Abstract
Summary
Chronic protein malnutrition leads to child mortality in developing countries. Spirulina alga (Spi), being rich in protein and growing easily, is a good candidate as supplementation. We showed that Spi completely prevents bone growth retardation and liver disturbances observed in young rats fed a low protein diet. This supports Spi as a useful source of vegetable protein to fight against protein malnutrition.
Introduction
Chronic malnutrition is a main factor of child mortality in developing countries. A low protein diet impairs whole-body growth and leads to fatty liver in growing rats. Spi has great potential as a supplementation as it has a 60 % protein content and all essential amino acids. However, its specific impact on bone growth and the related secretion of hepatokines have not yet been studied.
Methods
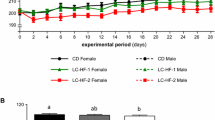
To address this question, 6-week-old female rats were fed isocaloric diets containing 10 % casein, 5 % casein, or 5 % casein + 5 % protein from Spi during 9 weeks. Changes in tibia geometry, microarchitecture, BMC, BMD, and biomechanical properties were analyzed. Serum IGF-I, FGF21, follistatin, and activin A were assessed as well as their hepatic gene expressions in addition to those of Sirt1, Ghr, and Igf1r. Hepatic fat content was also assessed.
Results
A low protein diet altered bone geometry and reduced proximal tibia BMD and trabecular bone volume. In addition, it increased hepatic fat content and led to hepatic GH resistance by decreasing serum IGF-I and increasing serum FGF21 without altering serum activin A and follistatin. Spi prevented low protein diet-induced bone, hepatic, and hormonal changes, and even led to higher biomechanical properties and lower hepatic fat content in association with specific InhbA and Follistatin expression changes vs. the 10 % casein group.
Conclusions
Altogether our results demonstrate the preventive impact of Spi on bone growth delay and hepatic GH resistance in conditions of isocaloric dietary protein deficiency.



Similar content being viewed by others
References
Muller O, Krawinkel M (2005) Malnutrition and health in developing countries. CMAJ 173:279–286
Schonfeldt HC, Gibson Hall N (2012) Dietary protein quality and malnutrition in Africa. Br J Nutr 108(Suppl 2):S69–76
Yahya ZA, Millward DJ, Yayha ZA (1994) Dietary protein and the regulation of long-bone and muscle growth in the rat. Clin Sci (Lond) 87:213–224
Van Duzen J, Carter JP, Zwagg RV (1976) Protein and calorie malnutrition among preschool Navajo Indian children, a follow-up. Am J Clin Nutr 29:657–662
Kwon DH, Kang W, Nam YS, Lee MS, Lee IY, Kim HJ, Rajasekar P, Lee JH, Baik M (2012) Dietary protein restriction induces steatohepatitis and alters leptin/signal transducers and activators of transcription 3 signaling in lactating rats. J Nutr Biochem 23:791–799
Frenk S, Gomez F, Ramos-Galvan R, Cravioto J (1958) Fatty liver in children; kwashiorkor. Am J Clin Nutr 6:298–309
Fournier C, Rizzoli R, Ammann P (2014) Low calcium-phosphate intakes modulate the low-protein diet-related effect on peak bone mass acquisition: a hormonal and bone strength determinants study in female growing rats. Endocrinology 155(11):4305–4315
Chevalley T, Bonjour JP, Ferrari S, Rizzoli R (2008) High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J Bone Miner Res 23:131–142
Fliesen T, Maiter D, Gerard G, Underwood LE, Maes M, Ketelslegers JM (1989) Reduction of serum insulin-like growth factor-I by dietary protein restriction is age dependent. Pediatr Res 26:415–419
De Sousa-Coelho AL, Marrero PF, Haro D (2012) Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J 443:165–171
Laeger T, Henagan TM, Albarado DC et al (2014) FGF21 is an endocrine signal of protein restriction. J Clin Invest 124:3913–3922
Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA (2008) Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8:77–83
Wei W, Dutchak PA, Wang X et al (2012) Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A 109:3143–3148
Kubicky RA, Wu S, Kharitonenkov A, De Luca F (2012) Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology 153:2287–2295
Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, Pedersen BK, Plomgaard P (2011) Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology 152:164–171
Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A (2006) Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res 21:626–636
Wang Y, Nishida S, Sakata T, Elalieh HZ, Chang W, Halloran BP, Doty SB, Bikle DD (2006) Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology 147:4753–4761
Wu S, Levenson A, Kharitonenkov A, De Luca F (2012) Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J Biol Chem 287:26060–26067
Eijken M, Swagemakers S, Koedam M et al (2007) The activin A-follistatin system: potent regulator of human extracellular matrix mineralization. FASEB J 21:2949–2960
Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E (2010) Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139:456–463
Arturi F, Succurro E, Procopio C et al (2011) Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab 96:E1640–1644
Yndestad A, Haukeland JW, Dahl TB et al (2009) A complex role of activin A in non-alcoholic fatty liver disease. Am J Gastroenterol 104:2196–2205
Ciferri O (1983) Spirulina, the edible microorganism. Microbiol Rev 47:551–578
Buono S, Langellotti AL, Martello A, Rinna F, Fogliano V (2014) Functional ingredients from microalgae. Food Funct 5(8):1669–1685
Roman GC (2013) Nutritional disorders in tropical neurology. Handb Clin Neurol 114:381–404
Maranesi M, Barzanti V, Carenini G, Gentili P (1984) Nutritional studies on Spirulina maxima. Acta Vitaminol Enzymol 6:295–304
Tranquille N, Emeis JJ, de Chambure D, Binot R, Tamponnet C (1994) Spirulina acceptability trials in rats. A study for the “MELISSA” life-support system. Adv Space Res 14:167–170
Salazar M, Chamorro GA, Salazar S, Steele CE (1996) Effect of Spirulina maxima consumption on reproduction and peri- and postnatal development in rats. Food Chem Toxicol 34:353–359
Voltarelli FA, de Mello MA (2008) Spirulina enhanced the skeletal muscle protein in growing rats. Eur J Nutr 47:393–400
Simpore J, Kabore F, Zongo F, Dansou D, Bere A, Pignatelli S, Biondi DM, Ruberto G, Musumeci S (2006) Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr J 5:3
Azabji-Kenfack M, Dikosso SE, Loni EG et al (2011) Potential of Spirulina platensis as a nutritional supplement in malnourished HIV-infected adults in sub-Saharan Africa: a randomised, single-blind study. Nutr Metab Insights 4:29–37
Moura LP, Puga GM, Beck WR, Teixeira IP, Ghezzi AC, Silva GA, Mello MA (2011) Exercise and spirulina control non-alcoholic hepatic steatosis and lipid profile in diabetic Wistar rats. Lipids Health Dis 10:77
Laib A, Barou O, Vico L, Lafage-Proust MH, Alexandre C, Rugsegger P (2000) 3D micro-computed tomography of trabecular and cortical bone architecture with application to a rat model of immobilisation osteoporosis. Med Biol Eng Comput 38:326–332
Jiang SD, Shen C, Jiang LS, Dai LY (2007) Differences of bone mass and bone structure in osteopenic rat models caused by spinal cord injury and ovariectomy. Osteoporos Int 18:743–750
Ju YI, Sone T, Okamoto T, Fukunaga M (2008) Jump exercise during remobilization restores integrity of the trabecular architecture after tail suspension in young rats. J Appl Physiol 104:1594–1600
Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD (2007) Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem 282:15872–15883
Hoang QQ, Sicheri F, Howard AJ, Yang DS (2003) Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 425:977–980
Dubois-Ferriere V, Brennan TC, Dayer R, Rizzoli R, Ammann P (2011) Calcitropic hormones and IGF-I are influenced by dietary protein. Endocrinology 152:1839–1847
Fujimoto M, Tsuneyama K, Fujimoto T, Selmi C, Gershwin ME, Shimada Y (2012) Spirulina improves non-alcoholic steatohepatitis, visceral fat macrophage aggregation, and serum leptin in a mouse model of metabolic syndrome. Dig Liver Dis 44:767–774
Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA (2009) Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem 284:19937–19944
Fazeli PK, Klibanski A (2014) Determinants of GH resistance in malnutrition. J Endocrinol 220:R57–65
Sos BC, Harris C, Nordstrom SM, Tran JL, Balazs M, Caplazi P, Febbraio M, Applegate MA, Wagner KU, Weiss EJ (2011) Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest 121:1412–1423
Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X (2009) Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9:327–338
Kreidl E, Ozturk D, Metzner T, Berger W, Grusch M (2009) Activins and follistatins: emerging roles in liver physiology and cancer. World J Hepatol 1:17–27
Guasti L, Silvennoinen S, Bulstrode NW, Ferretti P, Sankilampi U, Dunkel L (2014) Elevated FGF21 leads to attenuated postnatal linear growth in preterm infants through GH resistance in chondrocytes. J Clin Endocrinol Metab 99:E2198–2206
Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Savvides M, Papatheodorou A, Terpos E (2013) Circulating activin-A is elevated in postmenopausal women with low bone mass: the three-month effect of zoledronic acid treatment. Osteoporos Int 24:2127–2132
Brennan-Speranza TC, Rizzoli R, Kream BE, Rosen C, Ammann P (2011) Selective osteoblast overexpression of IGF-I in mice prevents low protein-induced deterioration of bone strength and material level properties. Bone 49:1073–1079
Garrett WS (2013) Kwashiorkor and the gut microbiota. N Engl J Med 368:1746–1747
Gordon JI, Dewey KG, Mills DA, Medzhitov RM (2012) The human gut microbiota and undernutrition. Sci Transl Med 4:137, ps112
Ohlsson C, Sjogren K (2015) Effects of the gut microbiota on bone mass. Trends Endocrinol Metab 26:69–74
Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27:1357–1367
Deng R, Chow TJ (2010) Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther 28:e33–45
Acknowledgments
The authors thank Mrs. S. Clément for her expert technical assistance with animals and biochemical measurements, and Mrs I. Badoud for her expert technical assistance with biomechanical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 12 kb)
ESM 2
Supplementary Fig. 1 Binary images created with ImageJ software representing the Oil red O staining of images in Fig. 3. (PPTX 114 kb)
ESM 3
(DOCX 13 kb)
ESM 4
(DOCX 16 kb)
ESM 5
Supplementary Fig. 2 Representative MicroCT images (3D) of the proximal tibia metaphysis for Con10, Con5, and Con5 + Spi5 groups. Scale bar: 1 mm. (PPTX 150 kb)
ESM 6
(DOCX 13 kb)
ESM 7
(DOCX 12 kb)
ESM 8
Supplementary Fig. 3 Correlations between the Ghr hepatic gene expression and serum FGF21 (A) or average size of hepatic lipid droplets (B) by using Pearson correlation tests. All the groups were used for these analyses. p < 0.05 was considered as statistically significant. (PPTX 40 kb)
Rights and permissions
About this article
Cite this article
Fournier, C., Rizzoli, R., Bouzakri, K. et al. Selective protein depletion impairs bone growth and causes liver fatty infiltration in female rats: prevention by Spirulina alga. Osteoporos Int 27, 3365–3376 (2016). https://doi.org/10.1007/s00198-016-3666-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3666-8