Abstract
Purpose
The diagnostic process in patients after painful total knee arthroplasty (TKA) is challenging. The more clinical and radiological information about a patient with pain after TKA is included in the assessment, the more reliable and sustainable the advice regarding TKA revision can be. The primary aim was to investigate the position of TKA components and evaluate bone tracer uptake (BTU) using pre-revision SPECT/CT and correlate these findings with previously published pain patterns in painful patients after TKA.
Methods
A prospectively collected cohort of 83 painful primary TKA patients was retrospectively evaluated. All patients followed a standardized diagnostic algorithm including 99m-Tc-HDP-SPECT/CT, which led to a diagnosis indicating revision surgery. Pain character, location, dynamics and radiation were systematically assessed as well as TKA component position in 3D-CT. BTU was anatomically localized and quantified using a validated localization scheme. Component positioning and BTU were correlated with pain characteristics using non-parametric Spearman correlations (p < 0.05).
Results
Based on Spearman’s rho, significant correlations were found between pain and patients characteristics and SPECT/CT findings resulting in nine specific patterns. The most outstanding ones include: Pattern 1: More flexion in the femoral component correlated with tender/splitting pain and patella-related pathologies. Pattern 3: More varus in the femoral component correlated with dull/heavy and tingling/stinging pain during descending stairs, unloading and long sitting in patients with high BMI and unresurfaced patella. Pattern 6: More posterior slope in the tibial component correlated with constant pain.
Conclusion
The results of this study help to place component positioning in the overall context of the "painful knee arthroplasty" including specific pain patterns. The findings further differentiate the clinical picture of a painful TKA. Knowing these patterns enables a prediction of the cause of the pain to be made as early as possible in the diagnostic process before the state of pain becomes chronic.
Level of evidence
Level III
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The causes for recurrent pain after total knee arthroplasty (TKA) are manifold and range from knee joint-related factors such as infection, arthrofibrosis, patellofemoral problems, malposition or malalignment, loosening or instability to non-knee joint-related causes such as psychological disorders, vascular pathologies, back or hip problems [5, 23].
The diagnostic process is challenging. Besides a detailed patient history, a thorough clinical examination, radiological, serological and microbiological investigations are part of a standardized diagnostic algorithm for unhappy patients after TKA [23, 35]. As radiological work-up conventional radiographs, stress radiographs, CT or single-photon-emission-computed-tomography/computer tomography (SPECT/CT) and magnetic-resonance imaging (MRI) are often indicated [10, 16]. After a thorough diagnostic work-up, a revision surgery should only be performed if the cause(s) of the complaints described were identified and fit the clinical picture. Revision surgery for unexplained pain has consistently been shown to result in poor outcomes [16, 23, 30].
In 2020, this study group was able for the first time to identify pain characteristics in unhappy patients after TKA and link these to specific underlying pathologies. Based on this, pain patterns were found [25] (Fig. 1). However, objective radiological findings, such as TKA component positioning, were not collected in this study, which constitutes a major limitation.
Illustration of pain patterns according to positive Spearman’s correlations among various pain characteristics and pathologies. E.g. instability correlates significantly with jumping/shooting, pricking/lancinating and tugging/wrenching pain character aggravated by chair raising or starting. Magnifier shows correlations between pain characters and dynamics. Reprinted with permission [25]
Over the past 10 years, SPECT/CT has become an increasingly recognized and appreciated diagnostic imaging modality in patients with pain after TKA. A considerable number of studies have been published proving its beneficial clinical use in establishing the diagnosis and providing guidance for further treatment [1, 11, 12, 14, 31]. SPECT/CT allows a combined assessment of structural, mechanical, and functional information [10]. In 2016, a retrospective study was performed on a series of 37 patients after bilateral TKA to evaluate the differences of bone tracer uptake (BTU) in symptomatic and asymptomatic knees after bilateral TKA and identify typical BTU patterns with regards to TKA component position and alignment. The authors could show a significant correlation of TKA component position and BTU and identified typical BTU patterns in symptomatic and asymptomatic knees [2].
To date, there exists no study that links findings of SPECT/CT to specific pain patterns in patients with pain after TKA. The more clinical and radiological information about a patient with pain after TKA is included in the assessment, the more reliable and sustainable the advice regarding TKA revision can be [34].
Therefore, the primary aim of this study was to assess the position of TKA components and evaluate BTU using pre-revision SPECT/CT and correlate these findings with previously published pain patterns in painful patients after TKA. It was hypothesized that specific TKA component positioning and BTU patterns can be correlated with recently identified pain patterns.
Materials and methods
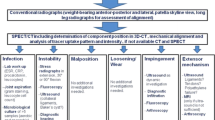
This study was approved by the local ethical committee (2017-02048) and was performed in accordance with the ethical standards of the responsible committee and with the guidelines of the Helsinki Declaration of 1975, as revised in 2008. A written informed consent was signed by every patient. A consecutive number of 83 patients, who underwent primary TKA from 1993 to 2017 and complained about unilateral persistent knee pain and who underwent a revision surgery after completing the diagnostic algorithm including SPECT/CT were prospectively collected and then included in this retrospective cohort study. The cohort is mostly consistent (83 of initially 97 patients) with that in the previously published study on TKA pain patterns [25]. Data were prospectively collected from a specialized knee centre in which the patients presented between 2012 and 2017 due to persistent pain after primary TKA. All patients followed a standardized diagnostic algorithm (Fig. 2) including detailed clinical examination, standardized (anterior–posterior and lateral weight bearing, patellar skyline view) and stress radiographs (anterior–posterior projection with full extension and 30° flexion for varus/valgus laxity; lateral projection in 15° and 90° flexion for anterior/posterior laxity using a Telos device with 15 kp) and 99 m-Tc-HDP-SPECT/CT. At the end of the standardized diagnostic process, the patient's pain was linked to one or more of the pathologies listed in Table 1, which set the indication for the proposed revision surgery. Patients, who have suffered a trauma, underwent revision surgery in other hospitals between primary TKA and presentation at our knee centre, periprosthetic joint infection, patients with exclusively neuropathic pain or not fully completed SPECT/CT protocol were excluded from this study (N = 46).
The “Bruderholz” standardized diagnostic algorithm for patients with pain after total knee arthroplasty. WB, weight bearing; SPECT/CT, single photon emission computed tomography/computer tomography. Reprinted with permission [25]
Data collection
Within the framework of the consultations at the knee centre, all the variables listed in Table 1 were described and documented in a consultation report by one expert knee surgeon (senior orthopedic consultant) for each patient in a standardized manner. The character of pain was described according to the sensory pain descriptors (dimension 1–10) used in the McGill Pain Questionnaire [27]. Pain dynamics were categorized according to the seven types of Laskin [19] and extended and adapted to the patient cohort. A member of this study group retrospectively collected and evaluated these criteria based on surgery and consultation reports meticulously and published the resulting pain patterns in 2021 (Fig. 1, [25]). Due to varying frequencies of pain characteristics reported by the patients, the N per dimension is not identical. Thereafter, another study group member evaluated all SPECT/CT images and recorded TKA component positioning and BTU.
Radiological imaging
All patients underwent 99m-Tc- hydroxymethyl diphosphonate (HDP) SPECT/CT imaging following a standardized and highly reliable protocol [10, 15, 31]. The mean time from primary TKA to SPECT/CT was 2.5 ± 3.0 years (range 0.04–19.7 years, in 20 cases < 12 months). All patients received a commercial 700MBq (18.92mCi) 99m-Tc-HDP injection (Malinckrodt, Wollerau, Switzerland). SPECT/CT was performed using a hybrid system (Symbia T16, Siemens, Erlangen, Germany), which consists of a pair of low-energy, high-resolution collimators and a dual-head gamma camera with an integrated 16-slice CT scanner (collimation of 16 × 0.75 mm). Planar scintigraphic images were taken in the perfusion phase (immediately after injection), the soft tissue phase (1–5 min after injection) and the delayed metabolic phase (at least 2 h after injection). SPECT/CT was performed with a matrix size of 128 × 128, an angle step of 32, and a time per frame of 25 s 2 h after injection.
Assessment of TKA position and mechanical alignment
Mechanical alignment and TKA position were assessed using a customized validated 3D-software which has been proven highly accurate (Fig. 3) [10, 15, 31]. For assessment of mechanical alignment, reconstructed images were displayed in orthogonal axial, coronal, and sagittal planes. Coronal (valgus–varus), rotational (internal–external rotation), and sagittal (flexion–extension, antero-posterior slope) TKA component position were measured in relation to standardized landmarks [31].
SPECT/CT images of a 74-year-old male patient with left painful total knee arthroplasty (TKA). Mechanical alignment and TKA position were assessed using a customized validated 3D-software “Orthoexpert®”. a–g show angle measurements of the femoral (b–d) and tibial (e–g) component. The increased internal rotation of 4° of the femoral shield (d) and varus positioning of 3° of the tibial component (f) causes pain in this TKA patient
For statistical analysis, angle cut-off values had to be determined to distinguish between conspicuous and inconspicuous. Based on the existing literature, the following values were defined as conspicuous [2,3,4, 6, 8, 9, 17, 36]: Femoral varus/valgus > 2°, flexion/extension > 10°/ < 0° and external/internal rotation > 10°/ > 5°. Tibial varus/valgus > 2°, posterior/anterior slope > 10°(CR), > 5° (PS)/ < 0° (CR, PS) and external/internal rotation > 10°/ > 5°. Tibiofemoral varus/valgus > 2°.
Measurement of BTU
For BTU, anatomically precise localization and quantification were recorded on the basis of a validated standardized localization scheme [11, 28, 31]. This localisation scheme (1 = medial, 2 = lateral and 3 = central) consisted of eight femoral (f-1sa, f-1ia, f-1sp, f-1ip, f-2sa, f-2ia, f-2sp, f-2ip), eight patellar (p-1sa, p-1sp, p-1ia,p-1ip, p-2sa, p-2sp, p-2ia,p-2ip), and 18 tibial zones (t-1astem, t-1atip, t-1atray, t-1pstem, t-1ptip, t-1ptray, t-2astem, t-2atip, t-2atray, t-2pstem, t-2ptip, t-2ptray, t-3astem, t-3atip, t-3atray, t-3pstem, t-3ptip, t-3ptray). It was used to accurately map the examined BTU volume in each anatomical area of interest (Fig. 4). Mean BTU values (mean ± standard deviation, median, and range) for each area of the localization scheme were recorded and normalized values calculated. For normalization, a specific area within the distal femoral shaft was used as reference region for all zones to obtain ratios of absolute measures. For statistical interpretation, based on factor analysis within femur, patella and tibia, areas were grouped into five major regions (femur total, patella total, tibial tray total, tibia stem and tip lateral, tibia stem and tip medial and central).
Standardized and validated scheme for localisation of bone tracer uptake after total knee arthroplasty. Reprinted with permission [12]
Statistical analysis
Results are presented as means, ranges and standard deviations (SD) or with numbers and percentages. To correlate binary variables, phi coefficients were calculated. Because the cut angles values and some BTU values are not normally distributed, ordinal Spearman correlations (rho) were used for all correlations. Two-sided p values < 0.05 were considered significant. All data were analyzed by an independent professional statistician using SPSS Statistics for Windows, version 26.0 (Armonk, NY: IBM Corp, USA). A post hoc analysis using G*Power, version 3.1.9 (University of Kiel, Germany) tested that, for the given N = 83, a correlation of rho = 0.28 can be found with a power of 80%. For the number of patients with pain characteristic values (N = 59), the same correlation has to be rho = 0.35.
Results
Patient demographics and TKA characteristics of all 83 patients included are shown in Table 2. Due to the set-up in a specialized knee centre with focus on painful TKA, most of the patients were referred from other surgeons to our clinic. Therefore, primary TKA was performed by a total of 55 different surgeons.
Table 3 provides an overview of the pain generators based on the standardized diagnostics. The majority of patients were diagnosed with more than one underlying pathology (77 patients = 91.6%). Characteristics that occurred in less than four patients (N < 4) were excluded from the statistical analysis (N total = 19; Tables 3 and 4).
The frequencies of all pain characteristics are shown in Table 4 and correspond to the findings of the preceding study [25]. Most patients described more than one dimension of each pain characteristic.
Measurements of TKA component position in 3D reconstructed CT and BTU in SPECT/CT are shown in Tables 5 and 6. The greatest variation in component positioning was found on the femur in the sagittal plane (1° extension, 19° flexion) and on the tibia in the axial plane (11° internal rotation, 19° external rotation). The highest BTU was found in the supero-lateral areas of the femur and patella, and in the postero-lateral tibial tray.
Numerous significant correlations were found between various pain and patients characteristics and SPECT/CT findings including TKA component positioning (Tables 7 and 8).
Based on the correlations found the following patterns (P1–P9) were identified (Fig. 5):
-
P1: More flexion in the femoral TKA component is associated with tender/splitting pain and patella-related pathologies (p < 0.05).
-
P2: More valgus in the femoral TKA component is associated with constant pain (p < 0.001), in particular at night (p < 0.05), and instability-related pathologies.
-
P3: More varus in the femoral TKA component is associated with dull/heavy and tingling/stinging pain during descending stairs, unloading and long sitting (p < 0.05) in patients with high BMI (p < 0.01) and unresurfaced patella (p < 0.05).
-
P4: More internal rotation of the tibial TKA component is associated with tugging/wrenching pain during unloading (p < 0.05) and radiation to the spine (p < 0.01).
-
P5: More varus in the tibial TKA component is associated with starting pain (p < 0.05) in patients with high BMI (p < 0.05).
-
P6: More posterior slope in the tibial TKA component is associated with constant pain (p < 0.05).
-
P7: More tibiofemoral valgus alignment is associated with constant pain, in particular at night, and by walking downhill and with low BMI (p < 0.05).
-
P8: More tibiofemoral varus alignment is associated with patients with high BMI (p < 0.001).
-
P9: Increased BTU laterally at the stem and tip of the tibial component is associated with constant pain (p < 0.05), whereas increased BTU in the area of the tray is associated with patients with pain aggravation in flexion (p < 0.01).
Illustration of pain patterns according to positive Spearman’s correlations with TKA component positioning and pathologies. E.g. More flexion in the femoral component correlates with tender/splitting pain character and patella-related pathologies. Fine, dotted red line: association not significant. Fem femoral; BMI body mass index
Discussion
The principal finding of this study is the assignment of pain patterns to TKA component positioning in painful patients after TKA. These patterns were identified with regards to pain character, location, dynamics and radiation and linked to specific component positions in all radiological planes.
The most important findings and implications of this study were the following:
First of all, in this study, component-positioning-related pathologies accounted for the greatest proportion (81.9%) followed by patella-related problems (56.6%) and instability (51.8%). This is in contrast to most register data published in recent years [20,21,22]. In most registries, aseptic loosening and infection followed by instability lead the list of TKA failures. An explanation for this distribution of frequencies might be the standardized, diagnostic work-up of our patients including routinely performed conventional and stress radiographs as well as 3D-SPECT/CT scans [26]. The latter was used to determine the specific component position in three radiological planes in all patients included. Thus, all possible causes for pain are systematically evaluated and in- or excluded in the course of the diagnostic process. It is, therefore, not surprising, that in most patients (91.6%), more than one pathology was found and TKA component-, instability- and patella-related problems are heading the list [26]. These findings correspond to the results of Hofmann et al. who found also high proportions of component- (54%) or alignment-related (41%) failure modes when malalignment were routinely examined [16]. In 50% of the revision cases, they held two or more reasons responsible for the implant failure [16].
Second, correlations were found between TKA component positioning, pain and TKA characteristics and other pathologies. More flexion in the femoral component was significantly associated with tender/splitting pain and patella-related pathologies (P1). More valgus in the femoral component and in the tibiofemoral alignment correlated with constant pain, in particular at night (P2, P7), whereas more varus in the femoral component correlated with dull/heavy and tingling/stinging pain during long sitting, descending stairs and by unloading in patients with unresurfaced patella (P3). Femoral component positioning in valgus (P2) and internal rotation was associated with instability-related pathologies, however, not significantly. More internal rotation of the tibial component correlated with tugging/wrenching pain during unloading and radiation to the spine (P4), whereas increased posterior slope in the tibial component caused constant pain (P6). Interestingly, a strong correlation was found between arthrofibrosis (other problems) and valgus positioning of the tibial component (Table 8). A clear explanation for this cannot be given. Although it is known that correct positioning in the sagittal plane of the prosthesis is imperative to achieve satisfactory ROM after TKA [24], there is no literature on the influence of component positioning in the coronal plane on arthrofibrosis. One speculation could be that excessive valgus in the tibial component leads to increased polyethylene wear and this might promote arthrofibrosis. Tibiofemoral not only varus alignment, but also varus positioning of the femoral and tibial component itself, correlated with patients with higher BMI (P3, P5, P8)—tibiofemoral valgus alignment with lower BMI. The association between obesity and increased risk of varus malalignment post-surgically has been described in literature in the past [7].
These findings play a crucial role in the diagnostics of patients with painful TKA. Component positioning represents a major challenge in this process and often confronts the clinician with the question whether a specific component position is considered as pathological or within the range of the “norm”. It is generally agreed, that TKA component positioning should not be viewed in absolute terms, but in the context of the complaints as a whole within the diagnostic process. However, reference values, which were also used in this study for statistical purposes to distinguish between conspicuous and inconspicuous TKA component positioning, are provided in the literature as follows [2,3,4, 6, 8, 9, 17, 36]: The femoral TKA component should be positioned with 0° ± 2° varus/valgus towards the mechanical axis. In the sagittal plane 5° ± 3° flexion can be accepted, as approximately 5–7° of flexion are built into the anterior flange of the femoral TKA component. The axial alignment should be between maximally 10° external and 5° internal rotation [2]. The tibial TKA component should be positioned with 0° ± 2° varus/valgus towards the mechanical axis. In the sagittal plane, it is generally aimed for a posterior slope of 5°–7° in a posterior cruciate retaining (CR) TKA and 0°–3° in a posterior cruciate substituting (PS) TKA. The rotational orientation should be set between 10° external and maximally 5° internal rotation. Femoral internal rotation of more than 5° may lead to patellofemoral overloading of the lateral patellar facet and lateral lift-off of the femoral condyle from the polyethylene inlay, which is called mid-flexion instability [2, 4, 8]. This association was seen in our data, too, but below significance level. This might be due to the fact, that the expression of internal rotation of the femoral component was not very strong (median -3°), but the most extreme value was -11°. The same can be applied to the not-significant association of valgus positioning of the femoral component and instability-related complaints. In the sagittal plane, flexion of the femoral TKA component increases the patellofemoral pressure and leads to a “pseudo” patella baja [33]. This correlation in combination with tender/splitting pain character was also found in P1. Anterior slope of the tibial TKA component may lead to a tight flexion gap and subsequent flexion deficit. Generally, it is aimed for a posterior slope of 0°–7° [3]. Clearly, this depends on the type of TKA implant [3]. In a posterior cruciate retaining (CR) TKA it is aimed for a posterior slope of 5°–7°, in a posterior cruciate substituting (PS) TKA 0°–3° [2]. Thus, this explains the correlation found in this study between anterior slope of the tibial component and PS inserts (Table 8). Tibial internal rotation may lead to patellofemoral maltracking, popliteal tendon impingement and anterior and posterior soft tissue pain [6, 36]. Based on the results of this study, more variables associated with internal tibial rotation can be added: tugging/wrenching pain character, aggravation when unloading and radiation to the spine.
However, there is an individual range of TKA component position in each direction, which is accepted by the patient, which we call the “envelope of TKA position” [2]. Awengen et al. postulated, that this envelope may be slightly different between patients. If this envelope is narrow a slight deviation from the optimal TKA position leads to pain after TKA. If this envelope is wide, a rather large deviation does not cause any problems [2].
A significant correlation between TKA component positioning and pain localisation could not be demonstrated in this study. However, this is not surprising as in the previously published pain patterns by Mathis et al., the anatomical location has been proven no being helpful in localizing the underlying problem [25].
Third, highest mean BTU in SPECT/CT was found in supero-lateral areas of the femur and patella, and in the postero-lateral tibial tray. Areas in the patella and around the tibial tray showed almost twice as high mean BTU values than femoral or stem and tip areas of the tibia. Therefore, one could argue that increased BTU at femoral areas and areas around the tibial stem or tip are more specific for identification of pathologies in patients with TKA. These findings are consistent with previous results of Awengen et al. who compared BTU distribution patterns in asymptomatic and symptomatic TKA patients [2]. However, in regards to correlations of BTU with pain or TKA characteristics, the results are less pretentious. Only an agreement between BTU and pain localisation could be shown. However, a finding which has already been described in the past [13]. Furthermore, the results suggested that increased BTU laterally at the stem and tip caused more constant pain, whereas BTU around the area of the tibial tray was increased in patients with pain aggravation in flexion. Men showed significantly higher BTU in the patella compared to women and CR knee systems showed higher BTU in femur and tibia. With regard to the latter, it can be speculated that CR knee systems generally allow for larger antero-posterior translation compared to PS systems based on the increased posterior slope (-12 to 3° in this cohort) [18], thereby generating more stress on the tibia and femur resulting in higher BTU [2, 14].
Several limitations of the present study have to be acknowledged. This was a retrospective series of prospectively collected 83 TKAs assigned to a specialized knee centre, operated by a team of different surgeons from various hospitals. Thus, a prospective study design including only painful TKAs from one surgeon would improve the comparability of the patient significantly. Another limitation is the restriction of underlying pathologies to anatomical and mechanical nature. It is a well-known fact, that psychological and social determinants play an important role in the perception of pain in patients after TKA [29]. However, these factors have not been assessed in this study. A more systematic and multidimensional pain assessment according to international guidelines should be aimed for in a future study.
Conclusion
The painful TKA remains a challenge for the surgeon [16]. Revision TKA for unexplained knee pain might harm even more. At 2 and 5 years after a TKA revision, pain is still reported three times more frequently than after a primary arthroplasty [32]. Therefore, before revising a TKA, it is inevitable to perform a conscientious and comprehensive clarification and evaluation of all eligible causes of failure. The clinician reviewing patients with a painful TKA should integrate as many variables as possible in the algorithm to reach the diagnosis. The evaluation of the TKA component positioning is an important part of this diagnostic process. The interpretation of the positioning, however, is very complex and often challenging even for experienced surgeons. The results of this study help to place component positioning in the overall context of the "painful knee arthroplasty" including specific pain patterns. Hence, these data support our hypothesis that specific TKA component positioning and BTU patterns can be correlated with typical pain characteristics.
The findings of this study add significant value to the previously published TKA pain patterns and further differentiate the clinical picture of a painful knee after TKA. Knowing these pain patterns to its utmost extent enables a prediction of the cause of the pain to be made as early as possible in the diagnostic process. If the causes of the described complaints are known, a decision for a necessary therapy can be made reliably and sustainably at an early stage before the state of pain becomes chronic.
Abbreviations
- TKA:
-
Total knee arthroplasty
- SPECT/CT:
-
Single-photon-emission-computed-tomography/computer tomography
- BTU:
-
Bone tracer uptake
- HDP:
-
Hydroxymethylen diphosphonate
- MRI:
-
Magnetic-resonance imaging
- P1-9:
-
Pattern 1–9
- CR:
-
Cruciate-retaining
- PS:
-
Posterior-stabilized
References
Al-Nabhani K, Michopoulou S, Allie R, Alkalbani J, Saad Z, Sajjan R et al (2014) Painful knee prosthesis: can we help with bone SPECT/CT? Nucl Med Commun 35:182–188
Awengen R, Rasch H, Amsler F, Hirschmann MT (2016) Symptomatic versus asymptomatic knees after bilateral total knee arthroplasty: what is the difference in SPECT/CT? Eur J Nucl Med Mol Imaging 43:762–772
Benjamin J (2006) Component alignment in total knee arthroplasty. Instr Course Lect 55:405–412
Berger RA, Rubash HE, Seel MJ, Thompson WH, Crossett LS (1993) Determining the rotational alignment of the femoral component in total knee arthroplasty using the epicondylar axis. Clin Orthop Relat Res 286:40–47
Bierke S, Haner M, Karpinski K, Hees T, Petersen W (2020) Midterm Effect of Mental Factors on Pain, Function, and Patient Satisfaction 5 Years After Uncomplicated Total Knee Arthroplasty. J Arthroplasty 35:105–111
Bindelglass DF (2001) Rotational alignment of the tibial component in total knee arthroplasty. Orthopedics 24:1049–1051
Estes CS, Schmidt KJ, McLemore R, Spangehl MJ, Clarke HD (2013) Effect of body mass index on limb alignment after total knee arthroplasty. J Arthroplasty 28:101–105
Fehring TK (2000) Rotational malalignment of the femoral component in total knee arthroplasty. Clin Orthop Relat Res 380:72–79
Gromov K, Korchi M, Thomsen MG, Husted H, Troelsen A (2014) What is the optimal alignment of the tibial and femoral components in knee arthroplasty? Acta Orthop 85:480–487
Hirschmann MT, Amsler F, Rasch H (2015) Clinical value of SPECT/CT in the painful total knee arthroplasty (TKA): a prospective study in a consecutive series of 100 TKA. Eur J Nucl Med Mol Imaging 42:1869–1882
Hirschmann MT, Henckel J, Rasch H (2013) SPECT/CT in patients with painful knee arthroplasty-what is the evidence? Skeletal Radiol 42:1201–1207
Hirschmann MT, Iranpour F, Konala P, Kerner A, Rasch H, Cobb JP et al (2010) A novel standardized algorithm for evaluating patients with painful total knee arthroplasty using combined single photon emission tomography and conventional computerized tomography. Knee Surg Sports Traumatol Arthrosc 18:939–944
Hirschmann MT, Konala P, Iranpour F, Kerner A, Rasch H, Friederich NF (2011) Clinical value of SPECT/CT for evaluation of patients with painful knees after total knee arthroplasty–a new dimension of diagnostics? BMC Musculoskelet Disord 12:36
Hirschmann MT, Mathis D, Rasch H, Amsler F, Friederich NF, Arnold MP (2013) SPECT/CT tracer uptake is influenced by tunnel orientation and position of the femoral and tibial ACL graft insertion site. Int Orthop 37:301–309
Hirschmann MT, Wagner CR, Rasch H, Henckel J (2012) Standardized volumetric 3D-analysis of SPECT/CT imaging in orthopaedics: overcoming the limitations of qualitative 2D analysis. BMC Med Imaging 12:5
Hofmann S, Seitlinger G, Djahani O, Pietsch M (2011) The painful knee after TKA: a diagnostic algorithm for failure analysis. Knee Surg Sports Traumatol Arthrosc 19:1442–1452
Hungerford DS (1995) Alignment in total knee replacement. Instr Course Lect 44:455–468
Ishii Y, Noguchi H, Sato J, Sakurai T, Toyabe SI (2017) Anteroposterior translation and range of motion after total knee arthroplasty using posterior cruciate ligament-retaining versus posterior cruciate ligament-substituting prostheses. Knee Surg Sports Traumatol Arthrosc 25:3536–3542
Laskin RS (1999) The painful knee. Orthopedics 22:869–870
No authors listed (2019) American Joint Replacement Registry (AJRR). Sixth AJRR Annual Report 2019 on Hip and Knee Arthroplasty Data. https://connect.ajrr.net/hubfs/PDFs%20and%20PPTs/AAOS_AJRR_2019_Annual_Report_Update_FINAL_150DPI.pdf?utm_campaign=2019%20AJRR%20AR&utm_medium=email&_hsenc=p2ANqtz-_R9e0yFLoCd0ilZVdi-Kx2fWPDNQKU55Z-MaFp5uUfeJUAIm2rJh-Lca146_R2hFgmvbRSs0CNPzjVEuIFjUh5cnj8k4QZYaNRm3lAyiu-JH3Pc2s&_hsmi=79114016&utm_content=79114016&utm_source=hs_automation&hsCtaTracking=f8fa4e79-6709-45d2-b51b-908382b7d5a7%7Cb61796dc-af0d-4a90-9b72-871845865e0b (accessed July 5, 2020)
No authors listed (2019) National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 16th Annual Report 2019. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf (accessed July 6, 2020)
No authors listed (2019) Swedish Knee Arthroplasty Register. Annual Report 2019. http://myknee.se/pdf/SVK_2019_1.0_Eng.pdf (accessed July 6, 2020)
Mandalia V, Eyres K, Schranz P, Toms AD (2008) Evaluation of patients with a painful total knee replacement. J Bone Jt Surg Br 90:265–271
Manrique J, Gomez MM, Parvizi J (2015) Stiffness after total knee arthroplasty. J Knee Surg 28:119–126
Mathis DT, Hauser A, Iordache E, Amsler F, Hirschmann MT (2021) Typical pain patterns in unhappy patients after total knee arthroplasty. J Arthroplasty. https://doi.org/10.1016/j.arth.2021.01.040
Mathis DT, Lohrer L, Amsler F, Hirschmann MT (2021) Reasons for failure in primary total knee arthroplasty—an analysis of prospectively collected registry data. J Orthop 23:60–66
Melzack R (1987) The short-form McGill Pain Questionnaire. Pain 30:191–197
Murer AM, Hirschmann MT, Amsler F, Rasch H, Huegli RW (2020) Bone SPECT/CT has excellent sensitivity and specificity for diagnosis of loosening and patellofemoral problems after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 28:1029–1035
Nunez-Cortes R, Chamorro C, Ortega-Palavecinos M, Mattar G, Paredes O, Besoain-Saldana A et al (2019) Social determinants associated to chronic pain after total knee arthroplasty. Int Orthop 43:2767–2771
Phillips JR, Hopwood B, Stroud R, Dieppe PA, Toms AD (2017) The characterisation of unexplained pain after knee replacement. Br J Pain 11:203–209
Rasch H, Falkowski AL, Forrer F, Henckel J, Hirschmann MT (2013) 4D-SPECT/CT in orthopaedics: a new method of combined quantitative volumetric 3D analysis of SPECT/CT tracer uptake and component position measurements in patients after total knee arthroplasty. Skeletal Radiol 42:1215–1223
Singh JA, Gabriel S, Lewallen D (2008) The impact of gender, age, and preoperative pain severity on pain after TKA. Clin Orthop Relat Res 466:2717–2723
Slevin O, Schmid FA, Schiapparelli FF, Rasch H, Amsler F, Hirschmann MT (2017) Coronal femoral TKA position significantly influences in vivo patellar loading in unresurfaced patellae after primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25:3605–3610
Tolk JJ, Waarsing JEH, Janssen RPA, van Steenbergen LN, Bierma-Zeinstra SMA, Reijman M (2020) Development of preoperative prediction models for pain and functional outcome after total knee arthroplasty using the dutch arthroplasty register data. J Arthroplasty 35:690–698
Toms AD, Mandalia V, Haigh R, Hopwood B (2009) The management of patients with painful total knee replacement. J Bone Jt Surg Br 91:143–150
Uehara K, Kadoya Y, Kobayashi A, Ohashi H, Yamano Y (2002) Bone anatomy and rotational alignment in total knee arthroplasty. Clin Orthop Relat Res 402:196–201
Acknowledgements
This work was funded by the Deutsche Arthrose Hilfe e.V, Saarlouis, Germany [grant number P458-A439, 2019] and Margarete and Walter Lichtenstein-Stiftung, Basel, Switzerland [grant number 3MS1036, 2019].
Funding
Open Access funding provided by Universität Basel (Universitätsbibliothek Basel). This work was funded by the Deutsche Arthrose Hilfe e.V, Saarlouis, Germany [grant number P458-A439, 2019] and Margarete and Walter Lichtenstein-Stiftung, Basel, Switzerland [Grant number 3MS1036, 2019].
Author information
Authors and Affiliations
Contributions
ST evaluated all SPECT/CT images and recorded TKA component positioning and BTU. HR supervised the radiological evaluation of ST. AH retrospectively collected and evaluated clinical pain criteria based on surgery and consultation reports. FA performed statistical analysis. MTH and DTM participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
One or more authors of this paper have disclosed potential conflict of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. Full disclosure statements have been provided for each of the authors.
Ethical approval
The study was approved by the local ethical committee (2017-02048) and was performed in accordance with the ethical standards of the responsible committee and with the guidelines of the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
A written informed consent was signed by every patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mathis, D.T., Tschudi, S., Amsler, F. et al. Correlations of typical pain patterns with SPECT/CT findings in unhappy patients after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 30, 3007–3023 (2022). https://doi.org/10.1007/s00167-021-06567-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06567-y