pmol/L), or (c) significantly impaired renal function as defined by a calculated endogenous creatinine clearance of < 30 mL/min/m2. Prior use of bisphosphonates or other osteoporosis drugs was allowed.
The primary study endpoint was the incidence of new radiographic vertebral fractures (VFx). A new VFx was defined as a vertebral body height loss of at least 20% (and 4 mm) of a vertebra that was unfractured at baseline, based on a 6-point placement of the vertebral bodies from T4 to L4, and confirmed by an increase by one or more severity grades according to the semiquantitative grading (SQ) scale by Genant et al. [7]. All VFx were centrally adjudicated by two radiologists blinded to treatment (BioClinica, San Francisco, CA, USA).
Key secondary endpoints included the incidence of pooled new and worsened VFx, clinical fractures (a composite of clinical VFx and non-vertebral fragility fractures [NVFFx]), NVFFx (excluding pathologic fractures and fractures of the skull, face, fingers, metacarpals, and toes), and a subgroup of major NVFFx involving the hip, radius, humerus, rib, pelvis, tibia, and femur [1].
A clinical VFx was defined as an episode associated with signs and symptoms highly suggestive of a VFx. These include severe back pain of acute onset; pain with little or no exertion; pain localized to a specific vertebra and associated with limited back mobility; pain relieved by bed rest; pain worsened when upright, coughing, or sneezing; limited back flexion; or paravertebral muscle tenderness secondary to spasms, confirmed with the detection of a radiographic VFx by the centralized X-ray imaging readers. Analyses of non-vertebral and pooled clinical fractures were based on all randomized patients who received at least one dose of the investigational product (full analysis set). Further study design, entry criteria, and methodology details are described by Kendler et al. [1] in their article summarizing the trial’s main efficacy and safety results.
Statistical analyses
The cumulative incidence of patients with at least one FRAX®-defined MOF during the 24 months of follow-up was estimated using Kaplan-Meier analyses. Treatment comparison was based on the stratified log-rank test adjusted for the two stratification factors used at randomization, i.e., the antecedent of a clinical vertebral fracture in the 12 months prior to study entry and recent use of bisphosphonates [1]. Patients who were lost to follow-up, died, or completed the study without experiencing a fracture were censored at the last date of contact.
The overall hazard ratio (HR) during the 24 months of follow-up was calculated as part of the stratified log-rank test calculations to estimate the treatment effect of teriparatide versus risedronate. Additionally, an extended Cox regression model was used to estimate the adjusted hazard ratios between both treatment groups at different times of follow-up. This model was adjusted by the following: treatment; antecedent of recent clinical vertebral fractures; recent use of bisphosphonate; age (years); baseline BMD at the femoral neck (gm/cm2); baseline 25-hydroxy-vitamin D concentration (ng/mL); geographical region defined as North America (US and Canada), South America (Argentina and Brazil), and Europe (all other countries). This model included a time-varying covariate treatment-by-time interaction.
Incidence rates expressed as a number of events per 100 patients/years, and corresponding incidence rate ratios (IRRs) with corresponding 95% confidence interval (CI) and p values were also estimated at different 6-month intervals. The incidence rate was calculated as the number of patients who experienced at least one fracture event in the respective time interval divided by the total amount of person-time contributed by the population at risk during each 6-month time period. Patients who had a fracture in a given period were not excluded from the analyses for incident rate estimates in later periods. Each patient contributed time at risk in each of the four time periods that were analyzed. These analyses were conducted in the full analysis set that included all randomized patients who received ≥ 1 dose of either investigational product. Analyses were performed under SAS 9.4.
Results
In total, 16 patients (cumulative incidence 2.6%) had one or more low trauma FRAX®-defined MOF in the teriparatide group compared with 40 patients (cumulative incidence 6.4%) in the risedronate group (overall HR 0.40; 95% CI 0.23–0.68; p = 0.001) (Fig. 1). The per-protocol analysis yielded similar results with 14 (2.7%) and 36 (6.8%) of the patients treated with teriparatide and risedronate sustaining a FRAX®-defined MOF, respectively (HR 0.39; 95% CI 0.23–0.68; p = 0.002).
At 24 months, clinical vertebral fractures were the most frequent fractures occurring in seven patients in the teriparatide group (cumulative incidence 1.1%) compared with 24 patients (cumulative incidence 3.9%) in the risedronate group (overall HR 0.29; 95% CI 0.14–0.58; p = 0.002). Refer to Table 1 for individual FRAX®-defined MOF locations.
The cumulative incidence for FRAX®-defined MOF, based on Kaplan-Meier estimates, and the adjusted HRs, estimated using the extended Cox model, is presented in Table 2. After 7 months of treatment, the HR with respect to the occurrence of FRAX®-defined MOF became statistically significant in patients treated with teriparatide compared with patients treated with risedronate (HR at 7 months 0.50; 95% CI 0.26–0.93; p = 0.03). Similar results were observed for the incidence of clinical fractures (i.e., clinical vertebral and NVFFx) (Table 3).
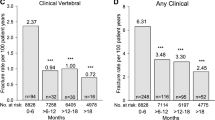
The incidence rates of FRAX®-defined MOF and clinical fractures by 6-month periods during 24 months of treatment are presented in Figs. 2 and 3, respectively. The overall incidence rate of FRAX®-defined MOF during the 24 months of follow-up (expressed as a number of fracture events per 100 patient-years) was lower in the teriparatide group than in the risedronate group (1.33 [95% CI 0.76–2.15] and 3.35 [95% CI 2.41–4.51], respectively), with an estimated overall IRR of 0.40 (95% CI 0.22–0.71; p = 0.002). Incidence rates of the clinical fractures, estimated overall and by 6-month periods, showed similar results, with a lower incidence rate in the teriparatide group compared with the risedronate group (2.52 [95% CI 1.71–3.55] and 5.18 [95% CI 4.00–6.57], respectively), with an estimated overall IRR of 0.49 (95% CI 0.31–0.75; p = 0.001). The largest reduction in the fracture incidence rate in the teriparatide group, when compared with the risedronate group, occurred at the 6- to 12-month period for both types of fractures (Fig. 2).
Incidence rates of FRAX®-defined major osteoporotic fracture (number of events per 100 patient-years) by 6-month periods and overall (0 to 24 months). Note: the incidence rates during each of the four 6-month period intervals are calculated as the number of patients who experienced at least one fracture event in the respective 6-month period, divided by the total amount of person-time contributed by the population at risk during the respective time interval. The overall incidence rate is estimated from 0 to 24 months
Incidence rates of pooled clinical fractures (clinical vertebral and non-vertebral) (number of events per 100 patient-years) by 6-month periods and overall (0 to 24 months). Note: data are presented as incidence rate. The incidence rate is calculated as the number of patients who experienced at least one fracture event in the respective 6-month period, divided by the total amount of person-time contributed by the population at risk during the respective time interval.
Discussion
In our analysis, patients treated with teriparatide had a 60% lower risk of sustaining FRAX®-defined major osteoporotic fractures during the 24-month treatment period, compared with those patients treated with risedronate. These results support and complement previous research indicating the increased antifracture efficacy of teriparatide over risedronate, alendronate, and placebo in postmenopausal women with severe osteoporosis and in glucocorticoid-induced osteoporosis [1, 8,9,10].
Our findings show similar fracture reductions using FRAX®-defined MOF compared with the predefined clinical fractures, which includes clinical vertebral and a more extended list of non-vertebral fracture sites.
Several other investigators have reported efficacy results using the FRAX®-defined MOF endpoint [3, 4, 11]. In the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE), Miller and colleagues [3] reported a 70% reduction in MOF in patients treated with abaloparatide compared with those who received placebo. This Phase 3 trial also compared abaloparatide with teriparatide for MOF, and at 18 months, the Kaplan-Meier-estimated event rate was 1.5% for the abaloparatide group versus 3.1% for the teriparatide group (HR 0.45; 95% CI 0.21–0.95; p = 0.03), with most of the MOF in this comparison being wrist fractures [3]. Similarly, in a large, multinational, randomized controlled trial involving postmenopausal women randomized to receive either romosozumab, an anti-sclerostin antibody, or alendronate over 12 months, Saag et al. [4] reported FRAX®-defined MOF event rates of 3.0% and 4.2%, respectively, with a statistically nonsignificant difference between the two drugs (HR 0.72; p = 0.053).
Early antifracture efficacy has been highlighted as a major need in osteoporosis care, particularly for those patients with recent fractures who are at imminent risk of fracture. In the current analysis, there was a statistically significant reduction in fractures between treatment groups as early as seven months for both categories of clinical fractures analyzed, with a statistically significant reduction in the IRR during the 6- to 12-month period.
These results add to the body of evidence suggesting a more rapid and greater fracture risk reduction in patients treated with anabolic therapies compared with antiresorptives [1, 4, 12, 13]. Our data confirm previous findings from the pivotal Phase 3 Fracture Prevention Trial, where a reduction in non-vertebral fracture risk was suggested starting after eight months of treatment with 20 or 40 μg of daily teriparatide [9]. This progressively increased, reaching statistical significance at the end of the study (i.e., at 20 months) in the Kaplan-Meier analyses [9]. Further to this early response, in a post hoc analysis of the Fracture Prevention Trial, Lindsay et al. [14] reported that increasing duration of teriparatide treatment was associated with reduced risk of NVFFx when compared with placebo. In that analysis, the Cox model showed that the relative hazard of specified NVFFx decreased by 9.5% for each additional month of teriparatide versus placebo (HR = 0.905; 95% CI 0.895–0.931, p = 0.002) [14]. Therefore, given its greater antifracture efficacy, early and more rapid onset of effect, teriparatide appears to be a superior clinical option to oral bisphosphonates for patients at high and imminent risk of fracture [15, 16]. No equivalent data comparing teriparatide with other antiresorptives are currently available.
This early efficacy of teriparatide compared with risedronate is also of clinical interest, as it has been suggested previously, using results from the combination of the multicenter Vertebral Efficacy with Risedronate Therapy (VERT) trials, that risedronate reduces the risk of clinical vertebral fractures in postmenopausal women with osteoporosis within 6 months of starting treatment (RR = 0.08; 95% CI 0.01–0.63) [17]. Results of that analysis suggest a 69% reduction in risk at one year (RR = 0.31; 95% CI 0.12–0.78) [17].
The current analysis has a number of strengths and limitations. The VERO study was the first randomized controlled trial that assessed fractures as the primary efficacy endpoint in a large subgroup of patients pretreated with bisphosphonates, thus reflecting real life, as in many countries worldwide, teriparatide use is limited to patients who develop fracture while receiving antiresorptive therapy. The VERO study population is based on patients with high fracture risk, a population that should be considered for osteoanabolic therapy. Furthermore, patient selection was based on strong predictors of fracture risk, given the inclusion of a combination of multiple or severe prevalent vertebral fractures with low BMD, which both are independent and additive indicators of high imminent fracture risk [18]. The analysis of the FRAX®-defined MOF endpoint was a post hoc analysis; however, the extended Cox regression model to assess the speed of the clinical fracture efficacy was predefined in the statistical analysis plan. Finally, given the patient population analyzed, these results may not be generalized to men, premenopausal women, or patients with less severe osteoporosis.
In conclusion, our post hoc analysis of the VERO trial shows that, in postmenopausal women with severe osteoporosis, teriparatide treatment was more efficacious than risedronate, with a 60% lower risk of FRAX®-defined MOF occurrence during the 24-month treatment period. Our study also detected a significant reduction of risk at seven months of treatment, confirming that this osteoanabolic treatment is particularly indicated for patients at imminent risk of fractures.
References
Kendler D, Marin F, Zerbini C, Russo L, Greenspan S, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A, Lespessailles E, Minisola S, Body J, Geusens P, Möricke R, Lopez-Romero P (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391(10117):230–240. https://doi.org/10.1016/s0140-6736(17)32137-2
Kanis J, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19(4):385–397. https://doi.org/10.1007/s00198-007-0543-5
Miller P, Hattersley G, Riis B, Williams G, Lau E, Russo L, Alexandersen P, Zerbini C, Hu M, Harris A, Fitzpatrick L, Cosman F, Christiansen C (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316(7):722–733. https://doi.org/10.1001/jama.2016.11136
Saag K, Petersen J, Brandi M, Karaplis A, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner P, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377(15):1417–1427. https://doi.org/10.1056/NEJMoa1708322
Strom O, Borgstrom F, Kanis J, Compston J, Cooper C, McCloskey E, Jonsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155. https://doi.org/10.1007/s11657-011-0060-1
European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on the evaluation of medicinal products in the treatment of primary osteoporosis. London, 16 November 2006. Doc. Ref. CHMP/EWP/552/95 Rev.2
Genant H, Wu C, van Kuijk C, Nevitt M (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148. https://doi.org/10.1002/jbmr.5650080915
Hadji P, Zanchetta J, Russo L, Recknor C, Saag K, McKiernan F, Silverman S, Alam J, Burge R, Krege J, Lakshmanan M, Masica D, Mitlak B, Stock J (2012) The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int 23(8):2141–2150. https://doi.org/10.1007/s00198-011-1856-y
Neer R, Arnaud C, Zanchetta J, Prince R, Gaich G, Reginster J, Hodsman A, Eriksen E, Ish-Shalom S, Genant H, Wang O, Mellström D, Oefjord E, Marcinowska-Suchowierska E, Salmi J, Mulder H, Halse J, Sawicki A, Mitlak B (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441. https://doi.org/10.1056/nejm200105103441904
Saag K, Shane E, Boonen S, Marín F, Donley D, Taylor K, Dalsky G, Marcus R (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357(20):2028–2039. https://doi.org/10.1056/NEJMoa071408
McCloskey E, Johansson H, Oden A, Harvey N, Jiang H, Modin S, Fitzpatrick L, Kanis J (2017) The effect of abaloparatide-SC on fracture risk is independent of baseline FRAX fracture probability: a post hoc analysis of the ACTIVE study. J Bone Miner Res 32(8):1625–1631. https://doi.org/10.1002/jbmr.3163
Diez-Perez A, Marin F, Eriksen E, Kendler D, Krege J, Delgado-Rodriguez M (2019) Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone 120:1–8. https://doi.org/10.1016/j.bone.2018.09.020
Kanis J, Harvey N, McCloskey E, Bruyère O, Veronese N, Lorentzon M, Cooper C, Rizzoli R, Adib G, Al-Daghri N, Campusano C, Chandran M, Dawson-Hughes B, Javaid K, Jiwa F, Johansson H, Lee J, Liu E, Messina D, Mkinsi O, Pinto D, Prieto-Alhambra D, Saag K, Xia W, Zakraoui L, Reginster J (2020) Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int 31(1):1–12. https://doi.org/10.1007/s00198-019-05176-3
Lindsay R, Miller P, Pohl G, Glass E, Chen P, Krege J (2009) Relationship between duration of teriparatide therapy and clinical outcomes in postmenopausal women with osteoporosis. Osteoporos Int 20(6):943–948. https://doi.org/10.1007/s00198-008-0766-0
Johansson H, Siggeirsdottir K, Harvey N, Oden A, Gudnason V, McCloskey E, Sigurdsson G, Kanis J (2017) Imminent risk of fracture after fracture. Osteoporos Int 28(3):775–780. https://doi.org/10.1007/s00198-016-3868-0
Geusens P, Marin F, Kendler D, Russo L, Zerbini C, Minisola S, Body J, Lespessailles E, Greenspan S, Bagur A, Stepan J, Lakatos P, Casado E, Moericke R, Lopez-Romero P, Fahrleitner-Pammer A (2018) Effects of teriparatide compared with risedronate on the risk of fractures in subgroups of ostmenopausal women with severe osteoporosis: the VERO trial. J Bone Miner Res 33(5):783–794. https://doi.org/10.1002/jbmr.3384
Roux C, Seeman E, Eastell R, Adachi J, Jackson R, Felsenberg D, Songcharoen S, Rizzoli R, Di Munno O, Horlait S, Valent D, Watts N (2004) Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin 20(4):433–439. https://doi.org/10.1185/030079903125003125
Siris E, Genant H, Laster A, Chen P, Misurski D, Krege J (2007) Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int 18(6):761–770. https://doi.org/10.1007/s00198-006-0306-8
Acknowledgments
We are indebted to Anja Gentzel-Jorczyk (Clinical Trial Project Manager), Estrella Crespo, and Laura Briones (Data Management) for their contribution to the study; to Emily Kelleher, for the editorial assistance; to the members of the investigational teams at the study centers; and to the women who participated in the study.
Funding
Eli Lilly and Company funded the VERO clinical trial.
Data Sharing AgreementLilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Jean-Jacques Body: consultant fees from Amgen, Sandoz, and UCB; research support from Lilly. Fernando Marin: employee, Lilly. David L. Kendler: honoraria, research grants, and/or consultant fees from Amgen, Lilly, Radius, and Pfizer. Cristiano A.F. Zerbini: research support from Lilly. Pedro López-Romero: employee, Lilly. Rüdiger Möricke: none. Enrique Casado: speaker and/or consultant fees from Amgen, Lilly, UCB, and Theramex. Astrid Fahrleitner-Pammer: speaker fees from Amgen, Alexion, BMS, Lilly, Fresenius, Sandoz, Shire, and UCB. Jan J. Stepan: none. Eric Lespessailles: speaker and consultant fees from Amgen, Expanscience, Lilly, and MSD; research grants from Abbvie, Amgen, Lilly, MSD, and UCB. Salvatore Minisola: speaker and/or consultant fees from Abiogen, Amgen, DiaSorin, Lilly, Italfarmaco, Fujii, MSD, and Takeda. Piet Geusens: consultant and/or speaker fees from Lilly, and research support from Pfizer, Abbott, Lilly, Amgen, MSD, Roche, UCB, BMS, Mylan, and Novartis.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The following investigators randomized at least one patient in the VERO trial: Argentina: A. Alvarisqueta, A. Bagur, C. Gómez, L. Maffei, F. Massari; Austria: E. Boschitz, A. Fahrleitner-Pammer, G. Höfle, H. Koller, C. Muschitz, E. Preisinger; Belgium: I. Beyer, J.J. Body, K. de Vlam, V. Gangji, P. Geusens, E. Gielen, S. Goemare, M. Leon, J.-Y. Reginster, M. Van den Berghe, R. Witvrouw; Brazil: J. Borges, M. Castro, L.A. Russo, C.A. Zerbini; Canada: J. Adachi, J.P. Brown, A. Cheung, S. Kaiser, A. Karaplis, D.L. Kendler, F. Morin, W. Olszynski, S. Seigel; Czech Republic: E. Dokoupilova, M. Ladova, R. Pikner, J. Stepan, V. Zikan; France: R. Chapurlat, J. Fulpin, C. Marcelli, M. Laroche, E. Lespessailles, T. Thomas; Germany: G. Dahmen, J. Fakler, I. Frieling, P. Hadji, R. Möricke, F. Thomasius, L. Unger, V. Ziller; Greece: M. Daniilidis, E. Foufoulas, G. Ioannidis, M. Kita, I. Kyrkos, I. Panagiotopoulos, S. Pnevmaticos; Hungary: E. Kanakaridu, K. Kudlak, P. Lakatos, K. Nagy, P. Somogyi, P. Suranyi, Z. Valkusz; Italy: G. Bianchi, M.L. Brandi, S. Giannini, G. Isaia, S. Minisola, G. Osella, M. Rossini; Poland: T. Blicharski, J. Brzezicki, P. Leszczynski, M. Mazurek, M. Rell-Bakalarska, J. Supronik; Spain: M.J. Amerigo, M. Bernad, E. Casado, N. Chozas, M. Díaz-Curiel, J. Malouf-Serra, J.A. Román, F.J. Tarazona; USA and Puerto Rico: N. Binkley, M. Bolognese, P. Bressler, M. Carroll, A. Chang, D. Cox, A. de la Llana, A. Dulgeroff, H. El-Kadi, M. Goldberg, S. Greenspan, H. Kenney, A. Kivitz, M.E. Lewiecki, M. Lillestol, P. Miller, A. Myers, P. Norwood, M. Perini, S. Rao, R.R. Recker, C.P. Recknor, H. Rodríguez, K.G. Saag, R. Sachson, C. Shuhart, O. Soto-Raíces, M. Spiegel.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Body, JJ., Marin, F., Kendler, D. et al. Efficacy of teriparatide compared with risedronate on FRAX®-defined major osteoporotic fractures: results of the VERO clinical trial. Osteoporos Int 31, 1935–1942 (2020). https://doi.org/10.1007/s00198-020-05463-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05463-4