Abstract
Summary
We explored relations between serum hepatocyte growth factor (HGF), disease activity, osteoproliferation, and bone mineral density (BMD) in ankylosing spondylitis (AS), in comparison with healthy controls. HGF was increased especially in male AS patients and smokers and associated with both lower BMD and more chronic radiographic changes in the spine.
Introduction
Ankylosing spondylitis (AS) is characterized by both osteoproliferation and increased bone loss. Biomarkers are requested to predict the processes. The aims of this study were to compare serum levels of hepatocyte growth factor (HGF), matrix metalloproteinase-3 (MMP-3), and vascular endothelial growth factor (VEGF) in AS patients with healthy controls (HC) and to explore the associations with disease activity, osteoproliferation, and bone mineral density (BMD).
Methods
Serum from AS patients (modified NY-criteria) and HC was analyzed for HGF, MMP-3, and VEGF with ELISA. Disease activity parameters were collected. Osteoproliferation was assessed with modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) and BMD was measured in femoral neck.
Results
Totally, 204 AS patients and 80 sex and age matched HC were included. Serum HGF was higher in the AS patients compared with the HC, whereas serum MMP-3 and VEGF were not. Serum HGF was also higher in smokers and in the male AS patients positively correlated with age, BASMI, and mSASSS, and negatively correlated with BMD. The biomarkers were all positively associated with ESR, CRP, and WBC. In multiple linear regression analysis serum HGF remained associated with higher mSASSS and lower BMD, after adjusting for age, sex, CRP, smoking, and body mass index.
Conclusions
Serum HGF was increased in male AS patients and associated with higher mSASSS and lower BMD. In addition, serum HGF was positively associated with risk factors for osteoproliferation such as age, CRP and smoking. HGF could be a potential biomarker of importance for the bone metabolism in AS.
Trial registration
NCT00858819.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease characterized by sacroiliitis, accompanied by inflammation of the spine and the entheses. Involvement of peripheral joints is common. Extra skeletal manifestations include anterior uveitis and intestinal inflammation. AS is also characterized by osteoproliferation, which can be seen as an enhanced reparative response to inflammation, mechanical stress or micro-damage. At the same time, AS patients are at increased risk of developing osteoporosis.
Disease activity assessment in AS is limited to patient self-reported questionnaires and standard laboratory measures such as acute phase reactants. Erythrocyte sedimentation (ESR) and C-reactive protein (CRP) do not entirely reflect the disease process in patients with AS [1]. There is a need for biomarkers assessing disease activity, disease progression, and response to therapy.
Hepatocyte growth factor (HGF), originally discovered as a mitogen of hepatocytes, is a multi-functional cytokine involved in embryogenesis, organogenesis, wound healing, and tissue repair, but it also plays a role in tumorigenesis and cancer invasion [2]. HGF is mainly expressed by stromal cells and binds to receptor-tyrosine kinase c-MET. HGF-MET signaling leads to a variety of cellular responses including morphogenesis, proliferation, cell survival, regeneration, and tissue protection in inflammatory diseases [3, 4]. In the immune system, HGF mainly has anti-inflammatory effects [5]. Interestingly, c-MET is also expressed on osteoblasts and osteoclasts indication that HGF may regulate bone metabolism [6].
Matrix metalloproteinases (MMPs) are proteins involved in the breakdown of extracellular matrix proteins and active during tissue remodeling in normal physiological processes as well as disease processes like arthritis and tumor metastasis. MMPs are produced in response to pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) [7, 8]. There have been promising results on the association between disease activity in AS and MMP-3 [9].
Vascular endothelial growth factor (VEGF) is a signaling protein involved in angiogenesis, an important process in the pathogenesis of chronic inflammatory disorders such as rheumatoid arthritis [10]. Histopathological examinations of sacroiliitis and peripheral arthritis in AS have also demonstrated increased vascularity in the synovial tissues, indicating the importance of angiogenesis in this disease [11, 12]. Earlier studies have reported significant correlations between serum VEGF and disease activity in AS [13, 14].
Aims
The aims of this study were to compare the serum levels of HGF, MMP-3, and VEGF in AS patients with healthy controls and to explore the associations between the biomarkers and disease activity, osteoproliferation, and bone mineral density in the AS patients.
Materials and methods
Patients and controls
A total of 204 patients were recruited from three rheumatologic sites in the west of Sweden, the Sahlgrenska University Hospital in Gothenburg, and the Rheumatology Departments in Borås and Alingsås. All patients fulfilled the modified New York criteria for AS [15]. Exclusion criteria were psoriasis, inflammatory bowel disease, dementia, pregnancy, and other concomitant rheumatologic diseases. Due to extensive questionnaires included in the study, patients with language difficulties were also excluded.
The healthy control group consisted of 80 blood donors, who were recruited when giving blood at the Sahlgrenska University Hospital. All blood donors answered a questionnaire stating that they were in full health and not on any medication, before blood samples were collected.
Written informed consent was obtained from each participant in the study. The study was approved by the regional ethics committee in Gothenburg and carried out in accordance with the Helsinki Declaration.
Clinical assessment
All patients underwent physical examination by the same physician (EK) and blood samples were drawn. Body weight and height were measured and body mass index (BMI) was calculated. Disease activity and function was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP), the Bath Ankylosing Spondylitis Patient Global Score (BAS-G), the Bath Ankylosing Spondylitis Functional Index (BASFI), and the Bath Ankylosing Spondylitis Metrology Index (BASMI) [16].
Laboratory assessment
The levels of ESR, CRP, hemoglobin (Hb), white blood cell count (WBC), and platelet count (PLT) were analyzed using standard laboratory techniques at the hospitals. Serum was stored at − 80° until analysis. The biomarkers, HGF (pg/mL), MMP-3 (ng/mL), and VEGF (pg/mL) were analyzed in serum using enzyme-linked immunosorbent assay (ELISA) kits (Quantikine® ELISA, R&D Systems, Inc., Minneapolis, MN, USA) following the manufacturer’s instructions. Serum carboxyterminal telopeptide of type 1 collagen (CTX-1), a biomarker for bone resorption, and serum osteocalcin, a marker of bone formation, were also measured in order to study their association with HGF, MMP-3, and VEGF. Serum CTX-1 and osteocalcin were analyzed with ELISA (Immunodiagnostic Systems, Tyne and Wear, UK). Absorbance was read at 450 nm in a spectrophotometer (SpectraMax 340PC384, Molecular Devices). The software SoftMax Pro 5.2 was used to calculate the biomarker concentrations. The limits of detection (LoD) and lower limits of quantification (LLoQ) for each biomarker were as follows: HGF: LoD = 40 pg/mL, LLoQ = 125 pg/mL. MMP-3: LoD = 0.002 ng/mL, LLoQ = 0.156 ng/mL. VEGF: LoD = 9 pg/mL, LLoQ = 31.2 pg/mL. CTX-1: LoD = 0.020 ng/nL, LLoQ = 0.020 ng/nL. Osteocalcin: LoD = 0.5 ng/mL, and LLoQ = 0.5 ng/mL.
Bone mineral density (BMD) and radiographic assessment
BMD was measured using dual-energy X-ray absorptiometry (DXA) (Hologic Discovery A, Hologic Inc., Bedford, MA, USA) in the femoral neck of the non-dominant hip. BMD of femoral neck was used since it is less affected by osteoproliferation than BMD of lumbar spine in AS and therefore more adequately reflects bone loss [17]. Lateral radiographs of the cervical and lumbar spine were acquired. Chronic radiographic changes related to AS in the spine were assessed by the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) [18].
Statistical analysis
All data was analyzed using SPSS Statistics version 25 (SPSS Inc., IBM, Chicago, IL, USA). Descriptive statistics are presented as median and inter-quartile range (IQR). In comparisons between groups, the Mann–Whitney U test was used for continuous variables and the chi-square test for categorical variables. Correlations were calculated using Spearman’s correlation (rs). In dichotomous variables, an event was coded 1 and no event was coded 0. Sex was coded 1 for women and 2 for men. Due to its skewed distribution, mSASSS was log-transformed before used as an outcome in linear regression analysis and patients with only sacroiliitis (mSASSS = 0) were excluded, leaving data from 148 patients. Linear regression with a stepwise method was run with 10Log mSASSS and BMD of femoral neck as outcomes. Covariates in the regression analyses were the studied biomarkers and variables known to be associated with osteoproliferation and osteoporosis: sex, age, BMI, smoking, and CRP. All tests were two-tailed and p < 0.05 was considered statistically significant.
Results
Characteristics of the participants
The characteristics of the 204 patients with AS are shown in Table 1. The median age of the AS patients was 49 (IQR, 41–62) years, median disease duration was 24 (13–34) years, and 57% (N = 117) were men. The healthy control group consisted of 80 blood donors, free from underlying disease and medication, with median age 48.5 (IQR 41–57) years and 66% (N = 54) were men. The distribution of age (p = 0.100) and sex (p = 0.202) was not significantly different between the patient and the healthy control group.
Serum levels of biomarkers in AS patients and controls
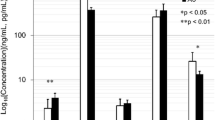
The male AS patients had higher serum levels of HGF, CTX-1 and osteocalcin compared with the healthy male controls, whereas no such differences were found between the female patients and female controls (Table 2).
Serum MMP-3 and VEGF were both evenly distributed over AS patients and controls. Men had however higher serum levels of MMP-3 than women both among the AS patients [28.64 (21.12–36.71) vs. 16.12 (12.41–22.11) ng/mL p < 0.001] and the healthy controls [23.31 (19.44–35.99) vs. 14.73 (11.06–19.54) ng/mL p < 0.001].
As shown in Table 3, the serum levels of HGF correlated positively with MMP-3 and VEGF in AS patients of both sexes, whereas serum MMP-3 was positively correlated with VEGF only in the female patients. Serum CTX-1 was positively correlated with serum osteocalcin (AS all patients rS = 0.493, p < 0.001; AS women rS = 0.520, p < 0.001; AS men rS = 0.469, p < 0.001), but no significant correlation was found between the serum levels of CTX-1 or osteocalcin and HGF, VEGF, or MMP-3.
Age and biomarkers
Increasing age was correlated with higher levels of serum HGF (rs = 0.316, p = 0.001) in the male AS patients, but not in the female AS patients. Serum VEGF was positively correlated with age in both the male (rs = 0.206, p = 0.026) and in the female AS patients (rs = 0.255, p = 0.017), whereas serum MMP-3, CTX-1, and osteocalcin were not associated with age in the AS patients. In the healthy control group, osteocalcin was negatively correlated with age among women (rs = − 0.494, p = 0.010), but none of the other biomarkers were associated with age in the healthy control group.
Biomarkers and disease activity in AS
The associations between disease activity parameters and the biomarkers are shown in Table 4. ESR, CRP, and WBC were all positively correlated with serum levels of HGF, MMP-3, and VEGF. Furthermore, both serum HGF and MMP-3 were positively correlated with swollen joints count. Serum HGF was positively associated with BASMI among the male patients (rs = 0.323, p < 0.001), but not among the female patients. Serum CTX-1 and osteocalcin were not associated with any parameters reflecting disease activity.
Biomarkers and smoking among AS patients
Smoking AS patients had higher serum HGF and CTX-1 than non-smokers [1715 (1431–2086) vs. 1458 (1235–1706) pg/mL; p = 0.002] and [0.31 (0.24–0.39) vs. 0.24 (0.17–0.35 ng/mL) p = 0.032] respectively, whereas serum MMP-3, VEGF, and osteocalcin were not associated with smoking status.
Biomarkers and osteoproliferation and BMD in AS
The AS men had significantly higher mSASSS than the AS women [8 (2–34) vs. 2 (0–10); p < 0.001].
mSASSS was positively correlated with serum HGF and MMP-3 (Table 4).
In the male patient group, serum HGF correlated with mSASSS (rs = 0.302, p = 0.001), but the association was non-significant among the female patients.
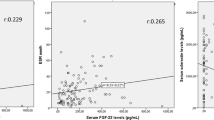
Serum HGF and VEGF were also correlated with lower BMD in the femoral neck. Whereas, serum CTX-1 and osteocalcin were not correlated with mSASSS or BMD of femoral neck.
Medication and biomarkers
The medication of the patients is demonstrated in Table 1. Totally, 76% of the patients on a tumor necrosis factor inhibitor (TNFi) were co-medicated with a conventional synthetic disease-modifying anti-rheumatic drug (csDMARD), mostly methotrexate 15 mg weekly.
Users of glucocorticoids had higher levels of HGF compared with non-users [2382 (1706–2927) vs. 1475 (1245–1714) pg/mL; p = 0.001]. Serum VEGF was significantly lower in users of TNFi compared non-users [249.4 (178.1–457.4) vs. 352.0 (224.3–558.4) pg/mL; p = 0.042]. Serum MMP-3 was not associated with use of glucocorticoids or TNFi and none of the biomarkers were associated with use of non-steroidal anti-inflammatory drugs (NSAIDs) or csDMARDs. No association was found between serum CTX-1, osteocalcin, and medication.
Multivariate analyses
The results of the linear regressions are demonstrated in Table 5. The analysis showed that 10 Log mSASSS was positively associated with male sex, age, and serum HGF. BMD of femoral neck was positively associated with male sex and BMI and negatively associated with age and serum HGF.
Discussion
The main findings of this study were that the serum levels of HGF were higher in the male AS patients than in the male healthy controls and associated with higher mSASSS and lower BMD of femoral neck after adjusting for other risk factors for osteoproliferation and osteoporosis. The serum levels of HGF increased with age in the male AS patients, but not in the male controls. The male AS patients also had higher serum levels of CTX-1 and osteocalcin than the male healthy controls, indicating both increased bone resorption and new bone formation in male AS patients.
AS is characterized by both osteoproliferation and increased bone loss. HGF also has dual effects on bone. Both osteoblasts and osteoclasts express c-MET and HGF is synthesized by osteoclasts [6]. HGF has been shown to promote osteoblastic differentiation of mesenchymal stem cells, osteoblast proliferation, and osteogenesis [19]. HGF treatment has led to enhanced fracture healing in animal models [20]. On the other hand, HGF also stimulates migration, cell division, and morphological changes in osteoclasts [21]. The association between serum HGF and male sex, age, smoking, and elevated CRP is intriguing, since these are all risk factors for osteoporosis and osteoproliferation in AS [17, 22]. Biomarkers for bone resorption, CTX-1, and new bone formation osteocalcin were however not associated with serum HGF, BMD, or mSASSS in the AS patients.
The elevated serum HGF in AS could also be a response to inflammation, since HGF was positively correlated with inflammatory parameters, especially WBC. HGF has pleiotropic effects in immunomodulation and inflammation. HGF is secreted by stromal cells of lymphoid organs and HGF and c-MET expression is enhanced by pro-inflammatory cytokines. HGF has been shown to have anti-inflammatory effects on monocytes, macrophages, dendritic cells, and B- and T-lymfocytes [5]. Animal models have also demonstrated that HGF mainly has anti-inflammatory effects, since HGF therapy in different animal models ameliorated collagen-induced arthritis, autoimmune encephalomyelitis, experimental colitis, pulmonary fibrosis, and renal injury in chronic renal disease and also prevented lupus nephritis [5, 23,24,25,26]. In addition, HGF has angiogenetic effects and counteracts the effects of TGF-β and other fibrogenic cytokines [3].
The results from the present study indicate that HGF could be involved in the process of osteoporosis and osteoproliferation in AS, although the pathophysiologic mechanisms are unknown. The increase in serum HGF in the AS patients was however modest and the study gives no support for serum HGF as a diagnostic tool.
One earlier study on AS assessing multiple cytokines and MMPs in serum showed that HGF was positively correlated with BASDAI, BASFI, and BAS-G in univariate analyses and that HGF together with MMP-8, MMP-9, and CXCL8 from a principal component analysis was associated with higher BASDAI and smoking status in multivariate analyses [27]. Smokers had however not higher HGF than non-smokers in the study.
Earlier studies on rheumatoid arthritis (RA) have shown increased levels of HGF in serum and synovial fluid in RA and that an elevated serum HGF is predictive of joint damage and radiographic progression [28,29,30]. Similar to our study, it is unclear if HGF contributes do damage or if the increased serum HGF is a response to inflammation. In patients with systemic lupus erythematosus (SLE), a high HGF expression and a low TGF-β expression in kidney biopsies was associated with a favorable response to cyclophosphamide and less chronic tubulointerstitial damage [31]. Interestingly, elevated serum HGF and mucosal overexpression of HGF and c-MET has also been reported in inflammatory bowel disease in young adults and children [32].
In the field of cancer research, aberrant HGF-MET signaling from cancer-associated fibroblasts has been shown to promote growth and survival of cancer cells, drug resistance, and metastatic spreading [33]. Elevated levels of serum HGF have been associated with poor prognosis in several tumor types and HGF and MET have emerged as interesting therapeutic targets in cancer treatment with several ongoing clinical trials [34,35,36].
In the present study, serum MMP-3 was positively associated with mSASSS, swollen joints count, ESR, CRP, and WBC. Previous studies have indicated that serum MMP-3 could be a useful marker for disease activity in AS, particularly in patients with peripheral synovitis [37,38,39]. Maksymowych et al. reported that higher serum levels of MMP-3 could be predictive of greater radiographic progression in AS, especially in patients with pre-existing syndesmophytes [40]. Other studies found that MMP-3 levels are reduced in response to treatment with TNFi [37, 38, 41, 42].
Serum VEGF was associated with higher ESR, CRP, and WBC and lower BMD of femoral neck in the present study. Serum VEGF was also lower in users of TNF-blockers compared with non-users. The finding is in accordance with the previous studies by Tošovský et al. [43] and Pedersen et al. [44] who also reported that AS patients treated with TNF inhibitors had lower serum VEGF compared to untreated patients. The association between VEGF and BMD has not previously been found in AS patients. Serum VEGF analyzed as a measure for progression of new bone formation has produced conflicting results [45, 46].
The strengths of the present study were the large and well-characterized cohort of AS patients and that the patients were evaluated with both radiography and DXA. A limitation was the cross-sectional design of the study. A longitudinal study is needed to show if serum HGF is a predictor for radiologic progression and osteoporosis. Despite being statistically significant, the increased in serum HGF in the AS patients was modest and the study gives no so support for serum HGF as a diagnostic tool.
Another limitation is the lack of information about smoking status in the healthy control group, since this may have affected the level of serum HGF in the control group. The results also need to be confirmed in other cohorts of patients with AS.
Conclusions
We show that serum HGF was higher in male AS patients and independently associated with higher mSASSS and lower BMD of femoral neck. Serum HGF was also positively associated with CRP, WBC, and smoking status. The findings suggest that HGF could be of importance for osteoproliferation and bone loss in AS, but an elevated HGF may also reflect the level of inflammatory activity.
In contrast, the serum levels of MMP-3 and VEGF were not elevated in the AS patients, but positively associated with standard inflammatory laboratory parameters. In addition, MMP-3 was associated with swollen joints count, and VEGF with lower BMD of femoral neck.
References
Spoorenberg A, van der Heijde D, de Klerk E, Dougados M, de Vlam K, Mielants H, van der Tempel H, van der Linden S (1999) Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol 26:980–984
Petrini I (2015) Biology of MET: a double life between normal tissue repair and tumor progression. Ann Transl Med 3:82
Nakamura T, Mizuno S (2010) The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci 86:588–610
Funakoshi H, Nakamura T (2003) Hepatocyte growth factor: from diagnosis to clinical applications. Clin Chim Acta 327:1–23
Molnarfi N, Benkhoucha M, Funakoshi H, Nakamura T, Lalive PH (2015) Hepatocyte growth factor: a regulator of inflammation and autoimmunity. Autoimmun Rev 14:293–303
Grano M, Galimi F, Zambonin G, Colucci S, Cottone E, Zallone AZ, Comoglio PM (1996) Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci U S A 93:7644–7648
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y (2000) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 59:455–461
Gao JW, Zhang KF, Lu JS, Su T (2015) Serum matrix metalloproteinases-3 levels in patients with ankylosing spondylitis. Genet Mol Res 14:17068–17078
Koch AE (1998) Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum 41:951–962
Kidd BL, Moore K, Walters MT, Smith JL, Cawley MI (1989) Immunohistological features of synovitis in ankylosing spondylitis: a comparison with rheumatoid arthritis. Ann Rheum Dis 48:92–98
Revell PA, Mayston V (1982) Histopathology of the synovial membrane of peripheral joints in ankylosing spondylitis. Ann Rheum Dis 41:579–586
Drouart M, Saas P, Billot M, Cedoz JP, Tiberghien P, Wendling D, Toussirot E (2003) High serum vascular endothelial growth factor correlates with disease activity of spondylarthropathies. Clin Exp Immunol 132:158–162
Goldberger C, Dulak J, Duftner C, Weidinger F, Falkenbach A, Schirmer M (2002) Vascular endothelial growth factor (VEGF) in ankylosing spondylitis--a pilot study. Wien Med Wochenschr 152:223–225
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Sieper J, Rudwaleit M, Baraliakos X et al (2009) The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 68(Suppl 2):ii1–i44
Deminger A, Klingberg E, Lorentzon M, Geijer M, Gothlin J, Hedberg M, Rehnberg E, Carlsten H, Jacobsson LT, Forsblad-d’Elia H (2017) Which measuring site in ankylosing spondylitis is best to detect bone loss and what predicts the decline: results from a 5-year prospective study. Arthritis Res Ther 19:273
Creemers MC, Franssen MJ, van’t Hof MA, Gribnau FW, van de Putte LB, van Riel PL (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 64:127–129
Frisch RN, Curtis KM, Aenlle KK, Howard GA (2016) Hepatocyte growth factor and alternative splice variants - expression, regulation and implications in osteogenesis and bone health and repair. Expert Opin Ther Targets 20:1087–1098
Matsubara H, Tsuchiya H, Watanabe K, Takeuchi A, Tomita K (2008) Percutaneous nonviral delivery of hepatocyte growth factor in an osteotomy gap promotes bone repair in rabbits: a preliminary study. Clin Orthop Relat Res 466:2962–2972
Blanquaert F, Delany AM, Canalis E (1999) Fibroblast growth factor-2 induces hepatocyte growth factor/scatter factor expression in osteoblasts. Endocrinology 140:1069–1074
Deminger A, Klingberg E, Geijer M, Gothlin J, Hedberg M, Rehnberg E, Carlsten H, Jacobsson LT, Forsblad-d’Elia H (2018) A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther 20:162
Benkhoucha M, Santiago-Raber ML, Schneiter G, Chofflon M, Funakoshi H, Nakamura T, Lalive PH (2010) Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 107:6424–6429
Tahara Y, Ido A, Yamamoto S, Miyata Y, Uto H, Hori T, Hayashi K, Tsubouchi H (2003) Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther 307:146–151
Okunishi K, Dohi M, Fujio K, Nakagome K, Tabata Y, Okasora T, Seki M, Shibuya M, Imamura M, Harada H, Tanaka R, Yamamoto K (2007) Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J Immunol 179:5504–5513
Kuroiwa T, Iwasaki T, Imado T, Sekiguchi M, Fujimoto J, Sano H (2006) Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft-versus-host disease. Arthritis Res Ther 8:R123
Mattey DL, Packham JC, Nixon NB, Coates L, Creamer P, Hailwood S, Taylor GJ, Bhalla AK (2012) Association of cytokine and matrix metalloproteinase profiles with disease activity and function in ankylosing spondylitis. Arthritis Res Ther 14:R127
Grandaunet B, Syversen SW, Hoff M, Sundan A, Haugeberg G, van Der Heijde D, Kvien TK, Standal T (2011) Association between high plasma levels of hepatocyte growth factor and progression of radiographic damage in the joints of patients with rheumatoid arthritis. Arthritis Rheum 63:662–669
Yukioka K, Inaba M, Furumitsu Y, Yukioka M, Nishino T, Goto H, Nishizawa Y, Morii H (1994) Levels of hepatocyte growth factor in synovial fluid and serum of patients with rheumatoid arthritis and release of hepatocyte growth factor by rheumatoid synovial fluid cells. J Rheumatol 21:2184–2189
Feuerherm AJ, Borset M, Seidel C, Sundan A, Leistad L, Ostensen M, Faxvaag A (2001) Elevated levels of osteoprotegerin (OPG) and hepatocyte growth factor (HGF) in rheumatoid arthritis. Scand J Rheumatol 30:229–234
Capuano A, Costanzi S, Peluso G, Zannoni G, Vellone VG, Gremese E, Zoli A, Scott C, Beltrami CA, Romano G, Ferraccioli G (2006) Hepatocyte growth factor and transforming growth factor beta1 ratio at baseline can predict early response to cyclophosphamide in systemic lupus erythematosus nephritis. Arthritis Rheum 54:3633–3639
Srivastava M, Zurakowski D, Cheifetz P, Leichtner A, Bousvaros A (2001) Elevated serum hepatocyte growth factor in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 33:548–553
Owusu BY, Galemmo R, Janetka J, Klampfer L (2017) Hepatocyte growth factor, a key tumor-promoting factor in the tumor microenvironment. Cancers (Basel) 9
Seidel C, Borset M, Turesson I, Abildgaard N, Sundan A, Waage A (1998) Elevated serum concentrations of hepatocyte growth factor in patients with multiple myeloma. The Nordic Myeloma Study Group. Blood 91:806–812
Bradley CA, Salto-Tellez M, Laurent-Puig P, et al (2017) Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat Rev Clin Oncol
Matsumoto K, Umitsu M, De Silva DM, Roy A, Bottaro DP (2017) Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci 108:296–307
Yang C, Gu J, Rihl M, Baeten D, Huang F, Zhao M, Zhang H, Maksymowych WP, de Keyser F, Veys EM, Yu DTY (2004) Serum levels of matrix metalloproteinase 3 and macrophage colony-stimulating factor 1 correlate with disease activity in ankylosing spondylitis. Arthritis Rheum 51:691–699
Vandooren B, Kruithof E, Yu DT, Rihl M, Gu J, De Rycke L, Van Den Bosch F, Veys EM, De Keyser F, Baeten D (2004) Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and down-regulation by tumor necrosis factor alpha blockade in spondylarthropathy. Arthritis Rheum 50:2942–2953
Chen CH, Lin KC, Yu DT et al (2006) Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in ankylosing spondylitis: MMP-3 is a reproducibly sensitive and specific biomarker of disease activity. Rheumatology 45:414–420
Maksymowych WP, Landewe R, Conner-Spady B, Dougados M, Mielants H, van der Tempel H, Poole AR, Wang N, van der Heijde D (2007) Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum 56:1846–1853
Pedersen SJ, Hetland ML, Sorensen IJ, Ostergaard M, Nielsen HJ, Johansen JS (2010) Circulating levels of interleukin-6, vascular endothelial growth factor, YKL-40, matrix metalloproteinase-3, and total aggrecan in spondyloarthritis patients during 3 years of treatment with TNFalpha inhibitors. Clin Rheumatol 29:1301–1309
Maksymowych WP, Rahman P, Shojania K, Olszynski WP, Thomson GT, Ballal S, Wong RL, Inman RD, Group MS (2008) Beneficial effects of adalimumab on biomarkers reflecting structural damage in patients with ankylosing spondylitis. J Rheumatol 35:2030–2037
Tosovsky M, Bradna P, Andrys C, Andrysova K, Cermakova E, Soukup T (2014) The VEGF and BMP-2 levels in patients with ankylosing spondylitis and the relationship to treatment with tumour necrosis factor alpha inhibitors. Acta Med (Hradec Kralove) 57:56–61
Pedersen SJ, Sorensen IJ, Garnero P, Johansen JS, Madsen OR, Tvede N, Hansen MS, Thamsborg G, Andersen LS, Majgaard O, Loft AG, Erlendsson J, Asmussen K, Jurik AG, Moller J, Hasselquist M, Mikkelsen D, Skjodt T, Lambert R, Hansen A, Ostergaard M (2011) ASDAS, BASDAI and different treatment responses and their relation to biomarkers of inflammation, cartilage and bone turnover in patients with axial spondyloarthritis treated with TNFalpha inhibitors. Ann Rheum Dis 70:1375–1381
Braun J, Baraliakos X, Hermann KG, Xu S, Hsu B (2016) Serum vascular endothelial growth factor levels lack predictive value in patients with active ankylosing spondylitis treated with golimumab. J Rheumatol 43:901–906
Poddubnyy D, Conrad K, Haibel H, Syrbe U, Appel H, Braun J, Rudwaleit M, Sieper J (2014) Elevated serum level of the vascular endothelial growth factor predicts radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis 73:2137–2143
Acknowledgments
We wish to thank all the patients who participated in the study.
Funding
This work was supported by grants from The Health and Medical Care Executive Board of the Västra Götaland, Rune and Ulla Amlövs foundation for Rheumatology Research, Göteborg’s Association Against Rheumatism, the Swedish Association Against Rheumatism, the Medical Society of Göteborg, and the Region Västra Götaland (agreement concerning research and education of doctors), COMBINE, the Margareta Rheuma research foundation and the Swedish Society of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
None.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Torres, L., Klingberg, E., Nurkkala, M. et al. Hepatocyte growth factor is a potential biomarker for osteoproliferation and osteoporosis in ankylosing spondylitis. Osteoporos Int 30, 441–449 (2019). https://doi.org/10.1007/s00198-018-4721-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4721-4